Introduction
Sitophilus oryzae L. (Coleoptera: curculionidae) commonly known as rice weevil is an invasive primary pest of stored food grains (Hill, Reference Hill1990). It infests the major stored cereals, such as wheat, rice, barley, corn and sorghum, causing high quantitative and qualitative losses. Matthews (Reference Matthews, Matthews and Hislop1993) reported that stored grain insect pests damage 10–30% of grains produced worldwide due to improper storage. Insecticides and fumigants, widely used to protect stored grains from insect infestations (Slavin, Reference Slavin2004), are toxic to mammals, cause environmental pollution and have lethal effects on non-target organisms. Further, the development of insecticide resistance leads to the ‘pesticide treadmill’ and necessitates in alternative control measures.
Successful control of insect pest (S. oryzae) requires a better understanding of its key metabolic components and associated enzymes (Govindan et al., Reference Govindan, Sundaram, Nagappan, Sundaram and Savarimuthu2000). The traditional bio-analytical procedures to study the biological effects and physiochemical properties are of little use to elucidate the different biochemical composition of the compounds actually occurring in the biomatrix (Dowell et al., Reference Dowell, Throne, Wang and Baker1999; Paliwal et al., Reference Paliwal, Wang, Symons and Karunakaran2004). Such disadvantages have been partially overcome by using NMR spectroscopic techniques in the study of complex bio-mixtures (Nicholson & Wilson, Reference Nicholson and Wilson1989). It is well established that insect pests become resistant relatively quickly and develop resistance mechanisms in due course of evolution against various xenobiotics and foreign chemicals. This resistance development mechanism is induced by stimulating the production of enzymes that can increase their tolerance to various insecticides. Insects' enzymes, hormones as well as the presence of cytochrome P-450 monooxygenases, all together are involved in the degradation of insecticides and other xenobiotics (René, Reference René1999). However, various dietary chemicals, both plant based and man-made, based on recognition systems, stimulate the production of enzymes that degrade insecticides and other xenobiotics within the insect's body (Terriere, Reference Terriere1984). Thus, an understanding of correlations among the biochemistry, physiology, endocrinology and nutrition of insects is necessary for the development of novel strategies against insect pests.
It has been observed that the ratios of amylase/proteinase in homogenates of isolated midguts of S. oryzae (L.), fed primarily on cereals or cereal products, are extremely high (Baker, Reference Baker1986, Reference Baker1988). Whereas, insects that feed and develop on animal products or foods with relatively high protein content have shown higher general proteinase (caseinolytic activity) and amino-peptidase activity and much higher proteinase/amylase ratios than the granivorous coleopterans (Liang et al., Reference Liang, Brookhart, Feng, Reeck and Kramer1991). 1H NMR spectroscopic metabolic profile studies of invertebrates, especially in arthropods, have not been researched much, although the possibilities for studying pathological and physiological processes are manifold (Higham et al., Reference Higham, Nicholson, Overnell and Sadler1986; Fan, Reference Fan1996). Previous 1H NMR spectroscopic studies (Nicholsan & Wilson, Reference Nicholson and Wilson1989; Thompson, Reference Thompson1990), on insects' haemolymph also suggest its feasibility on insect pest management by identifying the major metabolites, including the presence of amino acids (Fields & White, Reference Fields and White2002).
Here, we present a 1D-proton NMR spectroscopic assignment study on the rice weevil S. oryzae and demonstrate the pest's survival and adaptability to major cereal grains (rice, wheat and barley). In the present study, the key metabolic components and their associated enzymes as metabolic biomarkers have been identified using S. oryzae as the target insect to 1H NMR and TOCSY 1H-1H for designing novel strategies for pest control.
Materials and methods
Insects culture and sample processing
Sitophilus oryzae were originally obtained from the Department of Entomology, Chandra Shekhar Azad Agricultural University, Kanpur, India. The insects were cultured on rice, wheat and barley grains respectively; 50–55 live insects (randomly selected adults) were collected from culture from each cereal and frozen in liquid nitrogen for 5 min and then pulverized in a mortar. The addition of 5 ml perchloric acid (14%), was done while stirring continuously. After centrifugation at 10,000 rpm for 15 min, the supernatant was neutralized with 4M KOH between pH 7.2–7.4 and centrifuged once more for 15 min to remove potassium perchlorate. The samples were lyophilized and dissolved in 500 μl of D2O (Sigma-Aldrich) before NMR analysis. One-dimensional-proton NMR experiments were done on whole insects (mixed males and females). The insects cultured on different cereals were processed thrice and the experiments were done for each individual sample.
NMR experiments
1D-proton NMR
For the 1D-proton NMR experiments, insect samples were dissolved in 500 μl of D2O to provide a field-frequency lock for NMR spectrometer. One and two-dimensional 1H NMR experiments were performed using a Bruker (Fallanden, Switzerland) Avance DRX 300 MHz FT-NMR spectrometer (7.2 Tesla, 54 mm vertical-bore magnet) equipped with a 5 mm multinuclear inverse probehead with a Z-shielded gradient, operating at a proton frequency of 300.13 MHz. One-dimensional NMR spectra were obtained using a spin echo experiment (carr-purcell-meiboom-gill) CPMGPR water presaturation to remove resonances from macromolecules and other species with short T2 relaxation times, at 298 K with 32,768 data points, spectral width (SW) of 3591 Hz, 128 scans, with a total relaxation time of 7.68 s per scan; and the binomial 90° pulse was set to 32 μs. FIDs (Free induction decay) were multiplied by an exponential function with a line broadening of 0.01 Hz, prior to Fourier transformation (Nicholson & Wilson, Reference Nicholson and Wilson1989; Phalaraksh et al., Reference Phalaraksh, Lenz, Lindon, Farrant, Reynolds, Wilson, Osborn and Weeks1999).
2D-NMR
1H-1H total correlation spectroscopy (TOCSY) spectra were acquired using transients collected for 256 increments into 1024 data points per increment over a spectral width of 3172.589 Hz. A relaxation delay of two seconds was included. These raw data were multiplied by sine-bell squared apodisation functions prior to zero-filling to 512 points on the spin-coupling axis. The spin lock was achieved using the MLEV-17 sequence for a period of 60 ms, with the spinlock strength of 7 kHz; a relaxation delay of two seconds was included, and the water peak was suppressed as described above. In addition to chemical shifts, two- and three-bond scalar coupling connectivities (spin correlation) were obtained simultaneously for a number of metabolites by detecting off-diagonal cross peaks which are symmetric with respect to the diagonals.
Results
Complete assignments in the 1D-proton NMR spectra of S. oyzae infesting wheat, barley and rice are shown in figs 1, 2 and 3 and tables 1 and 2, respectively. Figures 4, 5 and 6, present the 2D-1H-1H COSY NMR spectrum in the range of 0–8 ppm in wheat, 1–5 ppm in barley and 1–5 ppm in rice-fed S. oryzae, respectively. Two-dimensional-TOCSY of wheat-fed (in the range of 0–8 ppm), barley-fed (in the range of 1–5 ppm) and rice-fed (in the range of 1–5 ppm) S. oryzae are shown in figs 7, 8 and 9, respectively. Amongst the various important metabolites, isoleucine, valine, leucine, β-hydroxybutyrate, lysine, glutamate, glutamine, proline, lactate, alanine, di-methylamine, α-glucose, β-glucose, choline, glycerophosphorylcholine and tyrosine were present in S. oryzae. In wheat-fed S. oryzae, the presence of threonine and absence of lactate was observed. However, in rice-fed S. oryzae, the presence of lactate signal and absence of threonine was evidenced. Barley-fed S. oryzae showed presence of tyrosine as well as lactate.

Fig. 1. (a) 1D-NMR of wheat-fed Sitophilus oryzae; (b) 1D-NMR of barley-fed Sitophilus oryzae.

Fig. 2. 1D-NMR of Sitophilus oryzae in chemical shift range (5.5–8.5): (a) barley fed and (b) rice fed.

Fig. 3. 1D-NMR of Sitophilus oryzae in chemical shift range (0.8–3.0): (a) barley fed and (b) rice fed.

Fig. 4. 2D-1H-1H COSY NMR spectrum in the range of 0–8 ppm in wheat-fed Sitophilus oryzae.

Fig. 5. 2D-1H-1H COSY NMR spectrum in the range of 1–5 ppm in barley-fed Sitophilus oryzae.

Fig. 6. 2D-1H-1H COSY NMR spectrum in the range of 1–5 ppm in rice-fed Sitophilus oryzae.
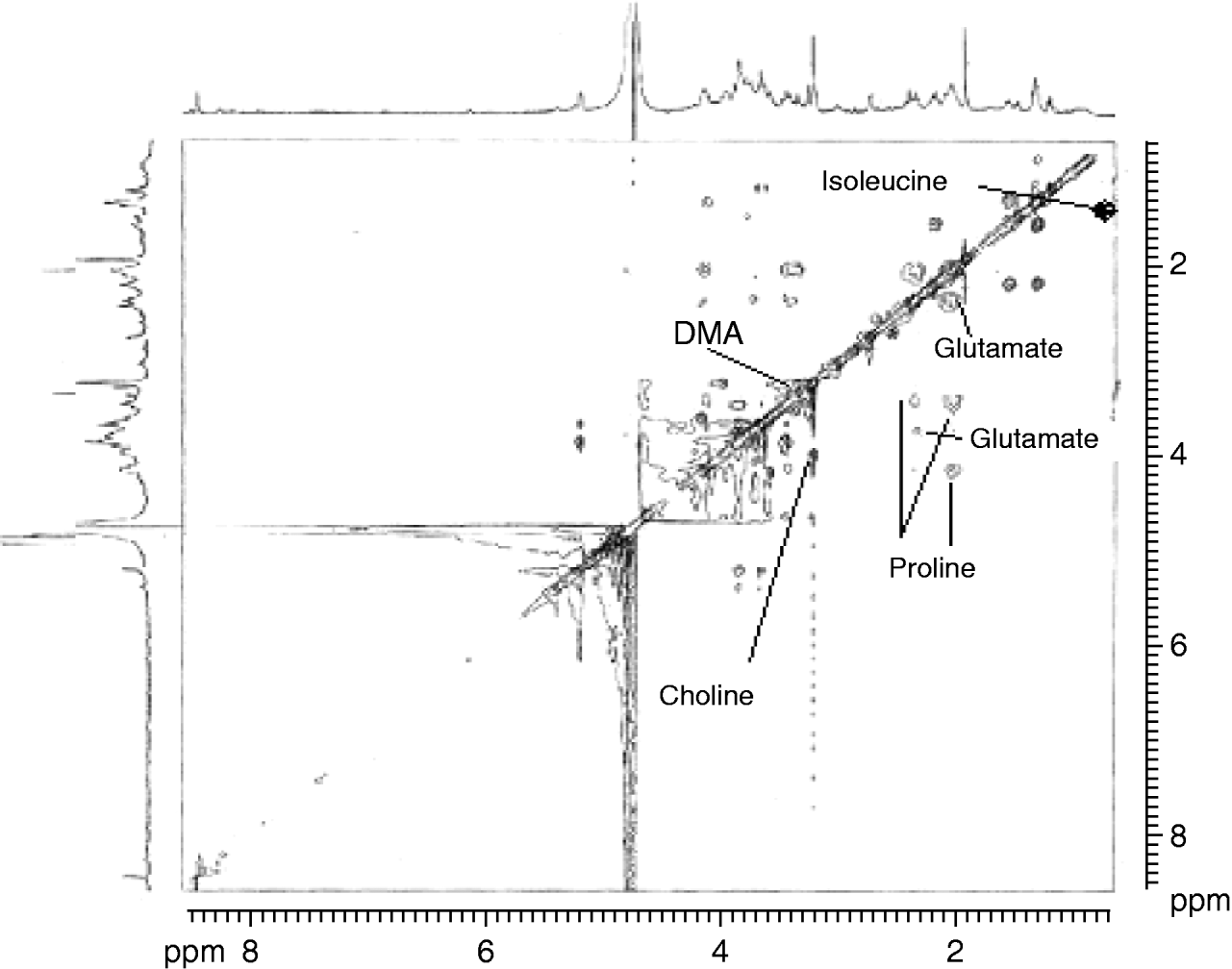
Fig. 7. 2D-TOCSY in the range of 0–8 ppm in wheat-fed Sitophilus oryzae.

Fig. 8. 2D-TOCSY in the range of 1–5 ppm in barley-fed Sitophilus oryzae.

Fig. 9. 2D-TOCSY in the range of 1–5 ppm in rice-fed Sitophilus oryzae.
Table 1. Assignation of different peaks of 1D-NMR on wheat-, barley- and rice-fed Sitophilus oryzae.

X, present;
**, absent.
Table 2. Assignments in the 1H NMR spectra of stored grain pest S. oryzae infesting different crops.

s, singlet; d, doublet; t, triplet; dd, double doublet; q, quartet; m, multiplet.
Wheat fed Sitophilus oryzae (L.)
Identification of the resonances resulting from the presence of a number of amino acids can be ambiguously achieved by comparison of 1D and 2D data. An organic acid, i.e. the threonine γ-CH3 doublet, is visible at 1.34 ppm, which is coupled with the α-CH at 3.58 ppm (TOCSY). Citrate gives a distinct doublet pattern in the spectra, which was visible at 2.52 and 2.69 ppm in the COSY spectrum. Also, acetate singlet is visible at 1.92 ppm. The β-CH multiplet of proline at 2.02 ppm was assigned through the coupling to the α-CH at 4.15, δ-CH at 3.75, δ-CH at 3.40, γ-CH at 3.33 and β-CH at 2.33 ppm (TOCSY). The δ-CH3 doublet of isoleucine was assigned at 0.92 ppm, and this was coupled with the γ-CH2 at 1.25 ppm (TOCSY). Alanine is assigned by its β-CH3 doublet at 1.47 ppm, which is coupled with the α-CH quartet at 3.78 ppm (TOCSY). Prominent resonances can be observed from glutamate with the β-CH2 at 2.08 and γ-CH2 at 2.34 ppm. The lactate β-CH3 doublet was visible at 1.33 ppm that was coupled with the α-CH3 at 4.1 ppm. Tyrosine presence in wheat is shown by the proton NMR signal at 6.7 ppm. Also, there was a presence of phenylalanine (NMR signal at 3.97 and 3.26 ppm), and lysine (NMR signal at 3.01 and 1.91 ppm). The prominent choline singlet was observed at 3.21 ppm from the –NMe+3 groups which were coupled with the O-CH2 at 4.01 ppm, visible in the TOCSY spectra. The resonance from glycerophosphorylcholine was visible at 3.23 ppm that coupled with the multiplet at 3.59 ppm and triplet at 4.18 ppm, visible in the 1D and TOCSY spectra. Presence of carbohydrates in the insect metabolites was also observed. These included resonance from α- and β-glucose that can be assigned through TOCSY spectra. α-glucose H4 double-doublet was observed at 3.44 ppm that was coupled with the H6 multiplet at 3.84 ppm, doublet of H1 at 5.22 ppm and multiplet on H6 at 3.70 ppm. Sharp H1 doublet of disaccharide trehalose was observed at 5.19 ppm that showed connectivity with the H2 at 3.62 ppm (TOCSY).
Barley fed Sitophilus oryzae
The identification of the resonances resulting from the presence of a number of amino acids can be ambiguously achieved by comparison of 1D and 2D data. These include β-CH3 multiplet of isoleucine assigned at 1.25 ppm that shows coupling with the δCH3 at 0.94, β-CH at 1.97 and with γ-CH2 at 1.01 ppm (TOCSY). The δ-CH3 doublet of leucine is assigned at 0.97 ppm that shows coupling with the β-CH2 at 1.72 ppm and with the α-CH at 3.7 ppm (TOCSY). Valine γ1-CH3 is assigned at 1.03 ppm, which was coupled with the β-CH multiplet at 2.29 ppm, γ-CH3 at 0.99 ppm and α-CH at 3.61 ppm (TOCSY). Alanine is assigned by its β-CH3 doublet at 1.47 ppm, which was coupled with the α-CH quartet at 3.78 ppm (TOCSY). Threonine γ-CH3 is visible at 1.34, which was coupled with the β-CH at 3.58 ppm (TOCSY). Tyrosine presence is shown by the proton NMR signal at 6.7 ppm. Phenylalanine dd signal is visible at 3.97 and 3.26 ppm. Prominent resonances of lysine are observed as multiplet of β-CH2 at 1.91 ppm that was well coupled with the δ-CH2 at 1.73 ppm, and ε-CH2 at 3.01 ppm (TOCSY). The resonance of glutamate can be observed as β-CH2 at 2.07 ppm and the resonances of glutamine can be observed from the β-CH2 at 2.16 ppm that is coupled with the α-CH at 3.77 ppm (TOCSY). The proline signals were assigned at γ-CH at 3.34 ppm coupled with the α-CH at 4.1 ppm. Among organic acids, β-hydroxybutyrate methyl doublet, visible at 1.20 ppm, was coupled to the signal at 2.59 ppm (TOCSY). The lactate β-CH3 doublet is visible at 1.33 ppm that was coupled with the α-CH3 at 4.1 ppm. Sharp H1 doublet of disaccharide trehalose was observed at 5.19 ppm; this shows connectivity with the H2 at 3.62 ppm, confirming the presence of carbohydrates.
Rice fed Sitophilus oryzae
Amino acids identification and assignments includes the δ-CH3 of isoleucine, assigned at 0.92 ppm, which is visible in the 1D and TOCSY spectra and was coupled to the signal at 2.29 ppm. The leucine β-CH2 at 1.72 ppm was coupled to the δ-CH3 at 0.97 and α-CH at 3.69 ppm (TOCSY). The valine γ1-CH3 doublet was visible at 1.04 ppm (TOCSY), and this was coupled to the β-CH at 2.29 ppm. Alanine is assigned by its β-CH3 doublet at 1.47 ppm, which was coupled with the α-CH quartet at 3.78 ppm (TOCSY). A triplet is present at 3.25–3.50 ppm for β-glucose. The resonance of glutamate can be observed as β-CH2 at 2.07 ppm, and the resonances of glutamine can be observed from the β-CH2 at 2.16 ppm that is coupled with the α-CH at 3.77 ppm (TOCSY). The proline signals were assigned at γ-CH at 3.34 ppm coupled with the α-CH at 4.1 ppm. Tyrosine presence is shown by the proton NMR signal at 6.7 ppm. Phenylalanine dd signal is visible at 3.97 and 3.26 ppm. Prominent resonances of lysine are observed as multiplet of β-CH2 at 1.91 ppm that was well coupled with the δ-CH2 at 1.73 ppm and ε-CH2 at 3.01 ppm (TOCSY). The nitrogen containing molecules were also observed in the NMR spectra, with dimethylamine giving a characteristic singlet at 2.74 ppm, visible in the 1D and TOCSY spectra. The prominent choline signal's singlet was observed at 3.22 ppm from the –NMe+3 group, which was coupled with the O-CH2 at 4.05 ppm, visible in the TOCSY spectra. The resonance from glycerophosphorylcholine was visible at 3.23 ppm that was coupled with the double-doublet at 3.52 ppm visible in the 1D and TOCSY spectra. Carbohydrates were also observed. These include resonances from α- and β-glucose that can be assigned through TOCSY spectra. α-glucose H4 double-doublet was observed at 3.45 ppm that was coupled with the H6 multiplet at 3.85 ppm and doublet of H1 at 5.24 ppm. β-glucose H3 multiplet was visible at 3.49 ppm that was coupled with the H1 doublet at 4.67 ppm. Sharp H1 doublet of disaccharide trehalose was observed at 5.19 ppm that shows connectivity with the H2 at 3.62 ppm. Among organic acids, lactate β-CH3 doublet is visible at 1.33 ppm, which was coupled with the α-CH3 at 4.1 ppm. A characteristic AB doublet pattern of citrate was visible at 2.69 and 2.80 ppm in the TOCSY spectrum.
Discussion
The success of Sitophilus spp. as a group is their capability to survive a wide range of environmental conditions, high fecundity, dietary habits and adaptability to thrive on different cereals. Their adaptation to different feed materials depends upon the digestive enzymes present in insects. Various components in the meal are hydrolyzed by enzymes belonging to different mechanistic classes, i.e. cathepsin-like (B-type, cistein-proteinase, or D-type, aspartic proteinases). Grain-feeding insects rely on amylases to hydrolyze starch and other sugars. To understand the metabolic profile and identification of key metabolites of the S. oryzae fed on different cereal crops, high-resolution 1D-proton NMR spectroscopy has been performed. High resolution NMR spectroscopy has shown that the various metabolites of differently fed S. oryzae contain a highly complex mixture of various metabolites as shown in figs 1–9. Despite of high degree of signal overlapping, many resonances could be assigned with the help of 2D-1H-1H COSY methods. Haemolymph of the tobacco hornworm, Manduca sexta, was evaluated for understanding the biochemical status in relation to physiological or environmental stress by NMR by Phalaraksh et al. (Reference Phalaraksh, Lenz, Lindon, Farrant, Reynolds, Wilson, Osborn and Weeks1999).
The structure and interaction of dietary components at the molecular or macromolecular stage characterize the dynamics of diet function (Cohen, Reference Cohen2003). NMR spectroscopy has shown marked differences in metabolites in insects infesting cereal grains of differing chemical constitutions. In other studies, the most abundant disaccharide trehalose concentration in the haemolymph was found to be higher than that of glucose (Thompson, Reference Thompson1990, Reference Thompson1998; Thompson & Borchardt, Reference Thompson and Borchardt1996), showing the highly active biochemical pest status of Manduca sexta. This relative stability shows the balance between synthesis and utilization; as trehalose is metabolized, more will be produced from glycogen (Gilmour, Reference Gilmour1965; Wigglesworth, Reference Wigglesworth1965; Chapman, Reference Chapman1982). The trehalose is derived from glucose via glucose-6-phosphate in the fat body. Since α-amylase is a predominant digestive hydrolase in S. oryzae, a major enzyme for hydrolyzing starch, the degree to which cereal diets affect amylase levels may indicate their suitability as potential hosts (Baker, Reference Baker1988; Anonymous, 1991). S. granarius (L.) showed higher growth rates and development of insects on wheat and barley compared to corn, oats or rice examined at 27.5°C and 75% RH (Schwartz & Burkholder, Reference Schwartz and Burkholder1991).
Conclusions
An understanding of the key insect metabolites, for the development of newer strategies of pest control is important. In this regard, advances in NMR spectroscopy has opened a new gateway of identifying metabolites in complex mixtures of biological substances. Analysis of crude S. oryzae metabolites, fed on different diets (e.g. wheat, barley and rice), through NMR revealed the presence of isoleucine, valine, leucine, β-hydroxybutyrate, lysine, glutamate, glutamine, proline, lactate, alanine, di-methylamine, α-glucose, β-glucose, choline, glycerophosphorylcholine and tyrosine. Wheat-fed S. oryzae showed the presence of threonine and absence of lactate. In rice-fed S. oryzae, the presence of lactate signal and absence of threonine was evidenced. Barley-fed S. oryzae showed the presence of both tyrosine and lactate. These differences in metabolites demonstrate the adaptability of the test species to various types of stored cereals. An understanding of S. oryzae's metabolism of different cereals may assist in designing molecules tailored to disrupt the enzymes systems leading to lethal effects.













