ECHINOCOCCUS AND ECHINOCOCCOSIS
Echinococcosis, caused by the larval stages of the cestode parasite of the genus Echinococcus, is a life-threatening disease of serious public health and economic concern of global proportion (Torgerson, Reference Torgerson2003; Eckert and Deplazes, Reference Eckert and Deplazes2004). Four distinct species within the genus Echinococcus have been identified i.e. Echinococcus multilocularis, E. granulosus, E. vogeli and E. oligarthrus (Thompson, Reference Thompson and Thompson1986). All Echinococcus species are potentially zoonotic, but only 2 are of significant medical importance in humans: E. multlilocularis (small fox tapeworm) being the most pathogenic parasite, and E. granulosus (dog tapeworm) being the commonest (Rausch, Reference Rausch, Thompson and Lymbery1995; McManus et al. Reference McManus, Wenbao, Jun and Bartley2003).
E. multilocularis infection causes alveolar echinococcosis (AE) in intermediate hosts (rodents) and humans, and is restricted to the northern hemisphere. In contrast, E. granulosus, the causative agent of cystic echinococcosis (CE) that occurs worldwide (Schantz et al. Reference Schantz, Chai, Craig, Eckert, Jenkins, Macpherson, Thakur, Thompson and Lymbery1995). The habitat of the adult worms is the small intestine of their respective definitive host (canids for E. granulosus, and canids and felids for E. multilocularis), where sexual reproduction and subsequent egg production take place. Eggs released in the faeces into the environment, where they are accidentally ingested by suitable intermediate hosts, such as small rodents for E. multilocularis, and mainly livestock for E. granulosus. Humans represent an aberrant intermediate host, acquiring the disease through the accidental ingestion of eggs, with serious consequences. Each egg contains an oncosphere (=first larval stage), which actively penetrates the intestinal lining and is transported via the blood and lymph to the sites of predilection/establishment. Affected organs in humans are mainly the liver for E. multilocularis and the liver and lung in the case of E. granulosus. In these sites, the oncospheres develop into metacestodes (=second larval stage). Within these metacestodes, via asexual reproduction, brood capsules and protoscoleces form in natural intermediate hosts. If the metacestode in/from an infected intermediate host is ingested by a suitable definitive host, the life-cycle is concluded. Protoscolex development in humans has only rarely been described (Gottstein and Hemphill, Reference Gottstein and Hemphill1997).
Metacestodes represent the disease-causing stage. They are fluid-filled cysts with cellular and acellular compartments (Fig. 1). The outer, acellular surface of the metacestode is formed by the laminated layer, a carbohydrate-rich structure, synthesized by the parasite, which, in terms of thickness, is much more prominent in E. granulosus than E. multilocularis metacestodes (Gottstein and Hemphill, Reference Gottstein and Hemphill1997). In addition, E. granulosus metacestodes are surrounded by a very prominent host-derived fibrous capsule, the adventitial layer, which is composed of host connective tissue. The laminated layer plays a crucial role in the survival strategy of the parasite by modulating immunological and physiological reactions of the host (Gottstein et al. Reference Gottstein, Dai, Walker, Stettler, Muller and Hemphill2002; reviewed in Siles-Lucas and Hemphill, Reference Siles-Lucas and Hemphill2002). The actual larval tissue is formed by the germinal layer, the distal part of which consists of the tegument which is directly associated with the inner surface of the laminated layer. The tegument is characterized by microvilli-like extensions termed microtriches, which protrude well into the matrix of the laminated layer and increase the resorbing surface of the parasite. In addition, the germinal layer contains highly differentiated cell types, including connective tissue, muscle cells, glycogen storage cells and many undifferentiated cells (Eckert et al. Reference Eckert, Thompson and Mehlhorn1983).
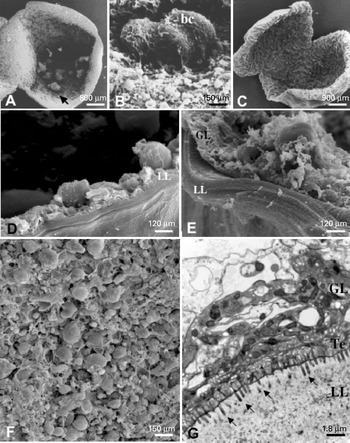
Fig. 1. Morphology and ultrastructure of Echinococcus metacestodes. (A) E. multilocularis metacestode, cut open to view the germinal layer with developing brood capsules (arrow); (B) SEM of brood capsules (bc) containing developing protoscoleces; (C) E. granulosus metacestode cultured in vitro; (D) SEM showing laminated layer and germinal layer of E. multilocularis metacestode; (E) SEM of laminated layer and germinal layer of E. granulosus metacestode; (F) SEM of germinal layer-associated tissue of E. multilocularis; (G) TEM of E. granulosus metacestodes showing laminated layer, tegument and germinal layer. GL=germinal layer, LL=laminated layer, Te=tegument, arrows in (G) point towards microtriches.
Typically, the fully mature E. granulosus metacestode (i.e. hydatid cyst) is a single-chambered or septated but usually unilocular cyst, which displays expansive growth, causing a compression of neighbouring tissue and resulting in organ dysfunction and disease (Kern, Reference Kern2003, Reference Kern2006). In E. multilocularis infection, the metacestode represents a multi-vesicular structure which reproduces (also asexually) by exogenous formation and budding of daughter vesicles, which resembles progressive tumour-like growth (Ohbayashi et al. Reference Ohbayashi, Rausch and Fay1971; Ali-Kahn et al. Reference Ali-Khan, Siboo, Gomersall and Faucher1983). This growth leads to the a large and heterogenous mass of the parasite characterized by mostly peripheral actively proliferating areas and, in many cases, centrally located necrotic tissue. Metastases can occur in other organs following the release of germinal layer cells into the blood or lymph system (Ali-Kahn et al. Reference Ali-Khan, Siboo, Gomersall and Faucher1983; Eckert et al. Reference Eckert, Thompson and Mehlhorn1983; Mehlhorn et al. Reference Mehlhorn, Eckert and Thompson1983).
LIMITATIONS OF CURRENT CHEMOTHERAPEUTICAL TREATMENT
Traditionally, the treatment of echinococcosis relies primarily on surgery and/or chemotherapy, and the treatment strategy depends largely on different factors, such as metacestode size and location, viability status, the interaction between the expanding parasite and the adjacent host tissue, complicating bacterial or fungal infection and potential complications related to cyst rupture and spillage of protoscoleces (Kern, Reference Kern2003, Reference Kern2006).
In CE, radical resection of the cyst mass represents the traditional treatment strategy and is, in many instances, accompanied by chemotherapy prior to and following surgery. Protoscolicidal substances are often applied, since there is a risk of spillage of cyst fluid containing protoscoleces, which can lead to metastases (Stey and Jost, Reference Stey and Jost1993; Pawloski, Reference Pawloski, Anderson, Ouhelli and Kachani1997; Kern, Reference Kern2003, Reference Kern2006). PAIR (puncture, aspiration, injection, re-aspiration) is a technique that was introduced in the mid-eighties, and includes percutaneous puncture of the cysts under ultrasonic guidance, aspiration of substantial amounts of cyst fluid, injection of protoscolicidal substance (e.g. 95% ethanol), and re-aspiration of the fluid cyst content after 15–20 min. Although the efficacy and potential risks have not been fully evaluated, and more long-term studies are needed, PAIR has been used in several hundred patients (Eckert and Deplazes, Reference Eckert and Deplazes2004; Brunetti et al. Reference Brunetti, Troia, Garlaschelli, Gulizia and Filice2004). For inoperable cases, chemotherapy applying benzimidazoles is the only option. There are over 2000 well-documented inoperable cases of CE, which have undergone treatment with benzimidazoles. When evaluated up to 12 months after initiation of chemotherapy, cysts disappeared in 30% of patients, cysts degenerated in 50–70%, indicating improvement, and in 20–30% of patients E. granulosus metacestodes did not respond to chemotherapy (Pawloski et al. Reference Pawloski, Eckert, Vuitton, Ammann, Kern, Craig, Dar, De Rosa, Filice, Gottstein, Grimm, MacPhearson, Sato, Todorov, Uchino, von Sinner, Wen, Eckert, Gemmell, Meslin and Pawlowski2001). Praziquantel (PZQ), a heterocyclic pyrazinoisoquinoline derivative has been proposed for use together with benzimidazoles in CE patients. PZQ exhibits a high efficacy against protoscoleces and metacestodes in animal experiments (Urrea-Paris et al. Reference Urrea-Paris, Moreno, Casado and Rodriguez-Caabeiro1999, Reference Urrea-Paris, Casado, Moreno and Rodriguez-Caabeiro2001), and the combined treatment with ABZ and PZQ given during the month prior to surgery has been shown to increase the number of human patients with non-viable protoscoleces as compared to therapy with ABZ alone (Cobo et al. Reference Cobo, Yarnoz, Sesma, Fraile, Aizcorbe, Trujillo, Diaz-de-Liano and Ciga1998).
For the treatment of AE, surgery, if applicable, is accompanied by chemotherapy using benzimidazoles, which should be maintained for at least 2 years following surgery, and the monitoring of patients should be continued for 10 years (Pawloski et al. Reference Pawloski, Eckert, Vuitton, Ammann, Kern, Craig, Dar, De Rosa, Filice, Gottstein, Grimm, MacPhearson, Sato, Todorov, Uchino, von Sinner, Wen, Eckert, Gemmell, Meslin and Pawlowski2001). Inoperable AE cases must undergo long-term, often life-long, chemotherapy with albendazole (ABZ) and/or mebendazole (MBZ) (Reuter et al. Reference Reuter, Jensen, Buttenschoen, Kratzer and Kern2000, Reference Reuter, Buck, Manfras, Kratzer, Seitz, Darge, Reske and Kern2004). Extensive animal experimentation and observations in human patients sufferering from AE have demonstrated that ABZ and MBZ exhibit a parasitostatic rather than a parasitocidal effect (El-On, Reference El-On2002; Reuter et al. Reference Reuter, Buck, Manfras, Kratzer, Seitz, Darge, Reske and Kern2004). Thus, benzimidazoles only prevent E. multilocularis growth, and the recurrence rates after interruption of therapy are high. Nevertheless, clinical studies have shown that chemotherapy has significantly increased the 10-year survival rate of inoperable or non-radically operated AE patients from 6–25% to 80–83% (Ammann and Eckert, Reference Ammann, Eckert, Thompson and Lymbery1995. Eckert and Deplazes, Reference Eckert and Deplazes2004). Spontaneous cure of AE, leading to calcified lesions is possible, but it is not known how frequently calcification occurs (reviewed by Gottstein and Hemphill, Reference Gottstein and Hemphill1997; Vuitton et al. Reference Vuitton, Zhang, Yang, Godot, Beurton, Mantion and Bresson-Hadni2006). Large surveys in endemic areas have shown that the number of patients with established AE is considerably lower than the number of seropositive humans exposed to the eggs of E. multilocularis (see Rausch et al. Reference Rausch, Wilson, Schantz and McMahon1987; Bresson-Hadni et al. Reference Bresson-Hadni, Laplante, Lenys, Rohmer, Gottstein, Jacquier, Mercet, Meyer, Miguet and Vuitton1994; Bartholomot et al. Reference Bartholomot, Vuitton, Harraga, Shi, Giraudoux, Barnish, Wang, MacPherson and Craig2002). This information suggests that immunity in humans is capable of killing the parasite after infection, which could facilitate the development of immunotherapeutical tools.
The clinical application of benzimidazoles for the treatment of AE and CE was accompanied by studies employing animal models, focusing on comparing the activities of different benzimidazole derivatives, and on different formulations and modes of application. The efficacies of oral administration were demonstrated to be dependent on the duration of treatment and the age of the parasite. Efficacy increases with the duration of treatment, but is decreased for infections which have persisted for long periods of time (over 6 months in mice) (Wangoo et al. Reference Wangoo, Ganguly and Mahajan1987). Increasing doses produced better results, although clear parasitocidal effects were not achieved (Taylor et al. Reference Taylor, Richards and Morris1989), and observations consistent with drug resistance were described (Morris and Taylor, Reference Morris and Taylor1990). There are conflicting reports of the most suitable mode of administration of benzimidazoles. It was postulated that parenteral administration of benzimidazoles resulted in a higher efficacy than other routes in animals experimentally infected with E. multilocularis (reviewed by Siles-Lucas and Hemphill, Reference Siles-Lucas and Hemphill2002). Drug combinations, normally consisting of one benzimidazole and one or more other compound, were tested in order to obtain better treatment efficacies. For instance, synergistic effects were reported for combinations of ABZ with the dipeptide methyl ester Phe-Phe-OMe (Sarciron et al. Reference Sarciron, Walchshofer, Walbaum, Arsac, Descotes, Petavy and Paris1997). In addition, novel formulations of benzimidazoles, either as pro-drugs (Walchshofer et al. Reference Walchshofer, Delabre-Defayolle, Paris and Petavy1990), liposome-entrapped compounds (Wen et al. Reference Wen, New, Muhmut, Wang, Wang, Zhang, Shao and Craig1996) or colloidal, intravenously injectable formulations (Rodrigues et al. Reference Rodrigues, Bories, Emery, Fessi, Devissaguet and Liance1995) were tested in rodents and showed enhanced efficacy at lower doses than the parental compounds. However, these studies have not yet been translated into clinical applications, with one exception: Chai et al. (Reference Chai, Menghebat, Deyu, Bin, Jincao, Chen, Xiong, Yiding, Xiuling, Dolikun, Yanchun, Fanghua and Shuhua2004) reported on improved efficacy of ABZ emulsion compared with ABZ tablets or capsules for the treatment of liver CE.
Adverse reactions against benzimidazoles under long-term chemotherapy include elevation of transaminases, proteinuria, hair loss, gastrointestinal disturbances, neurological symptoms (vertigo/dizziness), leukopaenia, headache, abnormal liver biopsy, abdominal pain, fever, urticaria, thrombocytopaenia, allergic shock (due to cyst collapse and release of E. granulosus cyst fluid) and toxicity to bone marrow. A study comprised of 3282 echinococcosis patients treated with ABZ showed that most side-effects were associated with the gastrointestinal tract (Pawloski, Reference Pawloski, Anderson, Ouhelli and Kachani1997; Kern, Reference Kern2003), but no fatal cases involving ABZ therapy have been described. In 3·8% of these cases, permanent discontinuation of treatment had to be undertaken. As suggested by animal experimentation (Horton, Reference Horton1989, Reference Horton1997), MBZ and ABZ may induce embryotoxic or teratogenic effects, and it is recommended that these drugs are not used for the treatment of pregnant women. Constant monitoring of drug levels in serum is suggested in order to prevent toxic reactions.
TOWARDS THE DEVELOPMENT OF IMMUNO-THERAPEUTICAL APPROACHES FOR THE TREATMENT OF ECHINOCOCCOSIS
Considerable effort has gone into elucidating the immunological basis of the host-parasite relationship during infection with Echinococcus. In both AE and CE, the initial immune responses during the primary phases of infection are characterized by a predominantly Th1-type response, whereas in the later stages of disease progression, the immune response switches to a Th2 polarized profile (Gottstein et al. Reference Gottstein, Haag, Walker, Matsumoto, Mejri and Hemphill2006; Vuitton et al. Reference Vuitton, Zhang, Yang, Godot, Beurton, Mantion and Bresson-Hadni2006; Zhang and McManus, Reference Zhang and McManus2006). In addition, one of the hallmarks of most helminth infections is the occurrence of a profound immuno-modulation and -suppression (Maizels and Yazdanbakhsh, Reference Maizels and Yazdanbakhsh2003). Hence, E. granulosus and E. multilocularis have a significant influence on the immune response in their hosts. For both E. multilocularis and E. granulosus, it has been shown that carbohydrate moieties or carbohydrate-rich fractions possess immunomodulatory properties (Dai et al. Reference Dai, Hemphill, Waldvogel, Ingold, Deplazes, Mossmann and Gottstein2001; Dematteis et al. Reference Dematteis, Pirotto, Marques, Nieto, Orn and Baz2001; Walker et al. Reference Walker, Baz, Dematteis, Stettler, Gottstein, Schaller and Hemphill2004a; reviewed by Baz et al. Reference Baz, Ettlin and Dematteis2006). This information is in accordance with findings of studies investigating the involvement of glycans in immunomodulation during helminth infections (Maizels and Yazdanbakhsh, Reference Maizels and Yazdanbakhsh2003), and the current challenge is to translate this knowledge into novel immuno-therapeutical applications.
Cystic echinococcosis
Some insights into the initial phases of CE have been obtained using experimental E. granulosus infection in sheep or mice (Baz et al. Reference Baz, Ettlin and Dematteis2006; Zhang and McManus, Reference Zhang and McManus2006). The initial low-level Th1-biased immune response following the infection is accompanied by an infiltration of macrophages and eosinophils and the development of a weak humoral immune response. Conversely, established cysts in experimentally infected animals and in human patients are capable of modulating the immune response, resulting in a Th2-polarized profile exhibiting the production of IL-4, IL-5, IL-6 and IL-10 and balanced by Th1 cytokines, such as IFN-γ. Upon the death of an E. granulosus cyst either naturally or due to chemotherapy, levels of Th2 cytokines decrease rapidly and the immune response polarizes towards Th1; upon relapse, Th2 responses regenerate within a few weeks. This Th1-Th2 co-existence and inter-conversion during CE is likely to be mediated by E. granulosus antigens. Examples of antigens which bear epitopes which can induce both Th1 and Th2 responses are Eg2HSP70, EgA31 and Eg/Trp, (Ortona et al. Reference Ortona, Margutti, Delunardo, Vaccari, Rigano, Profumo, Buttari, Teggi and Siracusano2003; Fraize et al. Reference Fraize, Sarciron, Azzouz, Issadi, Bosquet and Petavy2005). Another efficient strategy which contributes to the parasite survival strategy is the disruption of effector mechanisms of the host immune repsonse. In a study of E. granulosus, Diaz and co-workers showed that the activation of complement is inhibited by the binding of the host inhibitory Factor H to the hydatid cyst wall. The same authors investigated the consumption of complement components by molecular factors within the hydatid cysts (Diaz et al. Reference Diaz, Irigoin, Ferreira and Sim1999; Ferreira et al. Reference Ferreira, Irigoin, Breijo, Sim and Diaz2000, Reference Ferreira, Diaz, Fernandez and Sim2001). In addition, T cell responses were shown to be modulated by the intensity of infection, as demonstrated in mice infected with different doses of E. granulosus protoscoleces (Dematteis et al. Reference Dematteis, Rottenberg and Baz2003). The generation of T cell lines from patients exhibiting different courses of disease (harbouring active, transitional or inactive cysts) and subsequent stimulation of these cells with hydatid fluid and/?or? a defined E. granulosus antigen (AgB) has also shown that Th1 cells contribute to the inactive stage of hydatid disease, whereas Th2 cells have been shown to be more important in the active and transitional stages (Rigano et al. Reference Rigano, Buttari, De Falco, Profumo, Ortana, Margutti, Scotta, Teggi and Siracusano2004). Interestingly, patients undergoing chemotherapy with benzimidazoles exhibited a more pronounced Th1 cytokine profile, reflecting the regression of the metacestode (Rigano et al. Reference Rigano, Profumo, Ioppolo, Notargiacomo, Ortona, Teggi and Siracusano1995). Self cure of CE, leading to calcified cysts, is common in sheep and also in the human population in hyperendemic regions (MacPherson et al. Reference MacPherson, Kachani, Lyagoubi, Berrada, Shepherd, Fields and El Hasnaoui2004; Moro et al. Reference Moro, Garcia, Gonzales, Bonilla, Verastegui and Gilmanmd2005), and cytokines are likely to play a key role in these processes. Elucidating the factors involved in cyst calcification could lead to novel therapeutical approaches.
Alveolar echinococcosis (AE)
In accordance with E. granulosus infection in humans, Th1 responses dominate the early stages of E. multilocularis infection, with the immune response switching to Th2 at the chronic stage of disease (Zhang and McManus, Reference Zhang and McManus2006). Antibody levels are low initially, but levels of IgG1 and IgG3 increase during infection. In human patients, a strong cellular immune response is a characteristic hallmark, resulting in granulomatous infiltrate surrounding the parasite lesions (Vuitton et al. Reference Vuitton, Bresson-Hadni, Laroche, Kaiserlian, Guerret-Stocker, Bresson and Gilbert1989; Grenard et al. Reference Grenard, Bresson-Hadni, El Alaoui, Chevallier, Vuitton and Ricard-Blum2001). In patients with abortive or dead lesions, the cells contributing to this granuloma formation are mostly macrophages, myofibroblasts and a large number of CD4+T cells, and in patients exhibiting a progressive clinical course of AE, there is an increased number of CD8+T cells (Manfras et al. Reference Manfras, Reuter, Wendland and Kern2002). This corresponds to findings in mice, which have shown that E. multilocularis metacestodes exhibit a considerably increased growth in nude, TCR-β (−/−) and MHC-II (−/−) mice compared with wild type C57BL/6 mice, with the T cell-deficient mice dying 2 months after infection (Dai et al. Reference Dai, Waldvogel, Siles-Lucas and Gottstein2004). Thus, CD4+αβ+ T cells play a growth-limiting role in E. multilocularis infection.
A further hallmark of AE is the development of fibrosis surrounding the parasite. In human patients, fibrotic processes are frequently observed, even distant from the lesions caused by the parasite, suggesting a major role for cytokines in collagen synthesis and cross-linking (Vuitton et al. Reference Vuitton, Zhang, Yang, Godot, Beurton, Mantion and Bresson-Hadni2006). There are indications that fibrotic processes might be directed by the parasite itself. A parasite trans-glutaminase has been shown to be strongly expressed in and at the border of metacestodes, and this enzyme was capable of cross-linking human collagen in vitro (Grenard et al. Reference Grenard, Bresson-Hadni, El Alaoui, Chevallier, Vuitton and Ricard-Blum2001). Fibrosis itself, in addition to the outer acellular laminated layer, could be responsible for keeping cytolytic immune cells away from the parasite tissue.
The observation that a strong cellular immunity renders mice less susceptible to disease and that Th1 type immune responses, as assessed by corresponding cytokines, may kill the parasite or prevent its development suggests that immuno-therapeutical tools could be developed to combat echinococcosis. This notion is supported by evidence that the stimulation of the immune system with Bacille Calmette Guerin (BCG) has been reported to exhibit a profound reductive effect on the size and dissemination of E. multilocularis metacestodes in rodents (Rau and Tanner, Reference Rau and Tanner1975). However, despite encouraging clinical results, there are no reports on the limited BCG-trials in human patients (Vuitton et al. Reference Vuitton, Zhang, Yang, Godot, Beurton, Mantion and Bresson-Hadni2006).
Another immuno-stimulating compound, Isoprinosine™, has been shown to have a good efficacy against E. granulosus and E. multilocularis metacestodes and protoscoleces in vitro as well as in mice and jirds (Sarciron et al. Reference Sarciron, Delabre, Walbaum, Raynaud and Petavy1992, Reference Sarciron, Walbaum, Arsac, Raynaud and Petavy1993, Reference Sarciron, Walbaum and Petavy1995; Lawton et al. Reference Lawton, Walchshofer and Sarciron2001). The drug has been applied in patients with AE, and has shown a regression of parasite lesions in 2 patients (reviewed by Vuitton et al. Reference Vuitton, Zhang, Yang, Godot, Beurton, Mantion and Bresson-Hadni2006). This compound acts through stimulating cellular immunity, increasing IL-1, IL-2 and IFN-γ-production, and inhibiting Th2 type immune responses, including IgE-dependent reactions.
The pre-treatment of mice with IL-12 has been shown to efficiently limit the development of parasite lesions, resulting in aborted metacestode vesicles surrounded by infiltrative cells and fibrosis (Emery et al. Reference Emery, Leclerc, Sengphommachanh, Vuitton and Liance1998). However, IL-12 treatment has not been evaluated as a therapeutic agent in human AE patients because of potential side-effects.
Liance et al. (Reference Liance, Ricard-Blum, Emery, Houin and Vuitton1998) reported that the in vivo treatment of mice with a low dose (1 μg, twice a week for 3 weeks) of IFN-γ decreased metacestode growth and liver fibrogenesis. The effect was dose-dependent, as the treatment with a higher dose (5 μg, twice a week for 3 weeks) increased the number of metacestodes in the liver. Two case reports documented the clinical efficacy of IFN-γ-treatment. One patient suffering from severe side-effects caused by ABZ-therapy was treated with IFN-γ during a 3-day period once a month, and this treatment prevented disease progression but did not alter the Th2-dominated immune response against this parasite (Jenne et al. Reference Jenne, Kilwinski, Radloff, Flick and Kern1998). Another patient whose lesions were growing, despite mebendazole therapy, was treated with a combination of IFN-γ and mebendazole, which halted the progression of disease as revealed in a 1-year follow-up period (Schmid et al. Reference Schmid, Samonigg, Stoger, Auer, Sternthal, Wilders-Truschnig and Reisinger1995).
The treatment of mice with IFN-α-2a has been shown to largely prevent the formation of hepatic lesions in infected mice, with an inhibition of IL-10, IL-6 and IL-13 antigen-induced secretion in spleen cell cultures (Godot et al. Reference Godot, Harraga, Podoprigora, Liance, Bardonnet and Vuitton2003). One patient with AE and chronic hepatitis C was treated effectively with IFN-α-2a, suggesting that this cytokine limited parasite growth and reversed the cytokine profile to Th1 (Harraga et al. Reference Harraga, Godot, Bresson-Hadni, Pater, Beurton, Bartholomot and Vuitton1999). Thus, IFNα-2a seems to be the most promising candidate for, for instance, further clinical investigation, because it is already widely used for treating chronic viral infections. However, more studies are required to assess the ambiguous role of this cytokine.
SEARCHING FOR NOVEL CHEMOTHERAPEUTICAL OPTIONS
As discussed, novel and improved therapeutical tools are needed in order to optimize treatment of CE and AE. Both in vitro and in vivo laboratory models have been used for drug evaluation (reviewed by Siles-Lucas and Hemphill, Reference Siles-Lucas and Hemphill2002). Unfortunately, besides the initial development of benzimidazoles, the pharmaceutical industry has not expressed a keen interest in supporting the discovery of novel treatment options. Therefore, AE and CE must be regarded as neglected diseases.
Historically, the primary assessment of anti-echinococcal drug candidates has often been performed in rodents (mice or gerbils), which has led to the extensive use of animal experimentation. Subsequently, the in vitro culture of Echinococcus metacestodes has proven to be a suitable tool for the primary assessment of parasite susceptibility to certain compounds, with a focus on broad-spectrum anti-infective agents, and also represents an ideal model system for studies on drug uptake and associated metabolic changes imposed upon the parasite (Hemphill et al. Reference Hemphill, Stettler, Walker, Siles-Lucas, Fink and Gottstein2002). More recently, optimized in vitro culture conditions (Spiliotis et al. Reference Spiliotis, Tappe, Sesterhenn and Brehm2004) have allowed the dissection of the molecular nature of the signalling machinery within E. multilocularis metacestodes required for communication at the host-parasite interface (reviewed by Brehm et al. Reference Brehm, Spiliotis, Zavala-Gongora, Konrad and Frosch2006). These studies have provided extensive information on novel potential drug targets associated with the parasite signalling network. Although the availability of genomics and related technologies provides avenues for the application of modern approaches, the current genomic and expressed sequence tag (EST) information on Echinococcus is still limited. Nonetheless, the generation of more EST data is underway (see http://www.sanger.ac.uk/Projects/Echinococcus/) and will be an extremely valuable resource for gene expression studies.
The following strategies are currently being followed in order to identify novel alternative chemotherapeutical treatment options: (i) conventional primary in vitro testing of broad-spectrum anti-infective drugs, either in parallel with, or followed by, animal experimentation; (ii) studies on drugs which interfere with the uncontrolled proliferation of cancer cells and affect the viability of Echinococcus metacestodes and protoscoleces; (iii) exploitation of the similarities between the parasite and mammalian signalling machineries, with a special focus on targeting specific signalling receptors; (iv) in silico approaches, employing the current Echinococcus genomic database information to search for suitable targets for compounds of known mode of action.
(i) Chemotherapeutical activities of anti-infective drugs
In vitro chemotherapy studies of CE have mostly, but not exclusively, focused on protoscoleces, since these are easily cultured, and their differentiation into metacestodes is a time-consuming process that can easily take 4–6 months (Walker et al. Reference Walker, Rossignol, Torgerson and Hemphill2004b). Experimental prophylactic therapy of E. granulosus protoscoleces was carried out as a model that would mimic spillage during surgery, by treating protoscoleces with PZQ (Urrea-Paris et al. Reference Urrea-Paris, Casado, Moreno and Rodriguez-Caabeiro2001) or a combination of PZQ and ABZ (Casado et al. Reference Casado, Urrea-Paris, Moreno and Rodriguez-Caabeiro2001) prior to injection into mice. Respective findings are of substantial clinical relevance. Other promising compounds with in vitro protoscolicidal actions were cetrimide (Frayha et al. Reference Frayha, Bikhazi and Kachachi1981) and the ionophore monensin (Rogan and Richards, Reference Rogan and Richards1986), but these drugs were found to be rather ineffective against metacestodes. Levamisole and ivermectin, which are classically nematocidal, were shown to exhibit in vitro activities similar to benzimidazoles (Martinez et al. Reference Martinez, Perez-Serrano, Bernadina and Rodriguez-Caabeiro1999). Against E. granulosus infection in rodents, a combination of fenbendazol and netobimin (Garcia-Llamarez et al. Reference Garcia-Llamazares, Alvarez-de-Felipe, Redondo-Cardena, Voces-Alonso and Prieto-Fernandez1997) showed synergistic effects, allowing the administration of lower drug dosages. Oxfendazole, like ABZ, is a benzimidazole, used in veterinary medicine for the treatment of nematode infections, and has a similar antimicrobial spectrum but a longer half-life. Experimental treatments of naturally E. granulosus-infected sheep and goats suggested that oxfendazole may be as efficacious as ABZ, but does not require daily uptake of the drug because of its prolonged bioavailability (Blanton et al. Reference Blanton, Wachira, Zeyhle, Njoroge, Magambo and Schantz1998; Dueger et al. Reference Dueger, Moro and Gilman1999). Xiao et al. (Reference Xiao, Feng and Yao1995) studied the effects of several drugs on enzymes involved in carbohydrate metabolism and found that some of the corresponding host enzymes were not affected, thus identifying novel potential drug targets. Cyclosporin A, employed mainly as an immunosuppressant during the management of organ transplants, also exhibits anti-echinococcal activity. While the administration of cyclosporin A in 5 consecutive daily doses, beginning 2 days prior to the infection of mice with E. granulosus protsocoleces, resulted in a significant reduction in cyst numbers and cyst masses measured at 20 weeks after infection; no changes in cyst mass and numbers were recorded when the drug was administered 18 weeks after infection, but the wet weight was decreased by 42% compared with untreated controls. Ultrastructural examination of the germinal membrane and laminated layer of late-treated E. granulosus revealed abnormalities in all cysts studied, whereas control and early-treated hydatids were normal (Hurd et al. Reference Hurd, Mackenzie and Chappell1993).
In rodents infected with E. multilocularis metacestodes, mytomicin C, piperazine and quinolone derivates, alkylaminoethers and propargylic alcohols exhibited parasitostatic effects, at either a lower or comparable level as benzimidazoles (reviewed by Siles-Lucas and Hemphill, Reference Siles-Lucas and Hemphill2002). PZQ has been used for the treatment of AE, but experimental data in animals have shown that the efficacy of PZQ against E. multilocularis metacestodes was inadequate (Marchiondo et al. Reference Marchiondo, Ming, Andersen, Slusser and Conder1994). Also, the treatment of E. multilocularis-infected mice with alpha-difluoromethylornithine was not successful (Miyaji et al. Reference Miyaji, Katakura, Matsufuji, Murakami, Hayashi, Oku, Okamoto and Kamiya1993). In contrast to its use against CE, cyclosporin A did not have any anti-parasitic activity against E. multilocularis infection in experimentally infected mice, and its immunosuppressive activity was shown to be more effective than its parasitostatic effect (Liance et al. Reference Liance, Bresson-Hadni, Vuitton, Lenys, Carbillet and Houin1992).
Nitazoxanide (NTZ), a broad-spectrum anthelminthic also used for treatment against enteric bacteria, Giardia and Cryptosporidium (cf. Hemphill et al. Reference Hemphill, Mueller and Esposito2006), was identified as a compound inducing significant distortion of the germinal layer in vitro, and NTZ-treated E. multilocularis metacestodes were non-viable when introduced into susceptible mice (Stettler et al. Reference Stettler, Fink, Walker, Gottstein, Geary, Rossignol and Hemphill2003). NTZ was also found to induce severe damage to E. granulosus protoscoleces and the germinal layer of in vitro-cultured E. granulosus metacestodes (Walker et al. 2004 b). NTZ represents the parent compound of a class of drugs named thiazolides, which include modified variants of NTZ (Hemphill et al. Reference Hemphill, Mueller and Esposito2006; Esposito et al. Reference Esposito, Mueller and Hemphill2007). In vitro studies of E. multilocularis and E. granulosus employing NTZ-derivatives have shown that metacestodical and protoscolicidal activities of this class of drugs strongly depend on the presence of the nitro-thiazole moiety, suggesting that this nitro-group is instrumental for the activity of thiazoldies (Naguleswaran and Hemphill, unpublished findings). These promising results indicate the potential of NTZ and possibly other thiazolides as novel anti-echincoccocal compounds (Craig, Reference Craig2003).
Recently, Reuter et al. (Reference Reuter, Manfras, Merkle, Harter and Kern2006) investigated the efficacy of a series of compounds against E. multilocularis metacestodes, including ABZ, artemether, caspofungin, itraconazole (ITZ), ivermectin, methiazole (MTZ), miltefosine, NTZ, rifampicin and trimethoprim/sulfamethoxazole. They found that ABZ, ITZ, MTZ and NTZ effectively destroyed parasite vesicles in vitro. However, after drug discontinuation, re-growth of vesicles occurred, indicating a parasitostatic effect only. Combination treatment with ABZ/NTZ at concentrations between 1 and 10 μg/ml for 3 weeks yielded no re-growth of parasites during 8 months of drug discontinuation, and the subsequent evaluation in a bioassay in gerbils did also not result in viable parasite infections. These results indicated that combined ABZ/NTZ treatment exhibits a parasitocidal effect. In this respect, Stettler et al. (Reference Stettler, Rossignol, Fink, Walker, Gottstein, Merli, Theurillat, Thormann, Dricot, Segers and Hemphill2004) have shown that NTZ, applied orally to E. multilocularis-infected mice, either alone or in combination with ABZ, exhibited a profound anti-parasitic efficacy, with the ABZ/NTZ combination yielding the most promising outcome. Electron microscopical analysis of metacestode tissue obtained from treated mice suggested that the ABZ/NTZ combination exerted a synergistic effect. However, the pharmacokinetic analysis of corresponding serum levels in mice showed that the application of ABZ in combination with NTZ increased considerably the ABZSO-levels and also the half-life of ABZSO (Stettler et al. Reference Stettler, Rossignol, Fink, Walker, Gottstein, Merli, Theurillat, Thormann, Dricot, Segers and Hemphill2004). Therefore, the increased efficacy observed in mice could be the result of both direct effects of NTZ and ABZ metabolites and increased availability of ABZSO in mice receiving the combination treatment.
Amphotericin B desoxycholate (cAMB), an antifungal compound, was shown to effectively inhibit the growth of E. multilocularis metacestodes in vitro and in human patients in vivo (Reuter et al. Reference Reuter, Buck, Grebe, Nüssle-Kügele, Kern and Manfras2003a, Reference Reuter, Buck, Manfras, Kratzer, Seitz, Darge, Reske and Kernb). A major limitation of cAMB is its mode of administration (intravenous), which makes it unsuitable for prolonged use, except for salvage treatment (Reuter et al. Reference Reuter, Merkle, Brehm, Kern and Manfras2003b). Also, the effect of cAMB is only parasitostatic and the drug is nephrotoxic, limiting its widespread use. Nevertheless, prolonged application of cAMB for months to years may be feasible in some cases, as side-effects are mild and serious organ damage does not appear to occur (Reuter et al. Reference Reuter, Merkle, Brehm, Kern and Manfras2003b).
(ii) Anti-cancer drugs and echinococcosis
There are a number of similarities between cancer cells and some parasites, particularly Echinococcus (reviewed by Klinkert and Heussler, Reference Klinkert and Heussler2006). Similarities include features, such as the essentially unlimited proliferative capacity of protoscoleces/brood capsules, the potential to modulate the immune response, the secretion of proteolytic enzymes to reach their target sites or organs, and the formation of metastases. E. multilocularis metacestodes behave like malignant tumours, and there is an association between the uncontrolled proliferation and growth and the over-expression in metacestodes of a family of proteins named 14-3-3 (Siles-Lucas et al. Reference Siles-Lucas, Felleisen, Hemphill, Wilson and Gottstein1998, Reference Schumacher and Neuhaus2001). 14-3-3 proteins are found in all eukaryotic cells and participate in protein kinase signalling pathways. They function as phosphoserine/phosphothreonine-binding modules and have an effect on phosphorylation-dependent events, such as DNA-damage checkpoints and prevention of apoptosis (reviewed by Siles-Lucas and Gottstein, Reference Siles-Lucas and Gottstein2003). Some 14-3-3 proteins have been found to be aberrantly expressed in tumour cells, acting either pro-or anti-tumourogenically. Indeed, when Echinococcus 14-3-3 sequences are aligned with other 14-3-3 isoforms of other organisms, those over-expressed in metacestodes can be grouped with the tumour-growth related zeta-isoforms (Siles-Lucas et al. Reference Siles-Lucas, Nunes and Zaha2001). This information suggests that certain anti-tumour agents could interfere with the growth of Echinococcus metacestodes.
Doxorubicin, or hydroxyldaunorubicin, is a DNA-interacting drug used widely in the treatment of a wide range of cancers (Launchbury and Habboubi, Reference Launchbury and Habboubi1993). The parasiticidal properties of doxorubicin bound to polyisohexylcyanoacrylate nanoparticles (a colloidal biodegradable drug carrier) were assessed against the metacestode of E. multilocularis (see Liance et al. Reference Liance, Nemati, Bories and Couvreur1993). A reduction of the development of the parasite in the liver and a reduced viability of the metacestode were observed in mice injected with 5 mg/kg body weight, but 7·5 mg/kg body weight did not appear more efficient. Free doxorubicin or unbound nanoparticles had no anti-parasitic activity (Liance et al. Reference Liance, Nemati, Bories and Couvreur1993).
Another class of anti-cancer agents with proven anti-parasitic activities are isoflavonoids. Isoflavonoids are substances formed by plant tissue in response to physiological stimuli, such as infectious agents, with reported anti-oxidant, -bacterial, -viral and -fungal activities (Dakora and Philipps, Reference Dakora and Phillips1996). They are composed of a characteristic 15-carbon backbone ring structure connected by a heterocyclic pyrane (3-C) bridge (C6-C3-C6) (Reynaud et al. Reference Reynaud, Guilet, Terreux, Lussignol and Walchshofer2005), with the 2 aromatic rings generally containing a number of phenolic hydroxyl groups. Genistein, a major component of soya, is the most prominent isoflavonoid, and inhibits growth and metastasis of a number of cancer cell lines (breast, prostate, skin and colon) (Messina, Reference Messina1999). Genistein also stimulates the synthesis of TGF-β, which itself inhibits cancer cell proliferation (Messina, Reference Messina1999). Besides other targets, genistein acts on a number of signalling pathways by functioning as a kinase inhibitor (tyrosine kinase, MAP kinase, ribosomal S6 kinase). Recent studies have shown that genistein is highly effective against E. multilocularis metacestodes in vitro (Naguleswaran et al. Reference Naguleswaran, Spicher, Vonlaufen, Ortega-Mora, Torgerson, Gottstein and Hemphill2006). However, genistein has a disadvantage, in that it also exerts oestrogenic effects by binding to oestrogen receptor-β, which renders genistein unfavourable for long-term treatment. Binding to the oestrogen receptor-β has been proven to take place through the hydroxyl-group associated with the B-ring of the molecule. Therefore, a number of isoflavonoids that do not carry this hydroxyl-group have been tested in vitro and do not meet the steric requirements to bind to the oestrogen receptor-beta. One of these compounds, Rm6423, exhibits a pronounced anti-parasitic activity against E. multilocularis metacestodes as well as against E. granulosus metacestodes and protoscoleces (Naguleswaran et al. Reference Naguleswaran, Spicher, Vonlaufen, Ortega-Mora, Torgerson, Gottstein and Hemphill2006). Moreover, an examination of culture medium revealed increased leakage of parasite proteins into the medium during treatment, and zymography demonstrated a loss of the activity of metalloproteases. The molecular basis of the efficacy of genistein and its derivative Rm6423 has not yet been elucidated, but these compounds could interfere in signalling, for instance, through an inhibition of the tyrosine kinase activity associated with the epidermal growth factor receptor identified in E. multilocularis (see Spiliotis et al. Reference Spiliotis, Konrad, Gelmedin, Tappe, Bruckner, Mosch and Brehm2006).
An endogenous metabolite of oestrogen with both anti-angiogenic and anti-tumour effects, 2-methoxyestradiol (2-ME) (reviewed by Schumacher and Neuhaus, Reference Schumacher and Neuhaus2001) has been shown to down-regulate the pro-tumourogenic 14-3-3-ζ-isoform in a number of cancer cell types (Kumar et al. Reference Kumar, Garcia, Orsborn, Levin and Slaga2003). The pro-tumourogenic 14-3-3-ζ-isoform is also over-expressed in Echincoccus metacestodes (Siles-Lucas and Gottstein, Reference Siles-Lucas and Gottstein2003), and the exposure of 2-ME (2–10 μm) to E. multilocularis metacestodes in vitro severely damaged them in a dose-dependent manner. In parallel, drug treatment also down-scaled 14-3-3 transcription compared with actin expression (Naguleswaran et al. unpublished findings). Although it is not known how 2-ME exerts its effects on Echinococcus, the mechanism of action of 2-ME in cancer cells has been attributed to an interference with microtubule stability and a disregulation of the hypoxia-inducible factor (Klauber et al. Reference Klauber, Parangi, Flynn, Hamel and D'Amato1997; Mabjeesh et al. Reference Mabjeesh, Escuin, LaVallee, Pribluda, Swartz, Johnson, Willard, Zhong, Simons and Giannakakou2003) inducing cancers cells to undergo apoptosis via extrinsic and intrinsic pathways.
(iii) The Echinococcus signalling machinery as a novel drug target
More recently, the excellent work of Brehm et al. (Reference Brehm, Spiliotis, Zavala-Gongora, Konrad and Frosch2006) has shed light on the factors involved in host-parasite communication in AE. E. multilocularis metacestodes express a number of developmental factors which they share with other metazoans. These include signalling systems which employ receptor tyrosine kinases of the epidermal growth factor (EGF, Spiliotis et al. Reference Spiliotis, Kroner and Brehm2003, Reference Spiliotis, Konrad, Gelmedin, Tappe, Bruckner, Mosch and Brehm2006), the insulin/insulin-like growth factor (Ins/IGF)-receptor families (Konrad et al. Reference Konrad, Kroner, Spiliotis, Zavala-Gongora and Brehm2003) and the surface serine/threonine kinases of the closely-related transforming growth factor-beta (TGF-β) and bone morphogenetic protein (BMP)-receptor families (Zavalo-Gongora et al. Reference Zavala-Gongora, Kroner, Bernthaler, Knaus and Brehm2006). A cytokine of E. multilocularis which has significant homology to mammalian EGF is substantially upregulated in metacestodes cultured under conditions that promote growth and differentiation (Spiliotis et al. Reference Spiliotis, Kroner and Brehm2003). Also, EmSkip, a novel member of the SNW/SKIP family of transcriptional co-regulators was shown to be expressed in the Echinococcus metacestodes (including protoscoleces) during an infection of the intermediate host (Gelmedin et al. Reference Gelmedin, Zavala-Gongora, Fernandez and Brehm2005). EmSkip interacts with EmSmadA and EmSmadB, two TGF-β/BMP signal transducers of E. multilocularis (see Zavalo-Gongora et al. Reference Zavala-Gongora, Kroner, Wittek, Knaus and Brehm2003), indicating a role for this protein in TGF-β signalling processes in this parasite. In addition, the downstream signalling elements of the MAP kinase cascade have been identified and characterized (Spiliotis and Brehm, Reference Spiliotis and Brehm2004; Spiliotis et al. Reference Spiliotis, Tappe, Bruckner, Mosch and Brehm2005, Reference Spiliotis, Konrad, Gelmedin, Tappe, Bruckner, Mosch and Brehm2006). The Echinococcus MAP kinase cascade factors share molecular similarities to, but also differ in particular aspects from, their mammalian counterparts, and thus represent prime candidate targets for the development of novel anthelminthic drugs. This has been further substantiated via analysis of receptor activation, which has shown that the E. multilocularis insulin receptor EmIR interacts readily with insulin from the host, and TGF-βreceptor EmTR1 and possibly also the EGF-receptor EmER can interact with their corresponding host ligands (reviewed by Brehm et al. Reference Brehm, Spiliotis, Zavala-Gongora, Konrad and Frosch2006). Thus, parasite and host have evolved means of communication that would largely influence the developmental biology of both parasite and host. The evidence indicates that these receptor-ligand systems play a central role in host-parasite interaction processes and thus represent interesting drug targets (Brehm et al. Reference Brehm, Spiliotis, Zavala-Gongora, Konrad and Frosch2006). Cancer research has generated an enormous number of compounds which interfere with the functional activities of homologuous receptors or respective downstream kinases (see Sioud and Leirdal, Reference Sioud and Leirdal2007), and it will be the challenge now to identify drugs, or derivatives, which inhibit these receptors or the corresponding downstream enzymes in a parasite-specific manner.
(iv) In silico approaches
Mathis et al. (Reference Mathis, Wild, Boettger, Kapel and Deplazes2005) employed current genomic sequence information to define a drug target in Echinococcus in silico, and subsequently confirmed their hypothesis experimentally. In bacteria, the ribosomes are important antibiotic targets, and macrolides such as erythromycin and clarithromycin are agents which bind to the nascent peptide exit tunnel near the peptidyltransferase centre of large subunit ribosomal RNA (rRNA) (Rodriguez-Fonseca et al. Reference Rodriguez-Fonseca, Amils and Garret1995). Higher eukaryotes carry a guanine at position 2058 of both cytoplasmic and mitochondrial rRNAs, and this modification at this position has been demonstrated to confer the resistance of eukaryotic cells to macrolide antibiotics. In contrast, the mitochondrial rRNA of E. multilocularis carries an adenine at sequence position 2058, which would be predictive for susceptibility (Sander et al. Reference Sander, Prammananan, Meier, Frischkorn and Bottger1997), while the nucleus-encoded rRNA is characterized by a guanine at 2058 (Mathis et al. Reference Mathis, Wild, Boettger, Kapel and Deplazes2005). Upon in vitro culture of E. multilocularis metacestodes with clarithromycin, parasites, as expected, exhibited severely impaired growth characteristics, presented morphologically altered mitochondria and displayed a lack of microtriches, all in a dose-dependent manner. Adult worms were also severely affected, lost their motility and displayed morphological alterations, such as shortening and constriction of proglottids and increased vacuolization. Mathis et al. (Reference Mathis, Wild, Boettger, Kapel and Deplazes2005) were the first workers to employ a sequence-based in silico approach for the exploration of drugs whose mode of action was well studied at the molecular level and whose corresponding target was precisely defined. However, a prerequisite for such an approach is the availability of comprehensive genome sequence information for Echinococcus species.
CONCLUDING REMARKS
As outlined in this review, considerable efforts have been made to improve the therapeutical options for the treatment of CE and AE. The benzimidazole-based treatment regimens have improved considerably the prognosis of patients but, due to the obvious setbacks, new developments are needed. Compounds that not only act as parastostatics but also as parasitocidals in vivo have not been discovered to date, but it is conceivable that such compounds actually exist. Thus, the current situation for affected patients is far from satisfactory. Academic institutions can provide a scientific basis that could eventually lead to novel treatment options, but financial constraints constantly limit the further development of promising avenues. Thus, considerably more input and support is needed from the pharmaceutical and biotechnological industries as well as governmental agencies to provide solutions for these neglected diseases. The novel genomic resources developing the discovery of the receptor-ligand interactions and associated signalling pathways which influence the parasite-host interaction, and the further characterization of immuno-modulatory molecules provide new and exciting opportunities and promising targets for future studies of novel chemotherapeutical and immuno-therapeutical options for CE and AE.
We apologize to those authors whose contributions could not be cited in this paper. Norbert Mueller (Institute of Parasitology, University of Berne) is acknowledged for his great support and helpful comments. This work was supported through the National Science Foundation (31-111780), the Novartis Research Foundation, and the Swiss Secretariat for Ecudation and Science (grant No. C03.0007). M. Walker was a visiting scientist at Harvard School of Public Health, supported by an NSF fellowship.



