Introduction
There are plenty of challenges in the feedlot industry, but our number one health problem remains and is still in first place after more than a 100-year reign. That health problem is of course bovine respiratory disease or BRD. Morbidity rates vary between respective feed yards, but BRD likely remains the number one cause of illness and death.
One area on the BRD battlefield where we have been historically weak is in the area of ‘case definition’ or diagnosis. Numerous research findings demonstrate a weak correlation between lung lesions at harvest and treatment histories at the feedlot. ‘Our current methods of disease diagnosis are not adequate for evaluating management changes or product efficacy whether we are interested in understanding the cost-effectiveness of our decisions.’ (Bryant et al., Reference Bryant, Perino, Griffin, Doster and Wittum1999).
The industry has a poor track record of correct diagnosis of BRD, in part because we have lacked the specific chute side diagnostic tools. In most feedlot hospitals or processing barns, one will find a thermometer and nothing else. With no disrespect to the thermometer, it is not a specific diagnostic tool. The presence of a fever does not diagnose BRD. Analysis of hospital records measuring correlations between rectal temperatures and case fatality rates suggest that obtaining a rectal temperature does not constitute a thorough physical exam.
Methods
To analyze the effectiveness of current diagnostic tools (temperature, manual lung scores, and Whisper® lung scores), two data sets were collected. The first focused on discovering the correlation between rectal temperature, manual lung scores, and case fatality rate. The second data set focused on the improvement that Whisper® scores and rectal temperature could provide over either tool alone.
The first data set was collected from a single feedlot source; the data included the ‘at-pull rectal temperature’ and case fatality outcome for 3063 head that had temperatures recorded of greater than 100 °F and less than 109 °F. From the same feedlot source, 3112 head were analyzed for lung scores (on a 10-point scale) and case fatality outcome. Pearson correlation statistics were generated using Fisher's z transformation and weighted by within temperature or within lung score categories by frequency count. Alpha levels at 0.05 were imposed to interpret the results testing for a null hypothesis of a zero correlation. Analysis was done using SAS 9.4 CORR module.
For the second data set, predictive analysis was performed using a stepwise logistic regression approach (SAS 9.4 LOGISTIC module). This analysis included information from health records from 15,411 head pulled for BRD the first time across eight feedlots. There were 1339 (8.68%) post-treatment deaths and 14,072 survivors. To preserve clinical relevance and align established treatment protocols with temperature, cases were categorized into two groups: Fever group (temperature of 104.5 °F or greater) and No Fever group (temperature of <104.5 °F). To preserve consistency across feedlots, lung scores were assessed with Whisper® stethoscopes and software rendering a 5-point lung score scale. Receiver-operating characteristic (ROC) curves and R 2 estimates were used to assess model performances at each step. Wald confidence intervals on estimated odds ratio were calculated to interpret results. At each step, false-negatives (those deaths not classified as a positive diagnosis for a given diagnostic technique) were assessed and used to compare model performance and ultimately clinical usefulness of the diagnostic technique.
Results
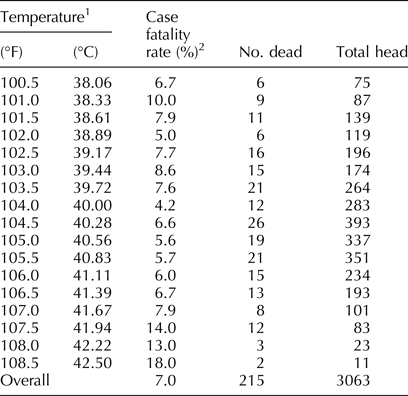
The first data set and analysis provided the motivation for the development of the lung-scoring technique. Table 1 describes the results of this analysis. Temperature was significantly correlated with the case fatality rate, but the correlation was not strong (r = 0.060; 95% confidence intervals (CI) 0.024–0.095). Fisher's z was 0.0601; P = 0.0009. This weak correlation between temperature and mortality inspired veterinarians to teach caregivers to use conventional stethoscopes to assign lung scores to pulled cattle. These manual lung scores were used to predict prognosis, select anti-infectives (AIF), assess pen rider skill, and assess disease severity in priority pens.
Table 1. BRD case fatalities associated with body temperature

1Rectal temperatures of 3063 feedlot cattle identified with signs of respiratory disease in a single feedlot.
2Case fatality rate was significantly but only weakly correlated with temperature at first pull (correlation = 6.0%, 95% CI for the correlation = 2.5–9.5%; P = 0.0008).
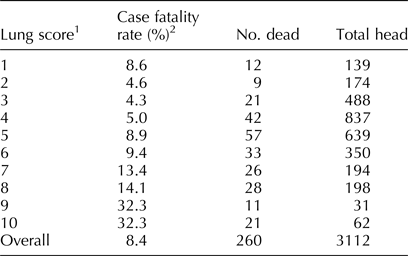
Table 2 gives a summary of the population where manual lung scores were collected. In contrast to temperature, lung score correlated more closely with case fatality rate (r = 0.799; 95% CI 0.785–0.811). Fisher's z was 1.0956; P < 0.0001. Both analyses indicated a Fisher's z statistic significant at an alpha level of <0.05 with no overlap in 95% CI.
Table 2. BRD case fatalities by lung score

1Lung scores were obtained by auscultation for 3112 cattle identified with signs of respiratory disease in a single feedlot.
2Case fatality rate was significantly correlated with lung score determined by auscultation with a stethoscope (correlation = 79.8%, 95% CI for the correlation = 78.5–81.1%; P < 0.0001).
The primary issue with manual lung scores is ensuring consistent scores across caregivers and locations. This lack of consistency motivated the use of Whisper® to evaluate just how much utilizing both tools can improve the ability to reduce unexpected mortality.
The logistic model with intercept was significant (Wald χ 2 = 3137.529; P < 0.0001). Stepwise regression analysis included Whisper® lung score at the first step. The ROC curve went from 0.5000 to 0.6434 and residual χ 2 was 199.8060 (P < 0.0001). At the second and final step, fever was entered into the model. The ROC curve went from 0.6434 to 0.6869, with Type 3 analysis of effects showing fever χ 2 = 192.1828 (P < 0.0001) and Whisper® lung score χ 2 = 294.8952 (P < 0.0001). The odds ratio for fever was 2.274 (95% CI 2.025–2.555). Whisper® lung score odds ratios were: score of 1 versus score of 5 (0.151, 0.119–0.191), 2 versus 5 (0.306, 0.247–0.379), 3 versus 5 (0.444, 0.357–0.554), and 4 versus 5 (0.561, 0.415–0.755). The parameter estimate for the intercept was −2.0693 (95% CI −2.1425 to −1.9976). The number of false negatives with fever as a predictor of death (i.e. those that died and did not have a fever) was 608 head. The number of false negatives with Whisper® lung score of 1 as a predictor of death (i.e. those that died and had a lung score of 1) was 398 fewer than for fever, at 210 head. The number of false negatives when the combination of fever and Whisper® lung score was used to predict death was 102 head, or 506 fewer head than when fever alone was used to predict death, and 108 fewer head than when Whisper® lung score alone was used to predict death (Table 3). This analysis indicates that the lung score is more likely to predict treatment outcomes than a thermometer, and that evaluation of lung score and identification of fever together most accurately predicts mortality.
Table 3. BRD severity diagnostic methods: 15,863 first-pulled animals and case fatality outcomes categorized by diagnostic results

1Cattle that were not observed to have a fever when first pulled and ultimately died from BRD.
2Cattle with a Whisper lung score of 1 (Whisper LS 1) when first pulled and ultimately died from BRD.
3Cattle with No Fever and Whisper LS 1 when first pulled were less likely to die than cattle only identified as afebrile or a Whisper LS 1. Animals categorized as likely to survive due to both absence of fever and Whisper LS 1 led to a decreased rate of cattle falsely predicted to survive by 83 or 51%, respectively, compared to the use of absence of fever or Whisper LS 1 alone.
Making a proper diagnosis remains a challenge in many feedlots. Caregivers select cattle to undergo hospital evaluation based on pen observations that include clinical depression, anorexia, exercise intolerance, and abnormal posture. The BRD complex can contribute to these signs but so can dehydration, fatigue, lameness, and digestive disorders. A significant number of cattle taken to hospitals do not have BRD and so should not be treated for BRD. More importantly, treatment success of cattle with BRD depends on an accurate diagnosis and accurate assessment of severity. The response to AIF can vary by feedlot and lung score. Treatment protocols can be designed to take into account BRD severity. Antibiotic response comparisons are more valid when stratified by lung score severity. Chute-side lung score data provide information that refines pen rider activities, guides hospital management decisions, and identifies priority pens during BRD epidemics.
Conclusion
Imagine that we had two human medical clinics staffed by two different doctors. At medical clinic ‘A’ the doctor spends little time with each patient and routinely provides a script for antibiotics. At clinic ‘B’ the doctor takes a bit more time and typically gets a good history, does an exam, requests blood work, radiographs, etc., to obtain a diagnosis. After each doctor has seen 1000 patients, which clinic would you expect to have the highest success rate and possibly the lowest mortality rate? Which doctor would you want to have for your physician?
The above example is a bit extreme, but the point is that the feedlot industry has tremendous opportunity to improve animal care, treatment success, lower mortality rates, and improve antibiotic stewardship. It all starts with understanding and embracing the opportunity provided by new diagnostic tools such as that provided by Whisper®.
Production animal medicine has plenty of obstacles, nuances, and challenges that are not to be found in human medicine. When used in a production animal setting, Whisper® is neither perfect nor infallible. However, the utilization of auscultation via Whisper® has resulted in better case definition, improved risk assessment, stratification of cattle by lung score, and targeted antibiotic treatments.