Introduction
Corn (Zea mays L.) and soybean [Glycine max (L.) Merr.] are the most widely grown grain crops in the USA, with production on over 69 million hectares in 2012 (NASS, 2012). In the upper Midwest, the growing season for corn and soybean crops typically lasts from mid-April to October. The absence of living plants during the late fall, winter and early spring, however, leaves corn and soybean fields susceptible to nutrient loss, soil erosion and the depletion of soil organic matter (Pimentel et al., Reference Pimentel, Harvey, Resosudarmo, Sinclair, Kurz, McNair, Crist, Shpritz, Fitton, Saffouri and Blair1995; Paustian et al., Reference Paustian, Collins, Paul, Paul, Paustian, Elliot and Cole1997; David et al., Reference David, Drinkwater and McIsaac2000). In particular, losses of soil, nitrogen and phosphorus from agricultural fields result in downstream environmental degradation (Burkart and James, Reference Burkart and James1999) and reduce the long-term productive capacity of fields (Pimentel et al., Reference Pimentel, Harvey, Resosudarmo, Sinclair, Kurz, McNair, Crist, Shpritz, Fitton, Saffouri and Blair1995). One effective strategy for preventing nutrient loss and improving soil protection is to have winter cover crop plants growing and taking up water and nutrients between harvest and planting in corn and soybean fields (Kaspar and Singer, Reference Kaspar, Singer, Hatfield and Sauer2011; Kladivko et al., Reference Kladivko, Kaspar, Jaynes, Malone, Singer, Morin and Searchinger2014).
In the upper Midwest, cereal rye (Secale cereale L.) is one of the few widely used cover crop species that can be readily established after corn and soybean harvest and can survive the minimum winter temperatures in this region without snow cover (Snapp et al., Reference Snapp, Swinton, Labarta, Mutch, Black, Leep, Nyiraneza and O'Neil2005; Singer, Reference Singer2008). Cereal rye has an extensive fibrous root system that reduces erosion (Kaspar et al., Reference Kaspar, Radke and Laflen2001), improves soil structure and lessens the effects of soil compaction over time (Kessavalou and Walters, Reference Kessavalou and Walters1999). Cereal rye has the ability to scavenge residual or newly mineralized nitrogen, typically holding 28–56 kg N ha−1 in its roots and shoots (Brandi-Dohrn et al., Reference Brandi-Dohrn, Dick, Hess, Kauffman, Hemphill and Selker1997; Kaspar et al., Reference Kaspar, Jaynes, Parkin and Moorman2007, Reference Kaspar, Jaynes, Parkin, Moorman and Singer2012). Cereal rye cover crops have been found to reduce nitrate leaching in drainage water by 48% over the course of 5 years (Kaspar et al., Reference Kaspar, Jaynes, Parkin, Moorman and Singer2012). Other benefits of cereal rye cover crops include the extra input of organic matter to soil, and the ability to suppress weeds (Teasdale et al., Reference Teasdale, Beste and Potts1991; Moore et al., Reference Moore, Wiedenhoeft, Kaspar and Cambardella2014). Farmers that value benefits such as these more highly are also more likely to employ cover crops (Arbuckle and Roesch-McNally, Reference Arbuckle and Roesch-McNally2015).
In contrast to these benefits, however, occasional corn yield decreases have been reported in some years following a cover crop of cereal rye or other winter cereals (Kaspar and Bakker, Reference Kaspar and Bakker2015). This suggests that along with the desirable impacts of cereal rye cover crops on soil health, one or more concurrent processes sometimes lead to negative impacts on corn performance. Understanding this risk of impaired corn performance is vital to improving the management of cover crops. Many farmers are uncertain regarding the risks that accompany cover cropping, and those that perceive greater risk are less likely to adopt cover cropping (Arbuckle and Roesch-McNally, Reference Arbuckle and Roesch-McNally2015). If properly understood, the risks associated with cover cropping before corn could be reduced by improved management practices, leading to a greater net benefit of cover cropping.
There are several plausible mechanisms have been proposed to explain how cereal rye cover crops could lead to reduced corn yield. For instance, corn populations may be reduced if the cover crop residue interferes with planter performance (Duiker and Curran, Reference Duiker and Curran2005). Depending on the timing of rainfall, rye cover crops could deplete soil moisture before corn planting, causing reduced germination or water stress (Munawar et al., Reference Munawar, Blevins, Frye and Saul1990). Rye residue may also delay the warming and drying of the soil, and its decomposition may immobilize soil nitrogen that is needed for the growth of the future corn crop (Kaspar and Singer, Reference Kaspar, Singer, Hatfield and Sauer2011). In some studies, cereal rye has been found to display allelopathic effects (Shilling et al., Reference Shilling, Liebl, Worsham and Thompson1985), although these chemically inhibitory effects are typically more detrimental to smaller weed seeds (Przepiorkowski and Gorski, Reference Przepiorkowski and Gorski1994) than to a large-seeded plants like corn.
In addition to these mechanisms that have been suggested previously, another possible cause of corn yield loss may be due to the cover crop maintaining and elevating the density of corn seedling pathogens in soil. It is known that cereal rye is a host to corn pathogens such as Fusarium spp. and Pythium spp. (White, Reference White1999; Bakker et al., Reference Bakker, Acharya, Moorman, Robertson and Kaspar2016). Furthermore, the presence of a live host plant over the winter can maintain or elevate pathogen populations in the spring compared with fallow ground (Smiley et al., Reference Smiley, Ogg and Cook1992). Additionally, because some pathogens are able to colonize and proliferate on plants that have been weakened or recently killed with herbicides (Levesque et al., Reference Levesque, Rahe and Eaves1987; Rosenbaum et al., Reference Rosenbaum, Miller, Kremer and Bradley2014), using herbicides to terminate a cereal rye cover crop may further increase pathogen populations (Bakker et al., Reference Bakker, Acharya, Moorman, Robertson and Kaspar2016).
Variable environmental conditions make it a challenge to initially use field trials to test the hypothesis that corn seedling disease plays a role in the occasional yield decline following cereal rye cover crops. In many years and locations, environmental conditions may not be conducive for disease development at the time of corn planting, or pathogen inoculum may be at low levels in some fields. At the other extreme, under very suitable conditions for disease development, baseline disease pressure may be so high that the presence of cereal rye cover crops does not increase disease incidence or severity appreciably. For these reasons, we designed a series of pot experiments under controlled environment conditions that would test the hypothesis that cereal rye cover crops enhance corn seedling disease pressure under suitable environmental conditions. Because fungicide seed treatment is one of the primary tools for dealing with corn seedling diseases, we included treated and untreated seed as an experimental factor in some experiments. Finally, we also contrasted effects of cereal rye cover crops on corn seedling growth under disease-conducive environmental conditions with effects of two other cover crop species: winter canola (Brassica napus L.) and hairy vetch (Vicia villosa Roth). These alternative cover crop species have the potential to overwinter in Iowa, and with additional development may become viable options for cover cropping in corn–soybean rotations in Iowa.
Materials and methods
Experiment 1: Effects of a rye cover crop and fungicide seed treatment on corn seedling growth
We designed a factorial experiment with four treatments testing the effects of rye cover crop presence by fungicide seed treatment. Preliminary work was used to determine appropriate temperature, water management, and experimental protocols. Once experimental conditions were established, two trials of the experiment were conducted. Plastic pots measuring 21.5 cm × 21.5 cm (height × diameter) were lined on the bottom with landscaping fabric. Approximately 4 kg of a sieved, field moist, Webster silty clay loam soil obtained from a continuous corn field at the Iowa State University Agronomy Research Farm just west of Ames, IA was mixed with 50 g of coarsely chopped corn residue and placed into each pot. The corn residue was added to the soil to improve soil drainage and structure, and to increase the availability of pathogen inoculum. In pots assigned to the cover crop treatment, 15 seeds of cereal rye (cultivar Elbon) were planted at a depth of 0.5 cm. In pots that were not planted with a rye cover crop, the soil surface was covered with perlite to reduce evaporation and heat absorption from radiant energy of the chamber lights.
Plants were grown in a controlled environment chamber (Conviron model E15). To account for variations in environmental parameters within the chamber, the chamber area was divided into five blocks; pots were divided among five blocks with treatment arrangement randomized within each block. The chamber was programmed to provide 13.5 h days (20 °C, 40% humidity) and 10.5 h nights (18 °C, 60% humidity). The light bank, consisting of both fluorescent and incandescent bulbs, was placed 55 cm above the surface of the pots. Pots were watered twice a week: once with 2 × c. 400 mL of deionized water (morning and afternoon), and once with 2 × 250 mL of nutrient solution (2.22 mg L−1 MiracleGro®; analysis 0.24 N, 0.08 P2O5, 0.16 K2O g g−1). All pots were sprayed with glyphosate [N-(phosphonomethyl) glycine; 6.6 g active ingredient L−1] with a hand-pump spray bottle at simulated field application rates at approximately 55 days after rye planting.
Seeds of commercial corn hybrids P0448 (untreated) and P0448AM1 (with fungicide seed treatment) were provided by DuPont Pioneer. The standard commercial seed treatment included fludioxonil [4-(2,2-difluoro-1,3-benzodioxol-4-yl)-1H-pyrrole-3-carbonitrile], mefenoxam [methyl N-(methoxyacetyl)-N-2,6-xylyl-d-alaninate], azoxystrobin [methyl (2E)-2-(2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl)-3-methoxyacrylate] and thiamethoxam [(EZ)-3-(2-chloro-1,3-thiazol-5-ylmethyl)-5-methyl-1,3,5-oxadiazinan-4-ylidene(nitro)amine]. Fludioxonil has activity against fungi, including Fusarium. Mefenoxam has activity against oomycetes, such as Pythium. Azoxystrobin has activity against both fungi and oomycetes. Thiamethoxam is an insecticide. Hybrid P0448AM1 is transgenic, with herbicide tolerance and insect protection genes. We were unable to obtain seed of hybrid P0448AM1 without the chemical seed treatment. Hybrid cultivar P0448 does not possess the transgenic genes, but is otherwise genetically similar to hybrid cultivar P0448AM1. Thus, the four treatments were: P0448 untreated following fallow, P0448 untreated following rye, P0448AM1 following fallow and P0448AM1 following rye.
Corn seeds were pre-imbibed (18 h, 20°C) between moist germination papers and were planted three days after glyphosate application, at which time temperature was reduced to 12°C during the 13.5 h days (40% humidity) and to 10°C for the 10.5 h nights (60% humidity). Corn was planted at a depth of 2.5 cm, with five seeds per pot. Seven days after glyphosate application, when the rye was completely dead, the rye cover crop was cut at the ground level and removed from the pots. At this time, the soil surface of all rye pots was covered, and the fallow control pots were covered again, with perlite to prevent evaporation and soil warming due to radiant energy from the lights.
Corn emergence rates and growth stage development were tracked. The corn plants were sampled at 45 days after planting. Corn shoots and roots were carefully washed and measured for radicle length, shoot dry weight, growth stage and incidence of radicle or mesocotyl disease. Growth staging followed the leaf collar method, in which the emergence of each new leaf collar signals the beginning of the next leaf growth stage (Abendroth et al., Reference Abendroth, Elmore, Boyer and Marlay2011). Incidence of radicle and mesocotyl disease was assessed as the presence of any tissue discoloration or necrosis that was visible to the naked eye.
Experiment 2: Effects of three cover crop species on corn seedling growth
A second experiment testing the effects of three cover crop species on corn seedling growth was run over two separate trials with the same design as the first experiment. The four cover crop species treatments that were contrasted were cereal rye (cultivar Elbon), winter canola (cultivar Riley), hairy vetch (variety not stated) and a no cover crop fallow control. Pots and growth conditions were as for Experiment 1. Seeding rates were 20 seeds per pot for rye, 30 seeds per pot for hairy vetch and 40 seeds per pot for winter canola. Cover crop seeds were planted a depth of 2.5 times their seed diameter. Cover crops were sprayed with glyphosate 45 days after planting, at the same rate as in the first experiment, and were cut at the ground level 7 days later. The cover crop growth period was shorter by 10 days in the second experiment compared with the first because of higher seeding rates, and due to considerations relating to the growth of multiple different cover crop species. Corn planting methods, chamber temperature settings and corn seedling growth assessment were the same as in Experiment 1. This experiment used only treated corn seed (hybrid P0448AM1).
In a change from the first experiment, we attempted to assess corn roots for infection rate by Fusarium spp. and Pythium spp., by plating root segments on semi-selective media. The radicle and seminal roots were cut into 2 cm segments, which were surface-disinfested, rinsed with sterile distilled water and blotted dry. Randomly selected segments were placed on Komada (Komada, Reference Komada1976) and P5ARP (Jeffers and Martin, Reference Jeffers and Martin1986) agar media. These agar plates were monitored for 7 days, and the formation of new fungal or oomycete colonies was observed and documented.
Because of problems encountered with culturing on selective media (namely, bacterial contamination and fungal growth of uncertain species identity), corn seedling tissues from the second trial were preserved for later analysis of pathogen density using quantitative PCR (qPCR). A 2 cm section (closest to the corn seed) of mesocotyl and of radicle was collected from each corn seedling within a given pot, and bulked. In some cases, where radicles were diseased, <2 cm of tissue were available for collection. Tissue samples were freeze-dried and pulverized by beating with a tungsten-carbide bead (3 mm diameter; Qiagen) on a MiniBeadbeater (Biospec Products). DNA was extracted using the DNeasy Plant Mini Kit (Qiagen), according to the manufacturer's directions.
Because root rot is a common problem in corn seedlings under cold and wet conditions, we targeted Pythium spp. in corn seedling tissue samples. We used the method of Acharya et al. (Reference Acharya, Bakker, Moorman, Kaspar, Lesnssen and Robertson2017) to quantify the abundance of Pythium clades B and F via qPCR. By both cultivation and amplicon sequencing approaches, species within these clades have been shown to comprise the majority of Pythium spp. in corn and rye roots in field settings (Acharya et al., Reference Acharya, Bakker, Moorman, Kaspar, Lesnssen and Robertson2017; Bakker et al., Reference Bakker, Manter, Moorman and Kaspar2017). qPCR was run on a CFX96 thermocycler and detection system (BioRad). All reaction conditions were as reported in Acharya et al. (Reference Acharya, Bakker, Moorman, Kaspar, Lesnssen and Robertson2017). Technical triplicates were run for all samples and standards. Each qPCR run included no-template controls and a standard curve of synthesized DNA (Invitrogen) spanning six orders of magnitude in template DNA concentration. Across qPCR runs, the standard curves always produced an R 2 > 0.99, and calculated PCR efficiencies were in the range of 87–99%. Non-detects were assigned a small non-zero value, defined as half of the calculated DNA content at a cycle threshold of 40. Measured pathogen densities in corn tissue were expressed as pathogen ITS gene copies per million copies of the corn Tua4 gene, and then were log10 transformed.
Statistical analyses
Data analyses were performed in R (R Core Development Team, 2011). Trials of each experiment were analyzed individually. Where measurements were made of individual plants, a single mean value per pot was carried forward for statistical analysis. For statistical comparison among treatments in Experiment 1, we used a model that incorporated the experimental block, presence of a cover crop, and seed treatment as main effects. The interaction of seed treatment and cover crop presence was not significant for any of the measured variables (P > 0.05) and was not included in the final model. For comparison among treatments in Experiment 2, we used an additive model that incorporated block and cover crop identity (rye, canola, vetch or none). Where significant differences were evident among treatments, Tukey's HSD was used for post hoc comparisons. For percentage data (i.e., disease incidence, measured as counts of diseased and of healthy plants on a per pot basis), we used logistic regression with a binomial model and a logit link (function glm in the stats package for R). Analysis of deviation from the mean was performed on the model fit using a chi-squared test (function anova.glm in the stats package for R).
Results and discussion
Experiment 1: Effects of a rye cover crop and fungicide seed treatment on corn seedling growth
In general, the presence of an herbicide-terminated cereal rye cover crop prior to corn planting reduced the growth of corn seedlings and increased corn seedling root disease, under the experimental conditions of low soil temperatures and high soil moisture status. Fungicide seed treatment had limited effectiveness in counteracting these effects.
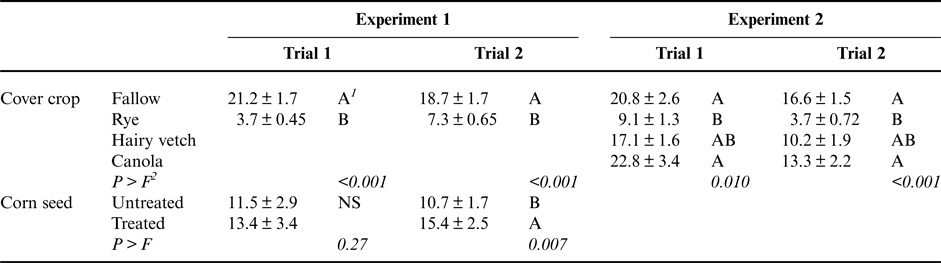
One of the most consistent impacts of the cereal rye cover crop was to reduce corn seedling radicle length. In both trials of Experiment 1, corn radicle length was significantly shorter following a rye cover crop than following a fallow control (Table 1). Reductions in radicle length compared with the control ranged from 61 to 83%. Fungicide seed treatment had a significant positive impact on radicle length only in the second trial, where radicles of plants growing from treated seeds were 44% longer than radicles of plants growing from untreated seeds (Table 1). Where radicles were shortened, the tip of the root was often necrotic.
Table 1. Main effects of cover crop presence or species and fungicide seed treatments on corn radicle length (cm) for Experiments 1 and 2.

1 Values followed by the same letter are not significantly different (shown are mean ± SE). ‘NS’ indicates that the given factor was not significant.
2 P > F indicates the significance of the experimental factor in an ANOVA test. In Experiment 1, factors consisted of block, cover crop identity and presence of fungicide seed treatment. In Experiment 2, factors consisted of block and cover crop identity.
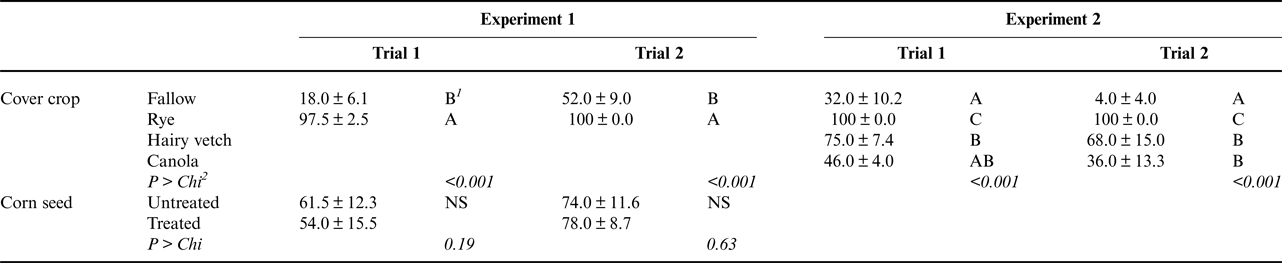
In many cases when radicles of corn plants from the rye treatment were very short, it was visibly apparent that the radicle tip and zone of elongation behind the tip had been destroyed and the root was no longer capable of elongation. Sometimes lesions were also visible behind the radicle tip. These symptoms were consistent with damage caused by corn root pathogens. Corn seedlings following a cereal rye cover crop had higher rates of visible radicle lesions or necrosis, which are normally symptoms of root disease, than corn plants from the fallow control (Table 2). Indeed, nearly every corn seedling grown following a rye cover crop had visible symptoms of radicle disease, while only 18–52% of corn seedlings that did not follow a cover crop had symptoms of radicle disease (Table 2). This is consistent with the reduction in radicle length observed following a rye cover crop (Table 1).
Table 2. Main effects of cover crop presence or species and fungicide seed treatments on corn seedling radicle disease incidence (%) for Experiments 1 and 2.

1 Values followed by the same letter are not significantly different (shown are mean ± SE proportion of plants in each pot with a diseased radicle). ‘NS’ indicates that the given factor was not significant.
2 P > Chi indicates the significance of the experimental factor in an analysis of deviance test on a generalized linear model with a binomial distribution and a logit link function (logistic regression). In Experiment 1, factors consisted of block, cover crop identity and presence of fungicide seed treatment. In Experiment 2, factors consisted of block and cover crop identity.
Somewhat surprisingly, treatment of corn seed with fungicides did not have a significant effect on the presence of symptoms of radicle disease. It is possible that scoring only presence of disease symptoms, rather than severity, may have masked fungicide efficacy, as small or large lesions were scored identically. It is also possible that the radicles from treated seed became infected later in the trial or after they had extended beyond the zone of protection afforded by the fungicide seed treatment. We would note that our plant growth conditions were deliberately set to be conducive toward root disease development, in order to reveal potential disease risks associated with cover cropping ahead of corn planting. That is to say, under optimal conditions for disease development, the efficacy of seed treatments may be expected to be somewhat limited. Notably, in the second trial, fungicide seed treatment did protect radicle length (Table 1) even though it did not reduce radicle disease incidence.
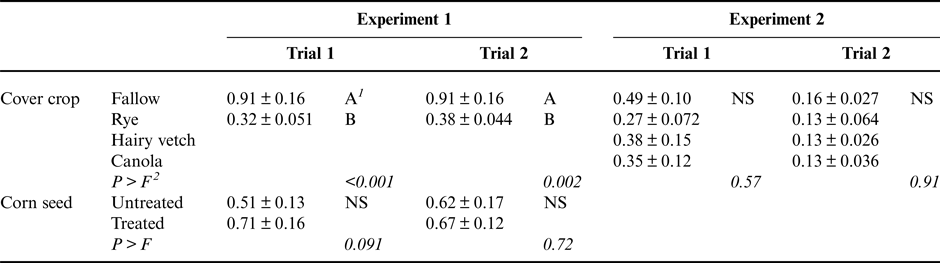
Corn seedlings following a cereal rye cover crop also had significantly higher rates of mesocotyl disease than corn plants in the fallow control (Table 3). Across trials, an average of 66–86% of corn plants following cereal rye had mesocotyl disease. In contrast, on average only 17–22% of corn plants that did not follow a rye cover crop showed symptoms of mesocotyl disease. Fungicide seed treatment was effective at reducing the incidence of mesocotyl disease in both trials. It is possible that fungicides were more effective at reducing mesocotyl disease incidence than radicle disease incidence because of directional transport of the active ingredients along with the movement of water through the xylem. One of the fungicides included in the seed treatment, mefenoxam, is reported to be systemic (Kennelly et al., Reference Kennelly, Gadoury, Wilcox and Seem2007). Other explanations may be found in soil moisture status or plant physiology differing between the radicle and the mesocotyl zone.
Table 3. Main effects of cover crop presence or species and fungicide seed treatments on corn seedling mesocotyl disease incidence (%) for Experiments 1 and 2.

1 Values followed by the same letter are not significantly different (shown are mean ± SE proportion of plants in each pot with a diseased mesocotyl). ‘NS’ indicates that the given factor was not significant.
2 P > Chi indicates the significance of the experimental factor in an analysis of deviance test on a generalized linear model with a binomial distribution and a logit link function (logistic regression). In Experiment 1, factors consisted of block, cover crop identity and presence of fungicide seed treatment. In Experiment 2, factors consisted of block and cover crop identity.
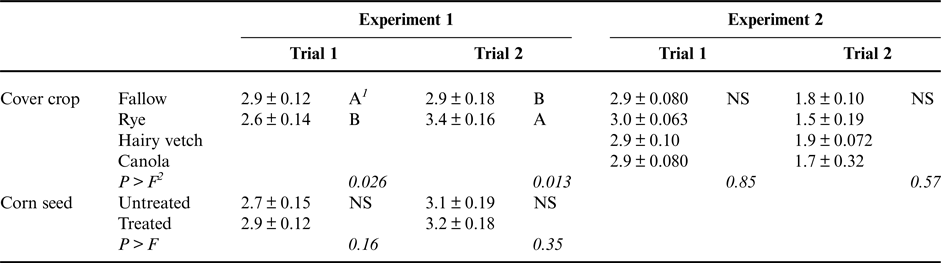
Above-ground measures of corn growth rate also indicated a slowing of corn development following a cereal rye cover crop. Corn shoot dry weight was significantly reduced, with plants following a rye cover crop producing approximately 60% less above-ground biomass in each trial (Table 4). Fungicide seed treatment did not significantly improve corn shoot dry weight in either trial.
Table 4. Main effects of cover crop presence or species and fungicide seed treatments on corn seedling shoot dry weight (g plant−1) for Experiments 1 and 2.

1 Values followed by the same letter are not significantly different (shown are mean ± SE). ‘NS’ indicates that the given factor was not significant.
2 P > F indicates the significance of the experimental factor in an ANOVA test. In Experiment 1, factors consisted of block, cover crop identity and presence of fungicide seed treatment. In Experiment 2, factors consisted of block and cover crop identity.
Corn seedling growth stage, an indication of the rate of plant development, was significantly affected by the cover crop treatment, but in an inconsistent way. In the first trial, corn following a rye cover crop developed more slowly than corn following a fallow control. However, in the second trial, corn following a fallow control developed more slowly than corn following cereal rye (Table 5). We have no explanation for this observation and the greater number of leaves for the rye treatment in the second trial is not consistent with the reduced shoot dry weight (Table 4). Fungicide seed treatment did not enhance the rate of corn seedling development in either trial.
Table 5. Main effects of cover crop presence or species and fungicide seed treatments on corn seedling growth stage (leaves plant−1) for Experiments 1 and 2.

1 Values followed by the same letter are not significantly different (shown are mean ± SE). ‘NS’ indicates that the given factor was not significant.
2 P > F indicates the significance of the experimental factor in an ANOVA test. In Experiment 1, factors consisted of block, cover crop identity, and presence of fungicide seed treatment. In Experiment 2, factors consisted of block and cover crop identity.
Experiment 2: Effects of three cover crop species on corn seedling growth
Comparing the impacts of a cereal rye cover crop on corn seedling growth with the impacts of other potential cover crop plant species revealed that cover crop species selection could reduce the disease risk to the following corn seedlings.
The plant species used as a cover crop significantly affected corn seedling radicle length in both trials of Experiment 2 (Table 1). Consistent with the results of Experiment 1, corn seedlings following cereal rye had radicles that were 56–78% shorter than the radicles of corn seedlings that did not follow a cover crop (Table 1). Notably, however, corn seedling radicles following a hairy vetch or winter canola cover crop were not different in length from radicles of corn seedlings in the fallow control. Corn radicles following hairy vetch, however, were also not significantly different from those following cereal rye. This suggests that some negative impacts of some cover crops on corn seedling radicle length may be avoided by selecting alternative cover crop species.
Neither corn shoot dry weight nor corn growth stage was significantly impacted by cover crop species treatments in Experiment 2 (Tables 4 and 5). This was unexpected, as cereal rye cover crops did impact these corn growth measures in Experiment 1. A possible explanation for this difference between experiments is that the cover crops were grown for 10 days longer in Experiment 1 compared to Experiment 2. It is possible the additional rye biomass produced in the longer growth period in Experiment 1 may have been important for revealing treatment impacts on corn shoot growth and development. Importantly, cover crop biomass production can be controlled by the timing of cover crop termination, which is a key management decision available to farmers and may be useful in mitigating potential risks associated with cover crops (Acharya et al., Reference Acharya, Bakker, Moorman, Kaspar, Lesnssen and Robertson2017).
The incidence of corn seedling radicle infection was significantly affected by cover crop species in both trials of Experiment 2 (Table 2). In general, corn seedlings in the fallow control had the lowest incidence of radicle infection, while corn seedlings following a hairy vetch or a winter canola cover crop had moderately higher incidence of radicle disease, and corn seedlings following a cereal rye cover crop had significantly higher incidence of radicle disease. This suggests that under favorable conditions, cereal rye may cause a greater risk of corn seedling root disease than the other two cover crop species tested. It might be expected that more pathogens capable of causing corn seedling disease would be hosted by roots of rye than by roots of hairy vetch or canola, given that corn is more closely related to cereal rye than to either hairy vetch or canola (Gilbert et al., Reference Gilbert, Briggs and Maharey2015).
The incidence of mesocotyl disease was significantly elevated in corn seedlings following any of the cover crop species compared to those seedlings that did not follow a cover crop, but only in the second of the two trials of this experiment (Table 3). In the first trial, incidence of mesocotyl disease was low for all treatments. Experiment 1 demonstrated that fungicide seed treatment could significantly reduce mesocotyl infection, and only treated seed was used in Experiment 2. Thus, it would seem that the seed treatment effectively prevented mesocotyl disease on corn seedlings following cover crops in the first trial of Experiment 2. In the second trial, however, infection rates of mesocotyls following any of the cover crop species were higher than infection rates of treated seed in Experiment 1.
Attempts to quantify infection rates by plating segments of corn radicles and mesocotyls on selective media in both experiments were complicated by bacterial overgrowth and by uncertainty about the identity of some fungi and oomycetes that grew out of corn tissue (data not shown). Thus, we used qPCR to quantify the density of Pythium spp. in corn seedling tissue from the second trial of Experiment 2. In contrast to culture-based methods on semi-selective media, qPCR offers a more quantitative measure of pathogen density and more stringent specificity for target organisms. We targeted two broad groups of species within Pythium: Clade B (which includes species such as P. torulosum, P. volutum and P. vanterpoolii) and Clade F (which includes species such as P. sylvaticum, P. irregulare and P. attrantheridium). Species within these clades have previously been shown to comprise the majority of Pythium spp. within corn and rye roots in our area (Acharya et al., Reference Acharya, Bakker, Moorman, Kaspar, Lesnssen and Robertson2017; Bakker et al., Reference Bakker, Manter, Moorman and Kaspar2017).
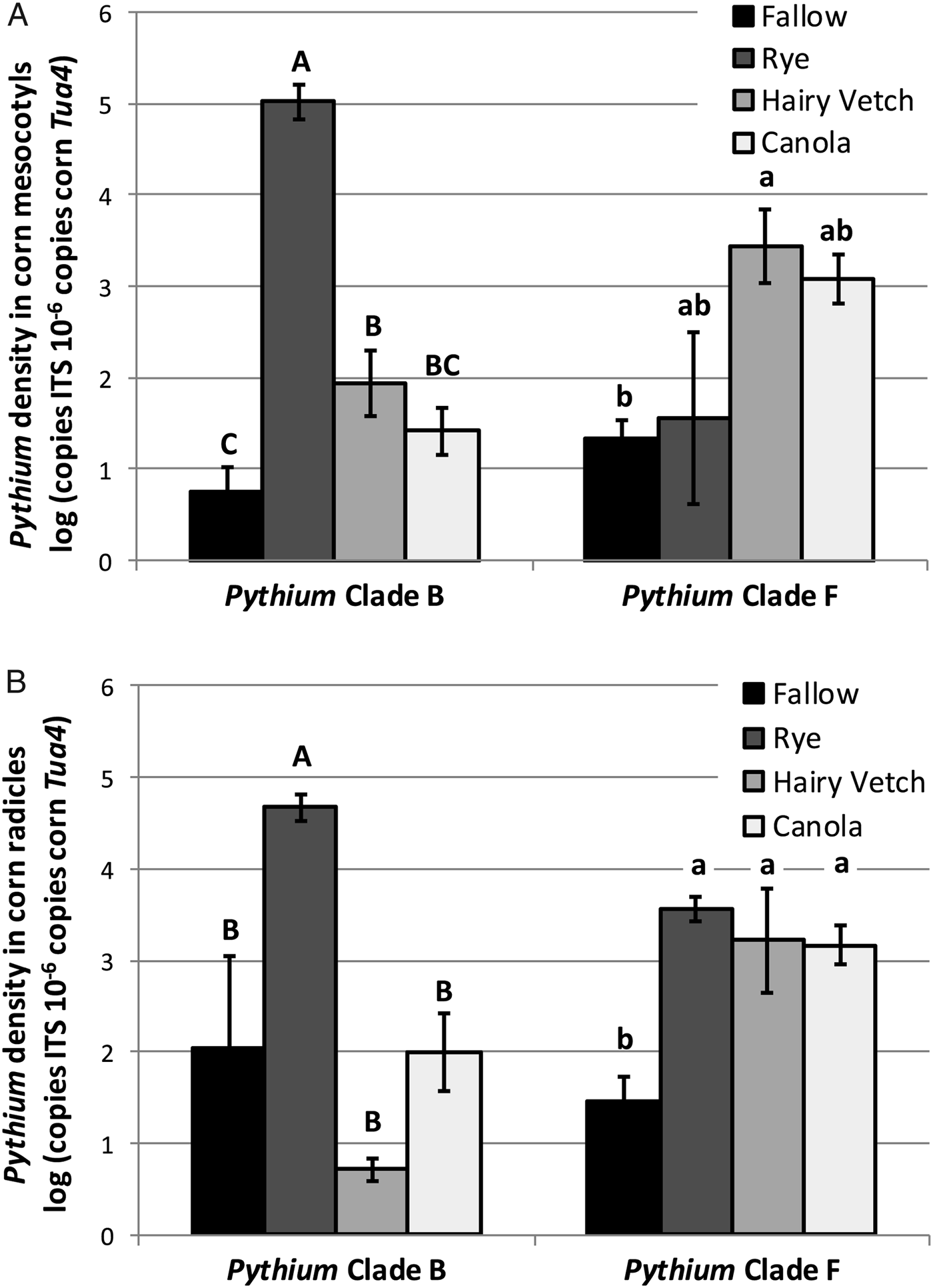
In corn radicles, Clade B Pythium spp. densities were elevated relative to the fallow control only by cereal rye (Fig. 1A). Clade F Pythium spp. densities in corn radicles were elevated by all three cover crop species compared to the fallow control (Fig. 1A). In corn mesocotyls, infection by Clade B Pythium spp. was elevated following a cereal rye cover crop (Fig. 1B), compared to all other treatments. Hairy vetch cover crops also significantly elevated densities of Clade B Pythium spp. in corn mesocotyls compared to a fallow control. In contrast, densities of Clade F Pythium spp. in corn mesocotyls were only significantly elevated relative to the fallow control following a hairy vetch cover crop (Fig. 1B). Clade F Pythium spp. densities in corn mesocotyls following cereal rye and canola were not significantly different from the fallow control or hairy vetch. Thus, cover crops grown preceding corn planting can elevate the densities of Pythium spp. in corn tissue, and different cover crop species may promote infection of corn seedlings by different species of Pythium. Additionally, different species of Pythium may exhibit different degrees of pathogenicity to corn seedlings. For instance, elevated densities of Clade F Pythium spp. in corn tissues following winter canola cover crops, without corresponding declines in corn seedling growth performance, suggest that Clade F Pythium spp. are not aggressive pathogens of corn.
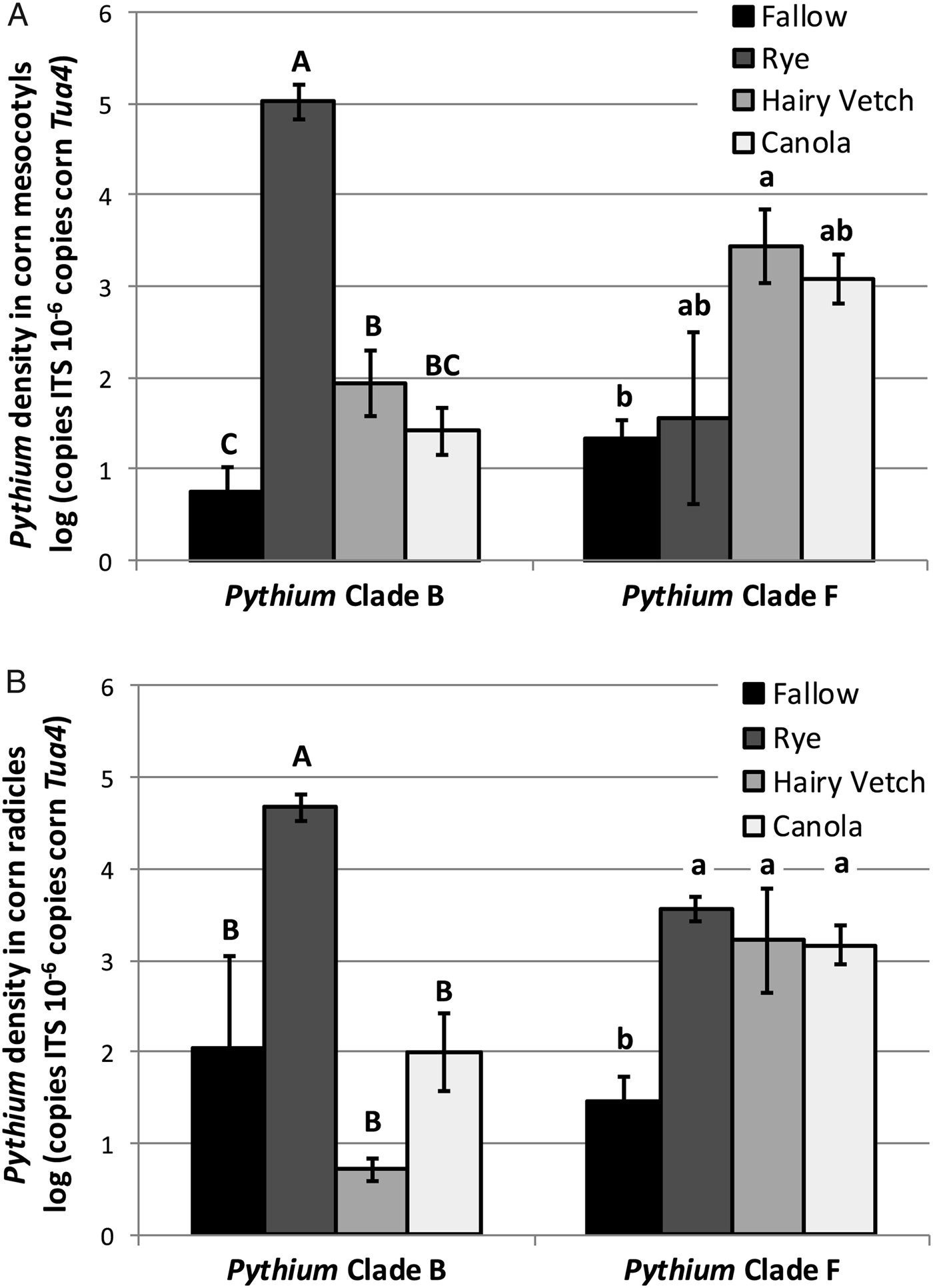
Figure 1. Density of Pythium spp. from Clade B and Clade F in corn (A) radicles and (B) mesocotyls following a fallow control or cereal rye, hairy vetch or canola cover crop treatments (Experiment 2, Trial 2). Bars topped by the same letter are not significantly different (P > 0.05; ANOVA with Tukey's HSD). Shown are mean ± SE.
Conclusion
In summary, this study indicates that soil-borne pathogens transferred from dying cover crop plants can infect corn roots and mesocotyls when environmental conditions are conducive to infection, leading to root disease and poor root and shoot growth. It should be noted that this study relied on controlled environment conditions and potted plants, under environmental conditions set to be conducive toward disease development; outcomes of cover cropping on corn seedling disease under field conditions may follow different dynamics than those observed in this study. Whether such effects at the seedling stage result in reduced yield later in the season would depend on environmental conditions later in the growing season. If disease pressure is strong and environmental conditions stressful enough to cause mortality, then corn populations may be reduced. If it is reduced below the optimum population for that growing season, yield could be impacted (Nafziger, Reference Nafziger1994). Additionally, corn plants with reduced or slowed growth because of seedling infection may be out-competed by neighboring corn plants (Ford and Hicks, Reference Ford and Hicks1992) and as a result may not develop an ear or develop an ear with fewer kernels, which could also impact yield.
Our hypothesis of elevated disease pressure in corn seedlings following cover crops is distinct from other proposed mechanisms regarding the risks cover crops may pose to corn performance and yield. For instance, reductions in corn radicle length following a hairy vetch cover crop have been reported previously (White et al., Reference White, Worsham and Blum1989), but were attributed to allelopathic effects. Our data suggest that fungal and oomycete pathogens may be involved, and this has been supported by complementary studies of field-grown cover crops (Bakker et al., Reference Bakker, Acharya, Moorman, Robertson and Kaspar2016). Correctly identifying the mechanisms by which cover crops may reduce subsequent corn performance is vital to improving management to allow corn yields to positively respond to soil quality improvements brought about by cover cropping.
In the future, we expect that cover crop species selection will be an important tool in managing this risk. Of the three cover crop species we tested, cereal rye seems to pose the greatest disease risk for corn seedlings. Unfortunately, at present few if any other cover crop species are as widely grown or better adapted to corn–soybean cropping systems in the upper Midwest than cereal rye. Cereal rye has superior winter hardiness and more growth at cool temperatures than most other potential cover crop species. Thus, testing, selection and breeding of other potential cover crop species or genotypes that could be used in place of cereal rye are essential. In the near term, additional research should develop management practices that reduce the risk of corn seedling disease following cover crops that are likely to host corn seedling pathogens. Possible management improvements include more efficacious seed treatments, lengthening the interval between cover crop termination and corn planting, and increasing the distance between cover crop plants and crop rows.
Acknowledgements
Partial funding for this work was provided by the Leopold Center for Sustainable Agriculture at Iowa State University (award E2012-03). Mention of trade names or commercial products is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer. This article was the work of U.S. Government employees engaged in their official duties and is exempt from copyright.