INTRODUCTION
Inland aquatic environments are under pressure in the humid tropics from population growth, land use and climate change. In South-East Asia, with four of 25 global biodiversity hotspots (Myers et al. Reference MYERS, MITTERMEIER, MITTERMEIER, DA FONSECA and KENT2000), an increase in emissions of N and S into the atmosphere (Ohara et al. Reference OHARA, AKIMOTO, KUROKAWA, HORII, YAMAJI, YAN and HAYASAKA2007, Streets et al. Reference STREETS, TSAI, AKIMOTO and OKA2001) could introduce new risks for the environment through eutrophication or acidification. An enhancement of atmospheric N deposition is particularly associated with a decrease in richness of species (Phoenix et al. Reference PHOENIX, HICKS, CINDERBY, JOHAN, KUYLENSTIERNA, STOCK, DENTENER, GILLER, AUSTIN, LEFROY, GIMENO, ASHMOR and INESON2006). Aquatic organisms vary widely in their sensitivity to acidification in temperate streams (Lovett et al. Reference LOVETT, TEAR, EVERS, FINDLAY, COSBY, DUNSCOMB, DRISCOLL and WEATHERS2009). However, uncertainty regarding the mechanisms for the delivery of material to tropical streams limits our ability to address how anthropogenic disturbances can alter streamwater chemistry (Wohl et al. Reference WOHL, BARROS, BRUNSELL, CHAPPELL, COE, GIAMBELLUCA, GOLDSMITH, HARMON, HENDRICKX, JUVIK and MCDONNELL2012) which is closely linked to the habitats of aquatic organisms. Better understanding of the mechanisms for the delivery of materials from atmosphere to stream is needed for the protection of biological diversity in tropical ecosystems against anthropogenic emissions.
In tropical forests with strongly weathered soils, throughfall carrying solutes is an important pathway of nutrients to the forest floor (Bruijnzeel Reference BRUIJNZEEL1991). The decomposition of litter and subsequent release of nutrients is also an important component in the rain-forest nutrient cycle (Nye Reference NYE1961, Vitousek & Sanford Reference VITOUSEK and SANFORD1986). In the soil of a tropical rain forest, the biotic activity accompanied by mineralization, nitrification or plant uptake is also pronounced because of the tight nutrient cycling derived from the high competition between plants and microbes (Lodge et al. Reference LODGE, MCDOWELL and MCSWINEY1994). Meanwhile the tightness depends on the export of Ca, Mg and K, which are affected by soil fertility in the forest catchment (Bruijnzeel Reference BRUIJNZEEL1991). Tropical ecosystems with high rainfall can have high weathering rates relative to temperate systems (Stallard & Edmond Reference STALLARD and EDMOND1983). The possibility of rapid release rates of cations in catchments has been reported in a lowland topical forest of Puerto Rico (McDowell & Asbury Reference MCDOWELL and ASBURY1994, Shanley et al. Reference SHANLEY, MCDOWELL and STALLARD2011) and Malaysia (Bruijnzeel et al. Reference BRUIJNZEEL, WATERLOO, PROCTOR, KUITERS and KOTTERINK1993, Gomyo et al. Reference GOMYO, WAKAHARA, SHIRAKI, KURAJIL, KITAYAMA and SUZUKI2012a). Studies on storm hydrology in the tropics have shown the importance of fast flow paths (Chappell Reference CHAPPELL2010, Elsenbeer & Lack Reference ELSENBEER and LACK1995, Reference ELSENBEER and LACK1996; Schellekens et al. Reference SCHELLEKENS, SCATENA, BRUIJNZEEL, VAN DIJK, GROEN and VAN HOGEZAND2004), which may lead to the rapid leaching of some solutes (Goller et al. Reference GOLLER, WILCKE, FLEISCHBEIN, VALAREZO and ZECH2006, Shanley et al. Reference SHANLEY, MCDOWELL and STALLARD2011) and rapid dilution of other solutes in streams (Elsenbeer & Lack Reference ELSENBEER and LACK1995). The rapid weathering rates of the humid tropics may often result in relatively high streamwater pH levels of more than 6.5, even with a low precipitation and topsoil pH. This is the case for basins underlain by volcanic rock (Bruijnzeel et al. Reference BRUIJNZEEL, WATERLOO, PROCTOR, KUITERS and KOTTERINK1993, McDowell & Asbury Reference MCDOWELL and ASBURY1994, Newbold et al. Reference NEWBOLD, SWEENEY and KAPLAN1995) and various sedimentary rocks (Elsenbeer & Lack Reference ELSENBEER and LACK1995, Elsenbeer et al. Reference ELSENBEER, WEST and BONELL1994, Wilcke et al. Reference WILCKE, YASIN, VALAREZO and ZECH2001). Meanwhile, not only abiotic processes (e.g. chemical weathering) but also biological processes (e.g. mineralization of organic materials followed by base cation leaching, nitrogen or sulphur reduction under anaerobic conditions) contribute to high pH in tropical streams. This has received less attention in forests established on strongly weathered and acid soil thus far.
Observation of the vertical fluxes of major cations and anions through each ecological compartment from rainfall to throughfall, litter layer, topsoil, subsoil and the stream may contribute to our understanding of the mechanisms of delivery of material to tropical streams and the comprehensive understanding of the pH neutralization processes. This is crucial for evaluating the future impact of anthropogenic deposition on the species richness of plants and aquatic organisms. Our hypothesis is that acidified rainwater is neutralized through the tropical rain-forest catchment mainly by rapid chemical weathering and reductive biological processes in deeper strata. To test this, we (1) monitored both streamwater chemistry and water discharge for a long period and (2) determined the balances between anion and cation fluxes from rainfall to a stream in the lowland dipterocarp forest on Borneo Island, Malaysia.
METHODS
Study site
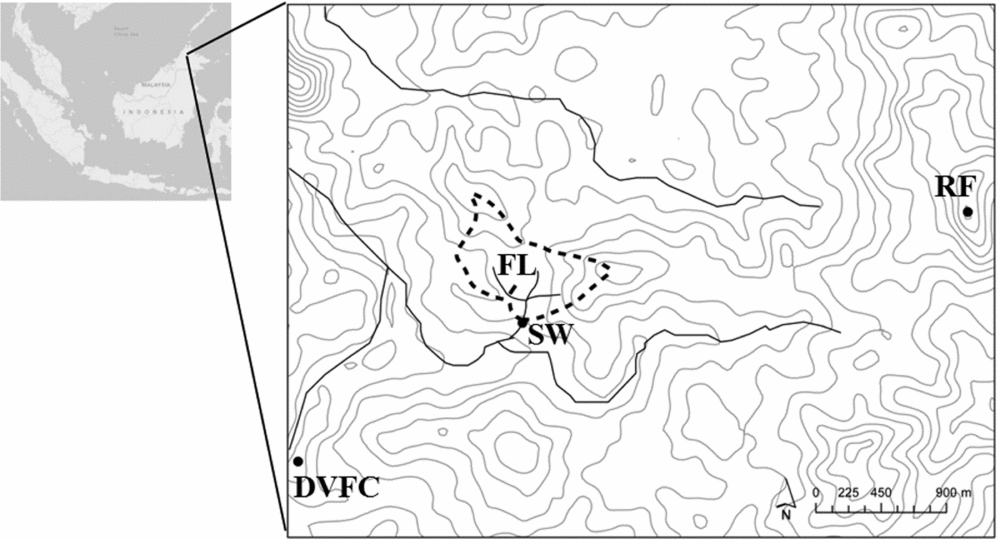
The experimental site (study catchment) is the Baru experimental catchment (Chappell et al. Reference CHAPPELL, MCKENNA, BIDIN, DOUGLAS and WALSH1999a), which is 0.441 km2 in area and lies to the north-east of the Danum Valley Field Centre (DVFC) in Sabah of Borneo island, Malaysia (5°01’N, 117°48.75’E, Figure 1). The catchment was 171–255 m asl. The climate of the DVFC is equatorial with modest annual seasonality but marked El Niño Southern Oscillation cycles (Chappell et al. Reference CHAPPELL, BIDIN and TYCH2001) and has a mean annual rainfall of 2778 mm. The study region is underlain by the Kuamut geological formation with Neogene or Jurassic-Cretaceous sedimentary rocks (Wakita et al. Reference WAKITA, OKUBO, BANDIBAS, LEI and SCHULTE2004), which is a melange largely composed of mudstones and sandstones (Clennell Reference CLENNELL, Hall and Blundell1996). A physicochemical analysis of soil profiles (Chappell et al. Reference CHAPPELL, TERNAN and BIDIN1999b) indicated that the FAO Haplic Alisol (Alh) unit dominates within the catchment (FAO-UNESCO 1990). The study catchment area is covered by lowland dipterocarp rain forest (Marsh & Greer Reference MARSH and GREER1992) which was ‘selectively logged’ during 1988 and 1989.

Figure 1. Topography and sampling locations in the 0.44-km2 Baru experimental catchment located near to the Danum Valley Field Centre, Sabah, Malaysian Borneo. SW: sampling point of streamwater at the Baru outlet weir, FL: sampling plot for ion fluxes via the throughfall, litter leachate and soil water pathways using the IER column, RF: sampling point on Bukit Atur for atmospheric deposition, DVFC: Danum Valley Field Centre, observation point for rainfall amount, Contour interval: 20 m.
Field sampling and sample analysis
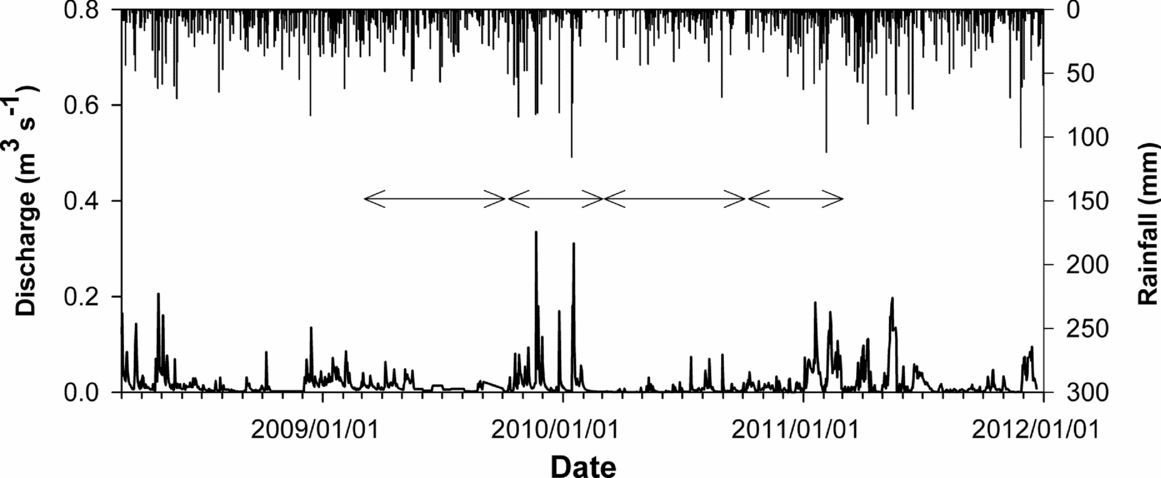
We collected streamwater from the gauging station at the outlet of the Baru catchment for 3.75 y, from April 2008 to December 2011. To examine the control of streamwater chemistry, vertical ion fluxes via rainfall, throughfall, litter leachate and soil water pathways were determined using an ion-exchange resin column (IER column) for 2 y, which were divided into four periods from March 2009 to February 2011 (Figure 2).

Figure 2. Rainfall amount and water discharge in Baru experimental catchment, Sabah, Malaysia. Arrows show observation periods for the internal nutrient fluxes using the IER column method (March 2009–September 2009, October 2009–February 2010, March 2010–September 2010 and October 2010–February 2011).
Rainfall and water discharge
Rainfall was measured using a tipping-bucket rain gauge at the DVFC at 210 m asl (Figure 1). From 1997 to 1998 the rainfall spatially fluctuated from 1198 to 1445 mm within five monitoring stations in the study catchment whereas the rainfall at the DVFC was 1524 mm (Bidin & Chappell Reference BIDIN and CHAPPELL2006). A 128° thin-plate V-notch weir was installed at the outlet of the catchment (Figure 1). This structure was built to a height of 1.2 m with a zinc plate and concrete retaining wall and pinned into the solid bedrock. The weir water level was measured with a pressure transmitter and levels calibrated to discharge using dilution gauging. The instantaneous water discharge (Q, m3 s−1) at the weir was combined with the chemical properties of each water sample taken at this location.
Chemical properties of the streamwater
Streamwater was collected at intervals of approximately 2 wk at the outlet of the catchment (Figure 1). The water samples were immediately transported to the laboratory, where they were stored in a refrigerator at 4°C until analysis. The pH and EC values were immediately measured with a glass electrode and a conductivity cell, respectively. The acid neutralizing capacity (ANC) was determined by strong acid (0.01 N H2SO4) titration with Gran plot analysis. Because the ANC is derived mainly from HCO3− in clean streamwater with a relatively low EC, the obtained ANC was assumed to be HCO3− in this paper. The water samples were filtered through a membrane filter (0.45 μm pore size), and then the concentrations of inorganic ions were determined by ion chromatography (DX-500, Dionex Corp., Sunnyvale, CA, USA). A Shimadzu total carbon analyser (model TOC 5000A) was used to measure the DOC concentrations. The SiO2 concentration was determined by the molybdosilicate method. The total dissolved N concentration was measured using the ultraviolet absorption method (220 nm) following wet digestion with a mixture of potassium peroxodisulphate and sodium hydroxide in an autoclave. The dissolved organic N (DON) was calculated as the difference between total N and mineral N.
Estimation of the vertical ion fluxes from rainfall to stream
PVC columns filled with cation and anion exchange resins were prepared to measure the ion fluxes via rainfall and throughfall pathways (Fenn & Poth Reference FENN and POTH2004, Simkin et al. Reference SIMKIN, LEWIS, WEATHERS, LOVETT and SCHWARZ2004) and via litter-leachate and soil-water pathways (Hart & Gunther Reference HART and GUNTHER1989, Szillery et al. Reference SZILLERY, FERNANDEZ, NORTON, RUSTAD and WHITE2006). For the fluxes via rainfall and throughfall pathways, the PVC tubes (10-mm diameter × approximately 400 mm height) with a 120-mm diameter funnel were filled with 30 g of Amberlite MB-1 (Rohm and Haas Co., Philadelphia, PA, USA) loaded with H+ or OH− with a total exchange capacity of 0.55 meq mL−1 wetted bed volume. For the flux via the soil-water pathways, ring-type PVC tubes (50-mm diameter × 20 mm height) were also filled with 30 g of MB-1: the top and bottom were covered with 1-mm and 0.2-mm nylon mesh lids, respectively. The capacity per resin column was approximately 21 meq (10 eq m−2) for both types. The resin columns were set out for consecutive half-year periods from March 2009 to February 2011 (March 2009–September 2009, October 2009–February 2010, March 2010–September 2010, October 2010–February 2011; Figure 2).
Three IER columns were installed for rainfall on an open hilltop at 455 m asl (Bukit Atur) 3 km north-east of the study catchment (Figure 1). This is the location of the Danum Valley Global Atmosphere Watch (GAW) Station, where monitoring on wet deposition has been conducted for the Acid Deposition Monitoring Network in East Asia (EANET, http://www.eanet.asia/). Nevertheless, we used IER columns for atmospheric deposition to compare with throughfall and soil water by the same method. Within the catchment 10 resin columns for throughfall were randomly located in the centre of the 20 × 20-m study plot (FL plot in Figure 1) on a west-facing slope of the western tributary of the Baru stream. The FL plot was chosen to obtain the typical forest in the study catchment. The forest in FL plot has understorey, closed-canopy layer and a few big stumps which would have been the emergent trees (pers. obs.). Soil physico-chemical properties in the nearest two soil pits from the FL plot (< 200 m) are also typical of this area (Chappell et al. 1999b). To quantify the litter-leachate and soil-water pathways, 20, 10 and 6 IER columns were installed at 0-cm (i.e. immediately beneath the litter layer), 20-cm and 60-cm soil depths, respectively, in the centre of the FL plot. The resin columns were placed so as to avoid overlapping vertically. On each sampling date (beginning of each period), new resin columns were placed at each location and depth. During the all-sampling period 196 columns were used at different compartments (i.e. rainfall, throughfall, litter-leachate and soil-water). Field blank had been installed at each compartment and period by placing the column into a plastic container. Bidin & Chappell (Reference BIDIN and CHAPPELL2006) reported that the rainfall amount at Bukit Atur (the site for atmospheric deposition) was similar to that of the monitoring station nearby FL plot.
The resins recovered from the field were extracted (1:50 w/v) with 1.0 m KCl for NH4+ and NO3− and 0.5 m HCl for SO42−, Na+, K+, Ca2+ and Mg2+. The extractions were repeated twice for 2-g resin samples taken from each extraction liquid. The NH4+ and NO3− concentrations of the extracts were determined using the indophenol blue method and the zinc reduction method by flow injection (Auto-Analyzer, BL TEC K.K., Osaka, Japan). Other ions were determined by ion chromatography (DX-500, Dionex Corp., Sunnyvale, CA, USA). Because the high ionic strength of the 0.5 m HCl sample matrix interfered with the IC peak resolution for SO42−, each sample was diluted 10-fold with ultrapure water using an automatic pipette. A standard solution of a similar matrix with 0.05 m HCl was prepared to quantify the SO42− concentration. In this condition, IC peak for SO42− concentration had been distinctly detected.
The vertical fluxes of ions in each period (kg ha−1 per period) via the pathways of rainfall, throughfall, litter layer and at 20-cm soil depth were estimated based on the collection area of the IER column (a 120-mm diameter funnel for the rainfall and the throughfall, a 50-mm diameter ring for the litter layer and soil). Surface of the column was set on the level (0°) to calculate the vertical flux per area (kg ha−1) at each compartment. In this estimation, the fluxes may contain the error derived from preferential flow to the column, which could be caused by the difference of conductivity between soil and IER (Lehmann et al. Reference LEHMANN, KAISER and PETER2001). At 20-cm soil depth we assumed that ions accumulated in the column were derived from only vertical/downward direction (90° angle to surface of the column). Meanwhile, at 60-cm depth we considered the effect of lateral flow from the upper part of the slope because the lateral flow is considerable at that depth in Danum Valley (Chappell et al. Reference CHAPPELL, SHERLOCK, BIDIN, MACDONALD, NAJMAN, DAVIES, Sawada, Araki, Chappell, LaFrankie and Shimizu2006). A tracer experiment using NaCl in a nearby area with a 21–23.5° slope reported that 60% flow in the subsoil was directed laterally and 40% vertically (Chappell & Sherlock Reference CHAPPELL and SHERLOCK2005). At a depth of 60 cm we assumed that 40% of all the ions caught by the column is derived from vertical flow; both vertical-downward flow and lateral-downward flow all passed through the surface of the IER column. In the above assumption, ion flux via vertical flow was estimated by the following simple equation:
Vertical flow at 60-cm depth (kg ha−1 per period) = M × 0.4 × 10000/b × 0.01 where M is weight of ion accumulated in the IER column (mg), b is the surface area of the column top (19.6 cm2), conversion factor from mg m−2 to kg ha−1 is 0.01. The annual fluxes (kg ha−1 y−1) were calculated as the sum of the fluxes in all periods divided by two, because the observation period was 2 y. To compare these fluxes with export via streams, for each period and annually (March 2009–February 2011), elemental exports via streamwater were determined by multiplying the average measured concentrations of dissolved chemicals in streamwater (SW) at the beginning and the end of the interval (i.e. about 2 wk) by the water discharge during the period (Likens & Bormann Reference LIKENS and BORMANN1995).
Statistical analysis
To characterize the streamwater chemistry, bivariate correlations between the pairs of all analysed properties were analysed by power regression or Pearson's correlation analysis (n = 90, P < 0.05). All statistics were conducted using the MASS and STATS packages on the R 2.5.0 statistical platform.
RESULTS
Streamwater chemistry
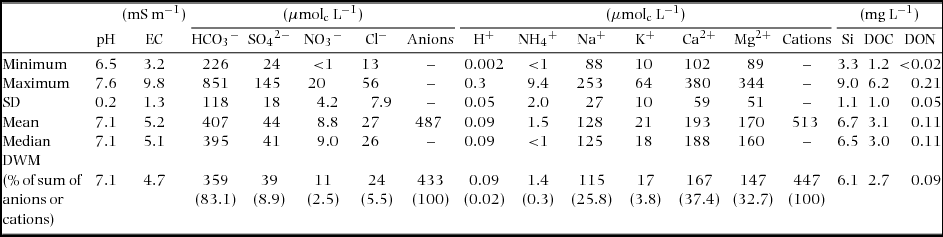
In the streamwater of the study catchment, the discharge-weighted mean of the pH was 7.1 and ranged from 6.5 to 7.6 (Table 1). Major cations and anions were well-balanced in the solutes which were dominated by HCO3− (83.1% of sum of anions), Na+, Ca2+ and Mg2+ (25.8%, 37.4% and 32.7% of sum of cations, respectively) based on the molecular charge. The mean concentrations of SO42−, NO3−, Cl−, NH4+ and K+ were lower than those of the dominant ions. The mean SiO2 (shown as Si in the figures and tables) and DOC values were 6.1 and 2.7 mg L−1 respectively, whereas almost all DON values were very low (< 0.2 mg L−1) and not detectable in some sampling periods (< 0.02 mg L−1).
Table 1. Summary of the solute concentrations in the streamwater from April 2008 to December 2011 (n = 90) in Baru experimental catchment, Sabah, Malaysia. Discharge-weighted mean (DWM) was calculated for April 2008–February 2011. Non-detected values (e.g. <1) are calculated as zero for statistical summary.

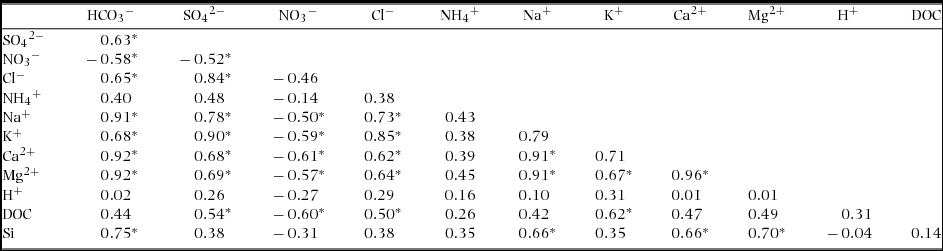
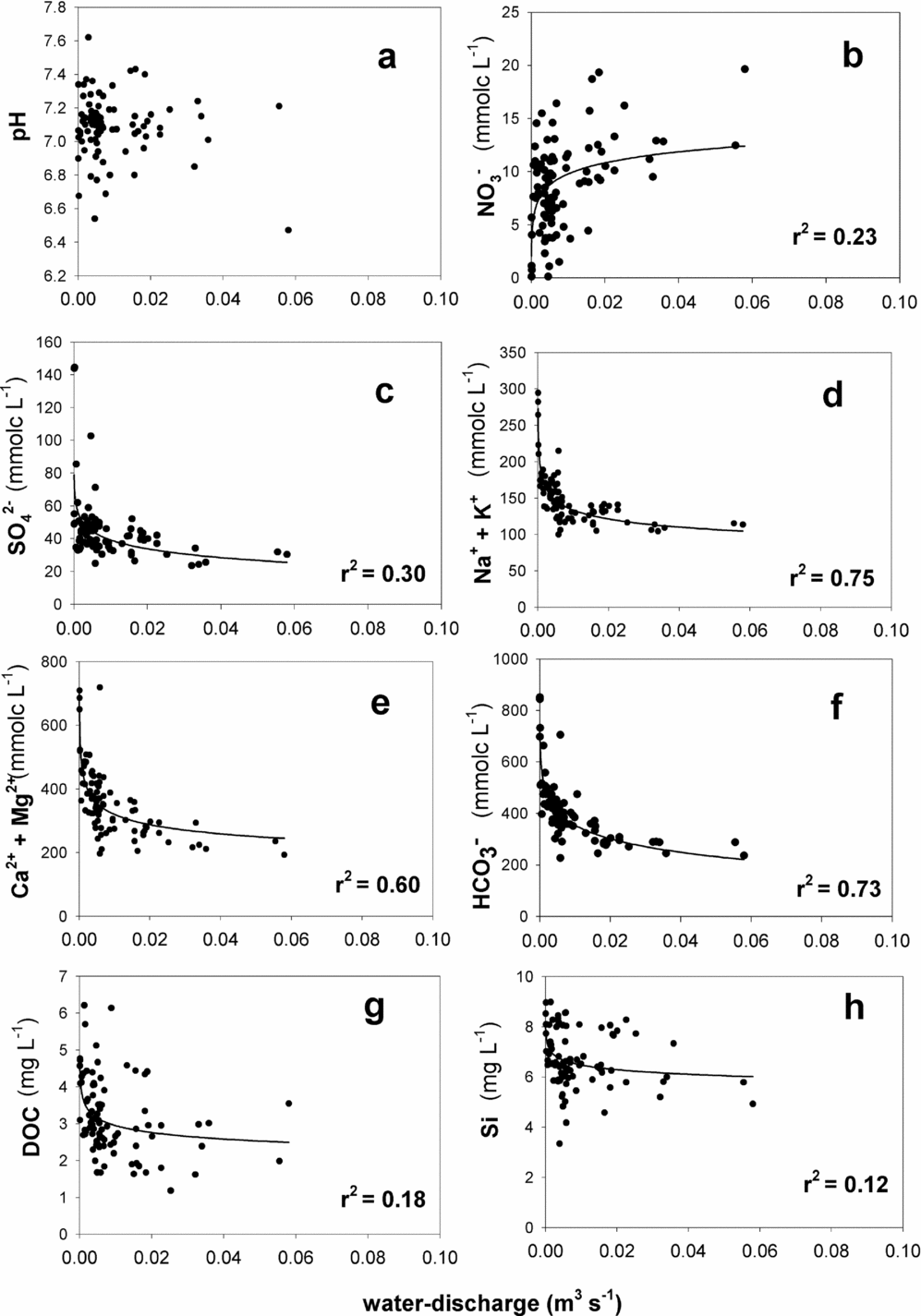
During the study period, several storm events were sampled primarily over the November–February period (Figure 2). Seasonal to inter-annual variation in water discharge, which increased in the first half of 2009, decreased in 2010 and increased again in 2011. Instantaneous water discharge at times when the water samples were taken was negatively correlated with concentrations of almost all dissolved constituents except NO3−, which was positively correlated with the water-discharge when using the power regression model (Figure 3). The relationships were stronger for concentrations of HCO3−, Na+ + K+ and Ca2+ + Mg2+ than for those of NO3−, SO42−, SiO2 and DOC. We did not find a significant correlation between the instantaneous water discharge and the pH. High correlation coefficients were observed among the concentrations of base cations, NO3−, SO42− and Cl− (Table 2). In addition, the concentration of SiO2 was significantly correlated with HCO3−, Na+, Ca2+ and Mg2+, whereas concentration of DOC was negatively correlated with NO3− concentration, and positively correlated with SO42−, Cl− and K+ concentrations.
Table 2. Correlation matrix of streamwater chemistry from April 2008 to December 2011 (n = 90) in Baru experimental catchment, Sabah, Malaysia. Each number shows Pearson's coefficients. Asterisk shows the coefficient is significant (P < 0.05).


Figure 3. Scatter plot between the instantaneous water discharge versus the pH (a), NO3− (b), SO42− (c), Na+ +K+ (d), Ca2+ +Mg2+ (e), HCO3− (f), DOC (g) and Si (h) concentrations in streamwater of Baru experimental catchment, Sabah, Malaysia. Solid lines show significant power regression for each coefficient, P < 0.05, b: NO3− = 24.9Q0.204, c: SO42− = 19.0Q−0.156, d: Na+ + K+ = 70.7Q−0.138, e: Ca2+ + Mg2+ = 88.7Q−0.042, f: HCO3− = 169Q−0.162, g: DOC = 1.91Q−0.095, h: Si = 5.33Q−0.042, where Q shows water discharge (m3 s−1).
Ion fluxes along rainfall, throughfall, litter, soil-water and streamwater pathways
The SO42−-S, NO3−-N, NH4+-N, Na+, K+, Ca2+ and Mg2+ fluxes for the solutes during the four periods were determined using the IER columns for rainfall, throughfall and soil-water, which were compared with the export in the stream (Table 3). In addition, the exports of H+ and Cl− in each period were shown with the atmospheric deposition data measured using a wet-only sampler in same period and place (cited from www.eanet.asia/product/index.html).
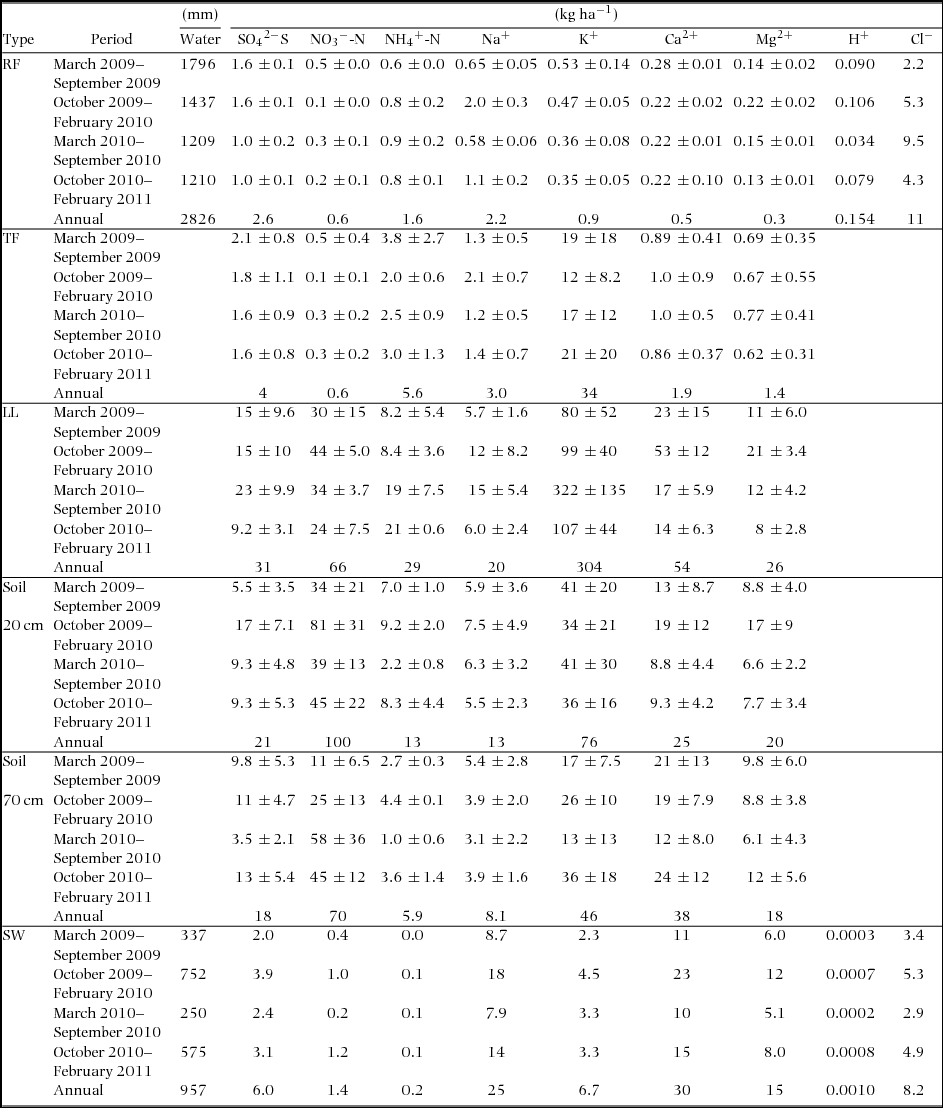
Table 3. Estimated periodic and annual vertical ion-fluxes via rainfall (RF), throughfall (TF), litter leachate (LL) and soil water (20 cm and 60 cm) pathways from March 2009 to February 2011 in Baru experimental catchment, Sabah, Malaysia. Fluxes via RF, TF, LL, 20-cm and 60-cm soil depths were estimated using IER columns with 3, 10, 20, 10 and 6 replicates, respectively. Export to SW was calculated based on water sampling twice a month at bottom of the study catchment. Each value show mean ± SD between replicates of IER-column in each period. H+ and Cl− fluxes via rainfall were from <http://www.eanet.asia/product/index.html>.

Water discharge in October 2009–February 2010 and October 2010–February 2011 (5 mo) was higher than that in March 2009–September 2009 and March 2010–September 2010 (7 mo). Rainfall amount was higher in 2009–2010 than 2010–2011. The periodic variation in rainfall amount was similar to the variation in SO42−-S and K+ fluxes for rainfall and throughfall. Although higher fluxes were observed in NO3−-N (20-cm soil depth in October 2009–February 2010), K+ (litter leachate in March 2010–September 2010) and Ca2+ (litter leachate in October 2009–February 2010) in comparison with other periods, as a whole, the differences among the four periods were not cleared for respective ion-fluxes in rainfall, throughfall, litter leachate and soil water. The difference of export of ions basically depended on water discharge. The large SD between the replicate columns in throughfall, litter leachate and soil-water fluxes were observed for almost all ions in comparison with IER columns in rainfall.
Annual discharge in the study period was 33% of the annual rainfall. The annual fluxes of NO3−-N markedly increased from rainfall and throughfall to the litter leachate and a depth of 20 cm in the soil-water, and it decreased from a depth of 60 cm (soil-water in the subsoil) to the streamwater (Table 3). A similar tendency was observed in SO42−-S, NH4+-N and K+. The NH4+-N and K+ fluxes decreased from the litter leachate to a depth of 60 cm more markedly than those of NO3−-N and SO42−-S. As for the exports from a depth of 60 cm to the stream, the decrease in SO42−-S was smaller than that of NO3−-N. In contrast, the Ca2+ and Mg2+ fluxes did not change from 60-cm depth to the streamwater, while the Na+ flux clearly increased. The annual exports in streamwater greatly exceeded the atmospheric deposition in Ca2+ and Mg2+ and moderately exceeded it for K+, NO3−-N and SO42−-S. As a result, the H+ export in the stream was smaller than the atmospheric deposition. These vertical variations in each ion flux from rainfall to streamwater were pronounced more than the difference between the periods and the variation in IER column replicates at each compartment.
DISCUSSION
Temporal variation of the streamwater chemistry
Other published work in tropical montane forests and tropical lowland rain forests demonstrates that the chemical properties of streamwater change markedly through storm periods (Boy et al. Reference BOY, VALAREZO and WILCKE2008, Elsenbeer & Lack Reference ELSENBEER and LACK1995, Newbold et al. Reference NEWBOLD, SWEENEY and KAPLAN1995, Shanley et al. Reference SHANLEY, MCDOWELL and STALLARD2011). Some of the samples in our study carried out over almost 4 y were collected during such storm periods and allowed the relationships between streamwater chemistry and stream discharge to be quantified. However, the highest value of stream discharge at the water sampling (0.058 m−3 s−1) was below the peak of each storm in the hydrograph for 3.75 y (0.2–0.3 m−3 s−1). This indicated that our sampling interval could not evaluate the notable increase and decrease in stream discharge during the large storm event in comparison with other published work which used continuous sampling in individual storms.
The negative correlations between the water discharge and base cation concentrations were mostly ascribed to a dilution of water from subsoil pathways by an increasing contribution of water from lateral flows from within the litter layer or topsoil during storms, which was reported in tropical rain forests elsewhere (Elsenbeer et al. Reference ELSENBEER, WEST and BONELL1994, Yusop et al. Reference YUSOP, DOUGLAS and NIK2006). The negative correlations of DOC and K+ with water discharge was inconsistent with existing reports that showed a positive relationship for DOC (Goller et al. Reference GOLLER, WILCKE, FLEISCHBEIN, VALAREZO and ZECH2006, Newbold et al. Reference NEWBOLD, SWEENEY and KAPLAN1995, Shanley et al. Reference SHANLEY, MCDOWELL and STALLARD2011) and K+ (Elsenbeer et al. Reference ELSENBEER, WEST and BONELL1994, Turvey Reference TURVEY1975); those reports indicated that both materials accumulated in the canopy, the litter and the topsoil could be leached out during the storm periods. The discrepancy between our result and those previous studies may be explained by the soil depth at which the lateral flow occurs during storms. In Puerto Rican forest, storm water flow may move rapidly through macropores in the top 20 cm of the soil profile (Schellekens et al. Reference SCHELLEKENS, SCATENA, BRUIJNZEEL, VAN DIJK, GROEN and VAN HOGEZAND2004), whereas the lateral flow in study catchment mainly occur at 60 cm soil depth (Chappell & Sherlock Reference CHAPPELL and SHERLOCK2005). A lateral flow at 60 cm soil depth is probably not crucial for the intensive leaching of DOC and K+ stored in the top soil.
The positive correlation between the water discharge and NO3− concentration might be ascribed to the enhancement of biotic activity during very wet soil conditions which may lead to greater NO3− leaching in the period with high discharge derived from high and continuous precipitation. Our sampling interval (twice a month for 3.75 y) probably does not cover the storm effect on DOC leaching but the lagged effect of biotic activity on gradual increase in NO3− concentration with an increase in stream base flow which occurs after continuous precipitation. The relationship of the NO3− concentration to the water discharge was unclear in the tropical forests of Puerto Rico and Ecuador (Goller et al. Reference GOLLER, WILCKE, FLEISCHBEIN, VALAREZO and ZECH2006, Shanley et al. Reference SHANLEY, MCDOWELL and STALLARD2011), whereas a positive correlation was found in Costa Rica (Newbold et al. Reference NEWBOLD, SWEENEY and KAPLAN1995).
Source for the solutes in streamwater
The atmospheric (bulk) deposition of most elements using the IER column in this study was almost at the lowest level among the existing studies within tropical rain forests across the globe. For instance, the NO3−-N and Ca2+ depositions of 0.6 and 0.5 kg ha−1 y−1 were lower than those of the lowland dipterocarp forest in Sarawak (1.9 and 22 kg ha−1 y−1, Gomyo et al. Reference GOMYO, WAKAHARA, SHIRAKI, KURAJIL, KITAYAMA and SUZUKI2012a), the nearest EANET station in Kuching City, Sarawak (3.3 and 4.6 kg ha−1 y−1 for the study period, www.eanet.asia/product/index.html), Brazil (1.2 and 1.1 kg ha−1 y−1, Lesack & Melack Reference LESACK and MELACK1996), Ecuador (1.1 and 5 kg ha−1 y−1, taken from bar-chart of Amelie et al. Reference AMELIE, CRESPO, FREDE and BREUER2011) and Puerto Rico (1.2 and 13.0 kg ha−1 y−1, McDowell Reference MCDOWELL1998). The SO42−-S deposition of 2.6 kg ha−1 y−1 was within the range of these published studies. NSS-Ca2+ (where NSS is ‘non-sea salt’) and NSS-SO42− accounted for 88% and 90% in the rainwater in study site (EANET station in Bukit Atur), respectively. Meanwhile, the volume-weighted pH of 5.32 of wet deposition in study site (EANET station in Bukit Atur) and period was comparable to the tropical montane forest of Gunun Silam, Sabah (5.4–5.9, Bruijnzeel et al. Reference BRUIJNZEEL, WATERLOO, PROCTOR, KUITERS and KOTTERINK1993) and EANET station in Kuching city, western Borneo (5.27 for the study period).
For most elements, the throughfall flux was higher than the atmospheric deposition. The higher leaching in K+ than Mg2+ or Ca2+ in the canopy was consistent with that observed in many other tropical forests, for example, in Ecuador (Wilcke et al. Reference WILCKE, YASIN, VALAREZO and ZECH2001), Puerto Rico (McDowell Reference MCDOWELL1998) and Brazil (Germer et al. Reference GERMER, NEILL, KRUSCHE, NETO and ELSENBEER2007). Major cations can be released due to the buffering capacity to H+ in the precipitation on the canopy (Shibata & Sakuma Reference SHIBATA and SAKUMA1996). Higher K leaching might be due to the high leachability of K+ from leaf tissue (Parker Reference PARKER1983, Zimmermann et al. Reference ZIMMERMANN, GERMER, NEILL, KRUSCHE and ELSENBEER2008). Tropical rainfall may also wash off dry deposition into the throughfall (Lindberg & Lovett Reference LINDBERG and LOVETT1985, Rodrigo & Avila Reference RODRIGO and AVILA2002).
An increase in ion flux from throughfall to litter leachate has been observed in other tropical forest ecosystems (Bruijnzeel et al. Reference BRUIJNZEEL, WATERLOO, PROCTOR, KUITERS and KOTTERINK1993, Goller et al. Reference GOLLER, WILCKE, FLEISCHBEIN, VALAREZO and ZECH2006, Lodge et al. Reference LODGE, MCDOWELL and MCSWINEY1994, Wilcke et al. Reference WILCKE, YASIN, VALAREZO and ZECH2001, Yamashita et al. Reference YAMASHITA, OHTA, SASE, LUANGJAME, VISARATANA, KIEVUTTINON, GARIVAIT and KANZAKI2010). This increase in flux through the litter layer is partly the result of the rapid turnover rate of the leaf litter in this forest, and a k factor (annual litter fall/annual litter mass) of 2.6 in the leaf litter was reported by Burghouts et al. (Reference BURGHOUTS, ERNSTING, KORTHALS and VRIES1992) in Danum valley. The rapid turnover was also suggested by the distinct Oi and Oe, and thin Oa in FL plot (pers. obs). Burghouts et al. (Reference BURGHOUTS, ERNSTING, KORTHALS and VRIES1992) also reported pH 4.4 in the litter layer. We speculate that the lower pH in the litter layer in comparison with rainfall is controlled by elevated NO3− from rainfall and strong organic acid although the mineralization of K, Ca and Mg in organic matter plays a role as H+ sink in the solution (Van Breemen et al. Reference VAN BREEMEN, MULDER and DRISCOLL1983). The large SD in litter layer flux in 20 resin columns in litter leachate suggested a large spatial variability of the release rate of each element (Burghouts et al. Reference BURGHOUTS, VAN STRAALEN and BRUIJNZEEL1998), which was also observed for NO3− in a tropical dry forest in Thailand (Yamashita et al. Reference YAMASHITA, OHTA, SASE, LUANGJAME, VISARATANA, KIEVUTTINON, GARIVAIT and KANZAKI2010).
From the litter leachate to a soil depth of 20 cm (lower topsoil), the increase of NO3− flux and decrease of NH4+ flux suggested that nitrification occurred in the topsoil. The decreasing trend in K+, NH4+ and Na+ fluxes from the litter leachate to 60 cm might be due to the plant uptake and the retention in the soils. The retention capacity of the soil in this site is probably high because of relatively high CEC (10–20 cmolc kg−1, Chappell et al. Reference CHAPPELL, TERNAN and BIDIN1999b). The relatively constant distributions of Ca2+ and Mg2+ fluxes with depth in the soil-water system may result from the simultaneous effect of the decrease due to the retention in the soil and the increase due to chemical weathering. In addition, the distribution of both fluxes with depth was similar to that of water-soluble base cations in two soil pits in the nearby FL plot (Chappell et al. Reference CHAPPELL, TERNAN and BIDIN1999b), which may primary reflect soil mineralogical composition along the profile (Bruijnzeel Reference BRUIJNZEEL1983). In those soil pits, pH was 4.41–4.57 and 5.70–6.45 at surface and subsoil (Chappell et al. Reference CHAPPELL, TERNAN and BIDIN1999b), which implies that main buffer substance is Al oxides and exchangeable base cations in the soil profile.
From a 60-cm depth in the subsoil to the streamwater, 84% and 79% of the fluxes of Mg2+ and Ca2+, respectively, were leached into the stream. The marked decrease of NO3− from the litter and soil-water to the stream might be caused by the denitrification process in the riparian zone (McClain et al. Reference MCCLAIN, RICHEY and PIMENTEL1994, McDowell et al. Reference MCDOWELL, BOWDEN and ASBURY1992, McSwiney et al. Reference MCSWINEY, MCDOWELL and KELLER2001) or the stream itself (Potter et al. Reference POTTER, MCDOWELL, MERRIAM, PETERSON and THOMAS2010). The denitrification process might occur below subsoil at more steady state than the NO3− leaching process with an increase in water discharge because the riparian zone was under reducing conditions through a year in study catchment. The presence of base flow through almost a year suggested the relatively long residence time of groundwater and the reducing conditions in the riparian zone. In the concentration of major ions of streamwater, a significant positive correlation among Si and base cations, which is strongly associated with the weathering of silicate minerals (Stumm & Morgan Reference STUMM and MORGAN1981), supported the large contribution of the chemical weathering to the base cations leaching to the stream.
A marked decrease from atmospheric H+ deposition to H+ leaching to the stream (from 0.154 to 0.001 kmolc ha−1 y−1) demonstrated high H+ buffering capacity to acid substances at catchment scale. The chemical weathering in the catchment was strongly supported by the fact that Na+, Ca2+ and Mg2+ leaching to the stream greatly exceeded the catchment input via atmospheric deposition. Very low base saturation in the litter, topsoil and subsoil of study catchment, which ranged 17.6%, 10.9%, 3.7% and 5.5% at 0-, 25-, 51- and 71-cm depths, respectively (Chappell et al. Reference CHAPPELL, TERNAN and BIDIN1999b), may suggest an even deeper source of base cations than the sampled 60-cm subsoil depth, perhaps at the subsoil-rock contact. Bruijnzeel (Reference BRUIJNZEEL1991) reported that annual nutrient discharge from a tropical forest catchment strongly depends on water discharge and soil fertility. The study catchment also followed this linearity and was classified with a moderate infertile soil group which still exports considerable nutrients to the stream. Somewhat higher SO42− discharge than the deposition might be affected by the sulphide minerals in the parent rock. In lowland dipterocarp forest of Ranbil hills (Sarawak state, Malaysia), Gomyo et al. (Reference GOMYO, WAKAHARA, SHIRAKI, KURAJI and SUZUKI2012b) stated that high SO42− discharge which might be derived from sedimentary rock including FeS2 distributed heterogeneously in the catchment.
Consequently, the streamwater pH value in this study catchment was high (relatively neutral) because of pronounced base cation leaching derived from the chemical weathering in the subsoil. Steep decreases in NO3− flux from soil to stream, which might be caused by denitrification, may also lead to an increase in stream pH. The ratio of total base cations and strong-acid anion fluxes (calculated from (K+ + Ca2+ + Mg2+)/(NO3− + SO42−) as equivalent concentration) were 0.58, 0.75 and 6.41 at 20 cm, 60 cm soil depth and stream discharge, respectively. An increase in the ratio may contribute to high pH in the stream because the pH is controlled by the balance between the supply of major base cations and strong-acid anions from soil to stream (Cosby et al. Reference COSBY, HORNBERGER, GALLOWAY and WRIGHT1985). Study forests in Puerto Rico, Ecuador and some sites of Malaysia had a streamwater chemistry with high pH values ranging from 6.5 to 7.5 (Bruijnzeel et al. Reference BRUIJNZEEL, WATERLOO, PROCTOR, KUITERS and KOTTERINK1993, Lodge et al. Reference LODGE, MCDOWELL and MCSWINEY1994, Wilcke et al. Reference WILCKE, YASIN, VALAREZO and ZECH2001). We assumed that the change of ion balance along the water pathways to the stream might be crucial to the neutralization processes.
CONCLUSIONS
The chemical properties of streamwater and stream discharge were monitored in a small catchment of lowland dipterocarp forest on Borneo Island, South-East Asia for nearly 4 y. This study also used IER to estimate the vertical ion fluxes along pathways from rainfall, throughfall, litter, topsoil (20 cm depth) and subsoil (60 cm depth). These approaches suggested that chemical weathering neutralized streamwater pH because the exports of Na+, Ca2+ and Mg2+ to the stream greatly exceeded those in atmospheric deposition, with SiO2 being significantly correlated with base cations in the streamwater. As a result, the precipitation pH of 5.3 was neutralized by soil-water from the subsoil to give 7.1 in the streamwater. Stoichiometric studies on chemical weathering and denitrification within the subsoil are needed to further develop our understanding of streamwater chemistry within natural streams in the humid tropics.
ACKNOWLEDGEMENTS
The authors thank the Yayasan Sabah Group, particularly their staff at the Danum Valley Field Centre for their support with the sampling and site access. We also thank Mr Sei Eng Yong at the Tawau branch office of the Malaysian Meteorological Department (MMD) for the rainfall sampler and shipping of samples, Dr Hideshige Toda at Shinshu University for technical assistance with the ion exchange resin method for sampling rainfall chemistry and Ms Leong Chow Peng, former deputy director general of the Asia Centre for Air Pollution Research for the initiation this collaborative study. The study was supported by the Environment Research and Technology Development Fund (B-0801), MOE and KAKENHI (20120012), MEXT, Japan and Asia-Pacific Network for Global Change Research (APN, ARCP2012–18NMY-Sase).