INTRODUCTION
Memory problems represent a key feature of cognitive decline during aging. Individual trajectories of cognitive change are highly heterogeneous, with some declining rapidly and others changing little or even improving (Reed et al., Reference Reed, Mungas, Tomaszewski Farias, Harvey, Beckett, Widaman and DeCarli2010; Wilson et al., Reference Wilson, Beckett, Barnes, Schneider, Bach, Evans and Bennett2002). Additionally, dissociations between brain pathology and clinical expression have been noted (Satz, Cole, Hardy, & Rassovsky, Reference Satz, Cole, Hardy and Rassovsky2011). Therefore, research has focused on identifying parameters that contribute to this heterogeneity, such as levels of education, occupational attainment, and genetic factors (Stern, Reference Stern2011).
Changes in cognitive performance associated with aging are the culmination of dynamic neurobiological processes that take place during the lifespan. These processes seem to be selective, affecting different areas of the brain at different rates and involve prefrontal cortical areas as well as the inferior and mesial temporal lobe areas, including the hippocampi. These areas sustain the greatest diminution of blood flow, neuronal loss and overall volume (Kramer et al., Reference Kramer, Mungas, Reed, Wetzel, Burnett, Miller and Chui2007). Furthermore, the availability or reuptake capacity of certain neurotransmitters seems to be affected by age, which in turn can result in a decrease in the speed and efficiency of synaptic signal transmission (Wu, Oh, & Disterhoft, Reference Wu, Oh and Disterhoft2002). These neuropathological processes provide a framework for understanding concomitant cognitive changes given that the prefrontal, inferior temporal, and hippocampal areas and associated networks are critical for organizing, categorizing, learning, and retrieving information (Constantinidou, Christodoulou, & Prokopiou, Reference Constantinidou, Christodoulou and Prokopiou2012; Glisky, Polster, & Routhieaux, Reference Glisky, Polster and Routhieaux1995; Lee, Yuen, Chu, & Chi, Reference Lee, Yuen, Chu and Chi2004; Salthouse & Ferre-Caja, Reference Salthouse and Ferrer-Caja2003).
The bulk of research on memory decline and aging has focused on verbal memory abilities (e.g., Rönnlund, Nyberg, Bäckman, & Nilsson, Reference Rönnlund, Nyberg, Bäckman and Nilsson2005; Salthouse, Reference Salthouse2009). Verbal memory assessment protocols typically incorporate tests of auditory attention span and working memory (such as digit span forward and backward tasks) and episodic memory capacity that include verbal learning tasks such as the Rey Auditory Verbal Learning Test or the California Verbal Learning Test.
Together, working and long-term memory measures capture key aspects of the complexity of memory systems, consistent with the working memory model proposed by Baddeley and associates (Baddeley, Reference Baddeley1986) and the long-term memory systems such as those proposed by Tulving (Reference Tulving1985), Squire (Reference Squire1992) and others. Tests that measure static spans (e.g., forward span of the WAIS or the WMS-III) are typically associated with the capacity of the phonological loop, which represents one of the many subcomponents of the entire working memory system, such as those responsible for the flexible control of attention (Baddeley, Reference Baddeley1995; Conway et al., Reference Conway, Kane, Bunting, Hambrick, Wilhelm and Engle2005; Unsworth & Engle, Reference Unsworth and Engle2007). The control of additional working memory components is believed to be exerted by specific executive functions (Salthouse, Reference Salthouse2005), such as those exemplified by dual processing, set shifting, and inhibition tasks (Colflesh & Conway, Reference Colflesh and Conway2007; Conway et al., Reference Conway, Kane, Bunting, Hambrick, Wilhelm and Engle2005; Kane & Engle, Reference Kane and Engle2002, Reference Kane and Engle2003). These additional mechanisms are facilitated by the memory “buffer” whose function is to integrate information from the phonological loop and the visuospatial sketchpad (Baddeley, Reference Baddeley2000) and also facilitate temporary retrieval from the episodic stores (Baddeley, Reference Baddeley2001). Memory tasks that critically depend on the active attentional control and the executive subcomponents of working memory likely include the digit span backward and verbal learning paradigms like the Rey Auditory Verbal Learning Test (RAVLT) or the California Verbal Learning Test (CVLT) (Conway et al., Reference Conway, Kane, Bunting, Hambrick, Wilhelm and Engle2005; Kane et al., Reference Kane, Hambrick, Tuholski, Wilhelm, Payne and Engle2004; Sander, Nakase-Richardson, Constantinidou, Wertheimer, & Paul, Reference Sander, Nakase-Richardson, Constantinidou, Wertheimer and Paul2007).
Age-related differences are generally noted on working memory tasks that pose heavy demands on the mental manipulation of information (e.g., recall of digits backward), or by requiring delayed recall, as compared to simple and more automatic information maintenance tasks such as forward digit span tasks (Craik, Reference Craik1991; Kasniak, Poon, & Riege, Reference Kasniak, Poon and Riege1986). Furthermore, when the amount of information to be remembered exceeds the typical auditory span (of five to nine items), then older adults perform worse than their younger counterparts (Swanson, Reference Swanson1999, cf. Bopp & Verhaeghen, Reference Bopp and Verhaeghen2005).
The Rey Auditory Verbal Learning Test (AVLT) has been used extensively in the aging literature as it provides the context for assessing multiple memory indices and corresponding memory phenomena, such as initial recall, learning slopes, proactive and retroactive interference, effortful retrieval, and recognition (Blachstein, Greenstein, & Vakil, Reference Blachstein, Greenstein and Vakil2012; Devanand et al., Reference Devanand, Liu, Brown, Huey, Stern and Pelton2012; List et al., in press; Okusaga et al., Reference Okusaga, Stewart, Butcher, Deary, Gerry, Fowkes and Price2013; Sander et al., Reference Sander, Nakase-Richardson, Constantinidou, Wertheimer and Paul2007). Prior research indicates that older adults over 60 perform worse than their younger counterparts on the five learning trials of the AVLT. Furthermore, demographic variables such as education level, socioeconomic status, and gender affect performance on this particular task (Constantinidou & Baker, Reference Constantinidou and Baker2002; Vakil & Blachstein, Reference Vakil and Blachstein1997). Older adults diagnosed with amnestic-Mild Cognitive Impairment (a-MCI) characteristically perform in the impaired range on this and similar episodic, verbal memory tasks. Interestingly, there is accumulating evidence that patients with a-MCI also show reduced performance on measures of attention span and working memory (Economou, Papageorgiou, Karageorgiou, & Vassilopoulos, Reference Economou, Papageorgiou, Karageorgiou and Vassilopoulos2007; Zheng et al., Reference Zheng, Dong, Sun, Xu, Ma and Wang2012), although the severity of these difficulties does not warrant diagnosis of the amnestic, multidomain variant of MCI. However, the vast majority of relevant studies focus on group differences at the mean-score level, and there is little research on the contribution of attention span and working memory ability on the age-related decline of episodic memory performance in healthy aging or in patients with clinically significant memory decline (e.g., a-MCI).
The present study attempts to fill this gap by testing moderation and mediation effects on verbal episodic memory performance in relatively large community and clinical samples. Specifically, the study aims to determine if age-related decline follows a linear trend or alternatively if the rate of decline in various indices of verbal episodic memory changes with age. The study will also determine if the rate of age-related decline is affected by participant demographics (formal education and gender) and clinical diagnosis (a-MCI). Finally the study assesses the extent to which age-related decline in verbal episodic memory is mediated by attention span and/or verbal working memory and the extent to which the magnitude of the hypothesized mediating effects vary with (a) education level and (b) the presence of episodic memory deficits in patients with mild cognitive impairment.
METHODS
The study was conducted in compliance with institutional standards for human research and in accordance with the Helsinki Declaration http://www.wma.net/e/policy/17-c_e.html.
Participants
Community sample
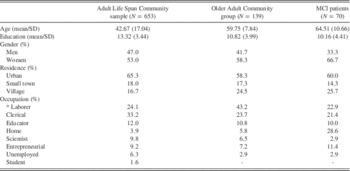
The effects of demographic variables on memory indices were assessed on data from a community sample of 653 native Greek speakers aged 17–86 years recruited from eight broad geographic areas of mainland Greece and the broad Nicosia area in Cyprus. Initial contact with prospective participants in each region was performed by trained psychologists in senior centers and internal medicine clinics. Inclusion criteria for participation in the study were the following: self-reported normal or corrected to normal vision and hearing, negative history of neurological (including traumatic brain injury accompanied by loss of consciousness of more than 10 min), or diagnosed psychiatric disorder. In addition, participants should have scored at least 23 points on the Mini Mental State Examination (Fountoulakis, Tsolaki, Chantzi, & Kazis, Reference Fountoulakis, Tsolaki, Chantzi and Kazis2000) and achieve scores lower than 7 points on the Short Form of the Geriatric Depression Scale (Fountoulakis et al., Reference Fountoulakis, Tsolaki, Iacovides, Yesavage, O’Hara, Kazis and Ierodiakonou1999) or 28 points on the Center for Epidemiological Studies Depression scale (CESD; Fountoulakis et al., Reference Fountoulakis, Iacovides, Kleanthous, Samolis, Kaprinis, Sitzoglou and Bech2001). There were no significant regional differences on age, education, and gender distribution. Sample demographic information is presented in Table 1.
Table 1 Demographic characteristics of the entire community sample, the MCI group and the age- and education level-matched older healthy comparison group
To further ensure that sensory deficits did not affect performance, examiners were trained to observe signs of hearing loss during the preliminary clinical interview and individuals who appeared to have trouble understanding the examiners’ queries at normal, conversational voice level were not included in the study.
a-MCI group
The clinical group included older adults aged 53–85 years who, based on comprehensive neurological, laboratory, and neuropsychological tests were eventually diagnosed with amnestic MCI (n=70). All patients were residing in the city of Heraklion and surrounding rural areas and presented to the Dementia Clinic of the University of Crete General Hospital with memory complaints. MCI was diagnosed according to the International Working Group (IWG) on MCI criteria (Winblad et al., Reference Winblad, Palmer, Kivipelto, Jelic, Fratiglioni, Wahlund and Petersen2004). Cognitive testing performed as part of the diagnostic procedure included the Greek adaptations of the Boston Naming and Peabody Picture Vocabulary tests (BNT & PPVT-R; Simos, Kasselimis, & Mouzaki, Reference Simos, Kasselimis and Mouzaki2011), the Trail Making Test A and B (Zalonis et al., Reference Zalonis, Kararizou, Triantafyllou, Kapaki, Papageorgiou, Sgouropoulos and Vassilopoulos2008), the Controlled Oral Word Association test (COWAT; Kosmidis, Vlahou, Panagiotaki, & Kiosseoglou, Reference Kosmidis, Vlahou, Panagiotaki and Kiosseoglou2004), the Symbol Digit Modality Test (SDMT; Constantinidou et al., Reference Constantinidou, Christodoulou and Prokopiou2012), and the Greek Passage Memory test (which is based on the Wechsler Memory Scale Logical Memory subtest; Simos, Papastefanakis, Panou, & Kasselimis, Reference Simos, Papastefanakis, Panou and Kasselimis2011). In addition to the clinical examination, all MCI patients had to meet the following diagnostic criteria: (a) Mini Mental State Examination (MMSE; Fountoulakis et al., Reference Fountoulakis, Tsolaki, Chantzi and Kazis2000) score >23, (b) age- and education-adjusted Z scores >-1.0 on PPVT-R, BNT, COWAT, TMT-A, & TMT-B, and SDMT, (c) scores lower than 9 points on the Geriatric Depression Scale or <40 points on the Center for Epidemiological Studies Depression scale, and (d) age- and education-adjusted Z scores <−1.5 on the Immediate and Delayed free recall indices of the Greek Passage Memory test.
Older adult community sample
A comparison group of 139 adults aged 52–86 years was selected from the greater community sample to match the a-MCI group on mean age and education level (p>.05). The two groups were also comparable on the distribution of gender, residence (i.e., urban, small town, village), and occupation type (p>.1 in all cases). Patient and control group demographics are shown in Table 1.
Measures
In addition to the neurocognitive battery used for MCI diagnosis, all participants were administered the following memory tests the results of which were not taken into account for diagnosis:
-
1. Digits Forward and Digits Reverse subtests from the Greek Memory Scale (Simos et al., Reference Simos, Papastefanakis, Panou and Kasselimis2011). The Digits Forward subtest provides a short-term memory index and requires an immediate replication of a series of digits orally presented to the participant. There are eight-difficulty levels ranging from two to nine digits. Successful repetition of all digits without additions and in the correct order is awarded with two points. Repetition of the entire set of digits without additions with a single switch in the order of two digits is scored with one point. The maximum score is 25 points. The Digits Reverse subtest provides a working-memory index and requires repetition of single digit sequences in reverse order. There are seven levels of difficulty ranging from two to eight digits. Scoring criteria are identical to those adopted in Digits Forward for a maximum score of 24 points. Test–retest correlations based on a random sample of 51 adults (23 men and 28 women, mean age=42.6 [SD=12.56], mean years of education=13.4 [SD=3.51]) were .692 and .688 for Digits Forward and Digits Reverse, respectively. Corresponding alphas were .78 and .81. Notably, the correlations between raw scores obtained using the currently reported scoring scheme (allowing for partial credit) and scores obtained using the standard scoring method (0 and 1 points only) were r=.963 and r=.957 for Digits Forward and Digits Reverse, respectively.
-
2. The Greek version of the Rey Auditory Verbal Learning Test (AVLT, Geffen & Geffen, Reference Geffen and Geffen2000). The AVLT was translated and adapted in Greek upon permission from the publisher (ACER). Forward and backward translation procedures were followed using blind professional translators. The following delayed recall and recognition indices were analyzed: Trials 1–5/Total Immediate Recall (the total number of words correctly recalled from List A during the five learning trials); Trial 6/Short Delay Recall (the number of words correctly recalled from List A immediately following recall of [interference] List B); Trial 7/Long Delay Recall (the number of words correctly recalled from List A after a 30-min delay during which participants were asked to fill out questionnaires assessing psychosocial characteristics); Retention: the proportion of words from AVLT list A recalled on Trial 5 (i.e., the last of the learning trials) which were also recalled on Trial 7 (range of scores 0–1.00); Recognition pA index: a non-parametric signal detection measure of delayed recognition correcting the recognition score of List A (Hit rate-HR) by taking into account the false positive rate (FP; distractors recognized as part of List A; Geffen, Butterworth, Forrester, & Geffen, Reference Geffen, Butterworth, Forrester and Geffen1994). PA was computed according to the following formula: pA=0.5 * (1 + HR – FP) with scores ranging from 0 (random performance) to 1.00 (perfect performance). Test–retest data (N=51) revealed acceptable reliability values for AVLT indices (Trials 1–5: r=.566, Trial 6: r=.633, Trial 7: r=.674, Retention: r=.59, Recognition pA: r=.64).
The short version of the Geriatric Depression Scale (Fountoulakis et al., Reference Fountoulakis, Tsolaki, Iacovides, Yesavage, O’Hara, Kazis and Ierodiakonou1999) was completed by some participants (n=139) and CESD by the remaining participants (n=584).
Data Analyses
The first aim of the study (determine the pattern of age-related decline in verbal episodic memory indices) was addressed through regression models that included linear and quadratic effects of age. Models were tested on data from the entire community sample (N=653). The linear term (effect of age) was entered in the first step and the quadratic (age2) in the second step of each model.
The second aim of the study (determine if the rate of age-related decline was affected by participant formal education and gender) was examined through moderated regression analyses (also on data from the entire community sample). These models assessed the hypothesis that the association between age and each of the verbal episodic memory indices was moderated by participant education and gender.
The third aim of the study addressed the following two hypotheses:
-
(a) Age-related decline in verbal episodic memory capacity was mediated, at least in part, by the effects of age on auditory attention span and/or working memory capacity, which in turn contributed significantly to the ability to form and maintain new episodic memories. This hypothesis predicted the presence of significant indirect effects of age on each immediate and delayed AVLT index through digits forward or digits reverse scores. Mediated regression models were fit to the data from the entire community sample to assess this hypothesis.
-
(b) The hypothesized indirect effect of age on AVLT indices through digit span scores varies with participant education level. Moderated mediation analyses were applied to the data from the entire community sample with digits forward or digits reverse scores serving as mediators of the association between age and each of the AVLT indices. Education level (in years of formal education) was entered into each model as a potential moderator of the three paths linking age and AVLT performance (age on digits score, age on AVLT score, and digits score on each AVLT index).
The fourth aim of the study tested the hypothesis that the strength of the paths between age, attention span and/or working memory capacity, and verbal episodic memory capacity is different in patients who present with episodic memory deficits (amnestic MCI) as compared to age- and education-level-matched older participants drawn from the community sample. Formally, this aim assessed the extent to which the mediating effects of digits forward or digits reverse score on the association between age and AVLT indices are moderated by clinical group. The moderated mediation model was similar to the one used to address Aim 3b, with the exception that Clinical group served as the moderating variable instead of education. The data fitted to this model were drawn from a group of 70 persons diagnosed with probable a-MCI and a matched comparison group chosen from the overall community sample (n=139; Older Adult Community group). In the presence of significant moderating effects of Clinical Group, simple mediation models would be applied to the association between Age and each delayed AVLT index (through digits forward or digits reverse) performed separately for each group. The alpha level was set to .05 for all statistical tests.
RESULTS
Age-Related Decline in Verbal Episodic Memory Capacity: Direct Linear and Quadratic Effects of Age
Zero order Pearson correlation coefficients between demographic variables (age, education, and gender) and each AVLT index are presented in Table 2 for the entire community sample, demonstrating significant linear effects of age on each index.
Table 2 Pearson correlations between AVLT indices, memory for digits and demographic variables in the Adult Life Span Community sample (N=653)

Note. Trials 1–5: Total number of words correctly recalled across Trials 1–5 (immediate recall); Trial 6: Short Delay Recall (number of words correctly recalled from AVLT List A immediately after recall of interference List B); Trial 7: Long Delay Recall (number of words correctly recalled from AVLT List A after 30-min delay); Retention: proportion of words from AVLT list A recalled on Trial 5 that were also recalled on Trial 7; Recognition pA: AVLT Delayed Recognition pA index.
* p<.01.
Next we examined the hypothesis that the age-related decline in verbal episodic memory indices changed significantly with advancing age. Two models of age-related change were contrasted (linear and quadratic) in hierarchical regression analyses, where age was entered as a predictor of each memory index in the first step and age squared in the second step. These analyses were, again, conducted on the entire community sample. Both predictor variables were centered to reduce colinearity. Results, summarized in Table 3, indicate that quadratic effects of age were significant for all indices. Given that both (linear and quadratic) regression coefficients were negative in each case, results are consistent with an increase in the rate of decline of verbal episodic memory capacity with increasing age. For instance, AVLT long-delay free recall score (Trial 7) is expected to drop by .079 points for every year over the age range of the community sample (17–86 years). Moreover, the rate of age-related decline in performance increased, on average, by .001 points for every year of life. Specifically, whereas the rate of decline in AVLT Trial 7 score was estimated to only .054 points/year at age 18 (1.5 SDs below the average age of 42.67 years in the present cohort), this rate rose to .079 points/year at 42.67 years, .104 points at 68.17 years, and .113 points at 76.67 years (corresponding to 1.5 and 2 SDs above the mean age of the cohort, respectively).
Table 3 Linear and quadratic effects of age on AVLT indices in the entire community sample (N=653)

Note. Trials 1–5: Total number of words correctly recalled across Trials 1–5 (immediate recall); Trial 6: Short Delay Recall (number of words correctly recalled from AVLT List A immediately after recall of interference List B); Trial 7: Long Delay Recall (number of words correctly recalled from AVLT List A after 30-min delay); Retention: proportion of words from AVLT list A recalled on Trial 5 that were also recalled on Trial 7. Unstandardized regression coefficients are shown.
* p<.001.
†p=.0001.
Moderators of Age-Related Decline in Verbal Episodic Memory: Education Level and Gender
This analysis tested the hypothesis that age-related, verbal episodic memory decline in the entire community sample was affected by demographic variables (gender and education level). Table 2 presents the expected pattern of associations between AVLT indices and education level (in years of formal education). Specifically, education was positively associated with all AVLT performance indices. Moreover, and with the exception of Recognition pA, Gender was also significantly associated with AVLT scores, suggesting higher scores for women than men. We further examined moderating effects of education and gender on the regression of memory performance on age.
Initially, gender and education were entered together as potential moderators of the (linear) effect of age on AVLT performance, using the SPSS macro developed by Hayes (Reference Hayes2013; model 2) for moderated regression. In each analysis the following equation was used to estimate the outcome variable (Y: individual scores on each of the four AVLT indices):
where X is the main predictor (age in years), M is gender (0 for men and 1 for women), and W is education (in years).
Results revealed that gender and education exerted, independently, significant direct effects on most AVLT indices, but failed to demonstrate evidence of a moderating effect of either demographic variable on the association between age and delayed AVLT scores. Thus, education level affected the absolute delayed episodic memory scores, although the rate of age-related decline was similar across education levels and between men and women. A relatively weak moderating effect was found for immediate recall (total words recalled on Trials 1 through 5): b=.0144, SE=.007, p=.028. The effect of age on total immediate recall was slightly stronger among participants averaging 8.4 years of education (b=-.37, SE=.03) than for participants averaging 15.6 years of education (b=-.26, SE=.03).
Is Age-Related Verbal Episodic Memory Decline Due to Progressive Deterioration of Attention Span and/or Working Memory, which Is in Turn Affected by Education Level?
As part of the third specific aim of the study, we sought to examine whether education moderates, instead, the indirect effects of age on verbal episodic memory through attention span and/or working memory. The general model tested is illustrated in Figure 1, where the mediation paths linking age and each of the AVLT indices (b11, b23) are allowed to vary with education (years of formal schooling). We used SPSS macros developed by Hayes (Reference Hayes2013; model 58), to assess moderated mediation. The mediator Mj (digits forward or digits reverse score, analyzed separately) was estimated using the following equation:
The following equation was used to estimate the outcome variable (Y: individual scores on each of the four AVLT indices):
Results presented in Table 4 reveal significant paths from Age to Digits Reverse (b11) and also between Digits Reverse and each of the three AVLT free recall indices (Trials 1–5/immediate recall, Trial 6/short delay, and Trial 7/long delay; b23). Importantly, results supported the hypothesis of significant mediation of age-related verbal episodic memory decline by verbal working memory: the total indirect effect of age through Digits Reverse was significant for each of the three free recall AVLT indices (normal theory-based test, p<0007). This conclusion was further supported by the fact that the 95% confidence interval obtained for the value of the standardized, total indirect effect through bootstrapping did not include 0. Inspection of the unstandardized regression coefficients in Table 4 indicates that Digits Reverse score is expected to drop by .09 points for every year in life in the entire community sample. Across ages, a one point reduction in Digits Reverse score was associated, for instance, with a .12-point decrease in Trial 7 free recall score. The magnitude of the estimated indirect effect of age suggests that for every year, delayed free recall score is expected to drop by .011 points solely due to the decline in verbal working memory capacity with age.

Fig. 1 Schematic illustration of the proposed moderated (by participant education level) mediation of the association between age and AVLT recall/recognition indices. *AVLT Trials 1–5, Trial 6, Trial 7, Retention, and Recognition pA were used as complementary indices of verbal episodic memory capacity.
Table 4 Unstandardized regression coefficients for moderated (by education) mediation of age on AVLT scores through Digit Span scores in the community sample (N=653)

Note. Trials 1–5: Total number of words correctly recalled across Trials 1–5 (immediate recall); Trial 6: Short Delay Recall (number of words correctly recalled from AVLT List A immediately after recall of interference List B); Trial 7: Long Delay Recall (number of words correctly recalled from AVLT List A after 30-min delay); Retention: proportion of words from AVLT list A recalled on Trial 5 that were also recalled on Trial 7.
* p<.01.
†p<.001.
With respect to Digits Forward, although age exerted a significant direct effect on this measure (path b11), the direct effects of Digits Forward on AVLT scores (b23) failed to reach significance (p>.5). Moreover, the total indirect effects of age through Digits Forward failed to reach significance for any of the AVLT indices.
The second hypothesis of Aim 3 concerned moderating effects of education on the indirect (mediated) effect of age on verbal episodic memory. Results failed to support this prediction: although Education affected attention span/working memory (b12) and verbal episodic memory indices (b22) directly, the moderating effects of Education were restricted to the conditional effect of Age on Digit Span Forward (b13).
Is the Relation between Age, Attention Span/Working Memory, and Verbal Episodic Memory Moderated by Clinical Group?
The fourth research aim of the current study was assessed on data from the group of a-MCI patients (N=70) and the control group of 139 neurologically intact controls, selected from the community cohort (Older Adult Community group). With this set of analyses we sought to determine if the mediation effects described in the previous section varied in the presence of clinical deficits in episodic memory capacity. The general model tested was similar in structure to the model illustrated in Figure 1, by substituting clinical group (controls, a-MCI) as the moderator variable (W), and including an additional moderation path from Group to the direct effect of Age on AVLT (b25). The conceptual model for these analyses is graphically illustrated in Figure 2 and implemented using Model 59 in Process (Hayes, Reference Hayes2013). The mediator Mj (digits forward or digits reverse score, analyzed separately) was estimated using the equation:
whereas the following equation was used to estimate the outcome variable:
As shown in Table 5, residual direct effects of age on each of the five AVLT indices (b21), after accounting for indirect and moderated effects of Group (paths b11, b23, b24, b22, b25), remained significant in all cases. In this respect, results in the combined elderly sample (patients and controls; N=209) were identical to those reported previously for the entire community sample. Moreover, the hypothesis of mediation of the age-related episodic memory decline by working memory capacity was supported, at least for free recall measures, by the following findings: First, each set of indirect paths linking Age and AVLT indices (b11, b23) was significant (p<.001; with the exception of Retention). Second, the total indirect effects of age on free recall AVLT indices (Trials 1–5/immediate recall, Trial 6/short delay recall and Trial 7/long delay recall) through Digits Reverse were significant according to the normal theory-based test (p<.0005) and associated with 95% CIs that did not include 0. As in the analyses on the entire community sample, the direct paths from Digit Span Forward to each of the AVLT indices (b23) and the total indirect effects of Age on AVLT indices through Digits Forward failed to reach significance (p>.05).

Fig. 2 Schematic illustration of the proposed moderated (by clinical group) mediation of the association between age and delayed AVLT recall/recognition indices. *AVLT Trials 1–5, Trial 6, Trial 7, Retention, and Recognition pA were used as complementary indices of verbal episodic memory capacity.
Table 5 Unstandardized regression coefficients for moderated (by clinical group) mediation of age on AVLT scores through Digit Span scores

Note. Trials 1–5: Total number of words correctly recalled across Trials 1–5 (immediate recall); Trial 6: Short Delay Recall (number of words correctly recalled from AVLT List A immediately after recall of interference List B); Trial 7: Long Delay Recall (number of words correctly recalled from AVLT List A after 30-min delay); Retention: proportion of words from AVLT list A recalled on Trial 5 that were also recalled on Trial 7.
* p<.01.
†p<.001.
With respect to Clinical Group there were three notable findings: First, all direct effects of Group on AVLT indices (b22) were significant. These effects reflected the expected reduced performance of a-MCI patients compared to the Older Adult Community group on AVLT, in the absence of significant effects of Group on either Digits Forward or Digits Reverse (path b12). Descriptive data on Digit Span and AVLT indices, separately for each group, are presented in Table 6.
Table 6 Mean (SD) for the clinical (n=70) and Older Adult Community groups (n=139) on AVLT and Digit Span scores

Note. Group main effect, †p<.0001. Trials 1–5: Total number of words correctly recalled across Trials 1–5 (immediate recall); Trial 6: Short Delay Recall (number of words correctly recalled from AVLT List A immediately after recall of interference List B); Trial 7: Long Delay Recall (number of words correctly recalled from AVLT List A after 30-min delay); Retention: proportion of words from AVLT list A recalled on Trial 5 that were also recalled on Trial 7. Unstandardized regression coefficients are shown.
Second, there was significant moderation of the direct effect of Age on three AVLT indices (Trials 1–5, Retention and Recognition pA) by Group (paths b25). The direction of the effect suggested stronger age effects among a-MCI patients than Controls. Given the surprisingly weak evidence of a moderating effect of education on the rate of age-related decline in verbal episodic memory indices in the entire community sample (which was restricted to total immediate recall), we examined the hypothesis that education may, instead, affect the moderating effects of clinical Group on the direct effect of age on AVLT scores. The model included two two-way interaction terms and the three-way interaction and was implemented using Model 3 in Process (Hayes, Reference Hayes2013). Results revealed a significant three-way interaction, b=-.122, SE=.041, p=.003, in the absence of significant two-way interactions (p>.1). As shown in Figure 3 there was a tendency for steeper slopes among participants with lower education (averaging 6 years), followed by slopes for persons with an average of 10 years of education, and even shallower slopes for the more highly educated participants (with an average of 14 years of formal education). These slopes were significant (p<.001) for both groups with the exception of the rate of age-related decline for the most highly educated controls (p=.09).

Fig. 3 Moderation of the rate of age-related decline in verbal episodic memory capacity (Total number of words recalled on Trials 1–5) by Clinical Group and Education level. All age-related slopes were significant (p<.001) with the exception of the age-related slope for Controls with the highest educational attainment (p>.09; dashed line, upper panel).
Third, the magnitude of the association between Digits Reverse and Recognition pA was moderated by Group (path b24). The interaction of Digits Reverse Score and Group on either immediate (Trials 1–5), short (Trial 6)- or long delay (Trial 7) free recall and, similarly, between Digits Forward and any of the four AVLT indices, did not reach significance (p>.05).
Follow up mediated regression analyses were further performed to probe into the significant moderating effects of Clinical Group. In these analyses, conducted separately for each Clinical Group, the mediator Mj (Digits Reverse score) was estimated using the following equation:
Equation 6 describes the model used to estimate the outcome variable (Y: individual scores on Recognition pA):
The conceptual model for these analyses is graphically illustrated in Figure 4 and implemented using Model 4 in Process (Hayes, Reference Hayes2013).

Fig. 4 Schematic illustration of the proposed mediated association between age and verbal recognition capacity through working memory capacity (Digits Reverse score) in separate analyses for each group (Older Adult Community group and a-MCI patients).
Results presented in Table 7 further support our initial impression that the direct effects of age on each of the two delayed measures of verbal episodic memory (Retention and Recognition pA; paths b21) were stronger in the a-MCI as compared to the Older Adult Community Group (unstandardized regression coefficients, indicating effect size, were -.02/-.13 and -.003/-.05, respectively). In addition, the strength of the association between Digits Reverse score and Recognition pA (path b22) was notably higher among a-MCI patients than in the Older Adult Community group (unstandardized regression coefficients indicating effect size were .27 and .07, respectively). This effect is illustrated graphically by the steeper slopes for the MCI group in Figure 5, suggesting that a one-point decrease in Digits Reverse is expected to result in as much as .27 points drop in Recognition pA in a-MCI patients and only .07 points reduction in Recognition pA among non-memory impaired older adults.

Fig. 5 Moderation of the association between working memory capacity (Digits Reverse score) and AVLT delayed recognition (Recognition pA index) by Clinical Group (Older Adult Community group: solid lines/circles; a-MCI patients: dashed lines/stars).
Table 7 Unstandardized regression coefficients for mediation effects of age on AVLT scores through Digit Span scores separately for the Older Adult Community group (N=139) and MCI patients (N=70)

* p<.01.
† p=.001.
Furthermore, in support of our hypothesis, the indirect effect of age on both AVLT indices through Digits Reverse, although significant for both groups (based on both the normal theory test [p<.01] and bootstrapped CIs), was substantially larger among a-MCI patients than in the Older Community Group. Unstandardized indirect effect estimates shown in Table 7, suggest that Recognition pA is expected to show a yearly reduction of .0243 points solely due to verbal working memory deterioration in a-MCI patients. By comparison this estimate was as low as .0077 points among memory non-impaired older adults. Importantly these group differences were present despite comparable direct effects of age on Digits Reverse scores, suggesting a yearly reduction of 11 points in the Older Adult Community Group and .09 points in the a-MCI group. The total indirect effects through Digits Forward failed to reach significance (p>.05).
DISCUSSION
The present study demonstrated that the effect of age on the capacity to maintain verbal information in long-term storage was mediated by working memory capacity as well as by accumulated age-related decline in attention span. Moreover, the presence of clinically significant memory difficulties (amnestic MCI) appeared to amplify the mediating role of working memory capacity among elderly participants. Educational attainment predicted higher scores on all cognitive measures although years of education affected the rate of age-related decline in immediate, and not in delayed, verbal episodic recall measures. Furthermore, education moderated the impact of clinical group on immediate word-list recall ability. These results are discussed in turn below.
Age, Education, Gender, and Verbal Episodic Memory
In agreement with numerous previous reports (e.g., Geffen, Moar, O’Hanlon, Clark, & Geffen, Reference Geffen, Moar, O’Hanlon, Clark and Geffen1990; Giogkaraki, Michaelides, Constantinidou, Reference Giogkaraki, Michaelides and Constantinidou2013; Rönnlund et al., Reference Rönnlund, Nyberg, Bäckman and Nilsson2005; Salthouse, Reference Salthouse2009; Vakil, Weise, & Enbar, Reference Vakil, Weise and Enbar1997), we documented a significant decline of verbal episodic memory capacity on all immediate/delayed word-list recall and recognition indices evaluated. More importantly, results suggested that the rate of decline in verbal episodic memory capacity is not static, but increases with advancing age through the 8th decade of life.
As predicted, educational attainment, as indexed by years of formal education, was positively related to immediate/delayed recall and recognition. Additionally, women performed better on the majority of AVLT indices than men across age groups, in agreement with previous studies (Vakil & Blachstein, Reference Vakil and Blachstein1997; Geffen et al., Reference Geffen, Moar, O’Hanlon, Clark and Geffen1990). However, while education and gender exerted independent effects on most AVLT indices, the moderated regression analyses provided only weak support to the hypotheses that the effects of age on memory varied with participants’ gender or education. Therefore, while women and those with higher education performed better on AVLT, the rate of age-related decline was similar across education groups and across genders for indices of delayed episodic memory. We did find, however, some evidence of reduced age-related decline rate in immediate verbal memory (AVLΤ Trials 1–5) among persons with higher formal education over the entire age-range covered in the present study (17–86 years). The role of education proved to be more complex, however, as described further below.
Does Education Moderate the Age-Verbal Episodic Memory Association Indirectly?
A further objective of the study was to examine whether education moderates the effects of age on verbal episodic memory performance indirectly, through attention span and/or working memory. To test this hypothesis, we first had to establish that age-related reduction in AVLT performance was mediated by performance on digits span forward and/or digits span reverse tasks. Mediated regression analyses supported this claim by revealing indirect effects of age on both immediate and delayed free recall performance (Trials 1–5, Trial 6, and Trial 7) through working memory capacity. This finding held in the analyses performed on the older adult section of the present sample and agrees with some previous reports (Hertzog, Dixon, Hultsch, & MacDonald, Reference Hertzog, Dixon, Hultsch and MacDonald2003).
Results pertaining to the moderating role of education level were again mixed: Consistent with the results of the moderated regression analyses reported in the previous section, education level did not affect the magnitude of either the direct or indirect paths linking age to verbal episodic memory through working memory. There was, however, evidence of accelerated age-related decline in attention span among persons with lower educational attainment. Thus, although education level may correlate with single-time estimates of working memory and episodic memory, higher educational attainment did not appear to exert a “protective” influence on the rate of decline of verbal episodic memory abilities which is mediated by attention span and/or working memory—at least when cognitively intact participants spanning a wide age range were considered (17–86 years).
Effects of Age on Delayed Verbal Episodic Memory Are Enhanced in a-MCI
Clinical Group was a significant moderator of the direct effect of Age on both immediate (Trials 1–5) and delayed episodic memory indices (Retention and Recognition), suggesting steeper decline of episodic memory capacity with age among a-MCI patients than among education-level-matched elderly participants who did not present with any objective cognitive difficulties. This finding is consistent with reports of accelerated rate of atrophy in mesial temporal gray matter in a-MCI (Small, Schobel, Buxton, Witter, & Barnes, Reference Small, Schobel, Buxton, Witter and Barnes2011) and the notion that reduced neural resources represent the key limiting factor in the performance of episodic memory tasks (Schneider-Garces et al., Reference Schneider-Garces, Gordon, Brumback-Peltz, Shin, Lee, Sutton and Fabiani2009).
Cognitive reserve (Stern, Reference Stern2009), as indexed by education level, was found to exert a further moderating role on the rate of episodic memory decline among older adults in agreement with numerous reports using similar memory tasks (e.g., Hall et al., Reference Hall, Derby, LeValley, Katz, Verghese and Lipton2007). In the a-MCI group, age-related decline remained significant across education-level groups although slopes were higher among patients with lower education. A similar trend was found in the Older Adult Community Control group, although the rate of age-related decline failed to reach significance among participants with the highest educational attainment. Given that the moderating effect of education was restricted to measures of immediate episodic memory, suggesting an effect on initial learning capacity rather than long-term maintenance of verbal episodic traces, our findings provide only partial support to the cognitive reserve hypothesis.
Arguably, education may be considered as an imperfect proxy for cognitive reserve given that the length of formal schooling depends on historic and social factors (especially for generations who experienced global catastrophic events such as WW-II, the ensuing civil war in Greece, and the effects of British colonization in Cyprus). Future studies using more comprehensive estimates of cognitive reserve may prove more sensitive to flush out such complex relationships. Such measures include reading ability as a measure of educational quality (Johnson, Flicker, & Lichtenberg, 2006), vocabulary knowledge (Giogkaraki et al., Reference Giogkaraki, Michaelides and Constantinidou2013) as a measure of index of accumulated semantic knowledge, and even more direct indices of age-related brain degeneration (Reed et al., Reference Reed, Mungas, Tomaszewski Farias, Harvey, Beckett, Widaman and DeCarli2010).
Clinical Pathology Moderates Working Memory-Episodic Verbal Memory Associations
Although medial temporal atrophy is the most outstanding anatomic feature of a-MCI, recent MRI morphometry studies have shown that atrophy also exists in other brain regions, such as in the lateral and anterior parts of the temporal cortex and dorsolateral prefrontal cortex and underlying white matter (Chang et al., Reference Chang, Jacobson, Fennema-Notestine, Hagler, Jennings, Dale and McEvoy2010; Hamalainen et al., Reference Hamalainen, Tervo, Grau-Olivares, Niskanen, Pennanen, Huuskonen and Soininen2007; Huang, Friedland, & Auchus, Reference Huang, Friedland and Auchus2007). Furthermore, the degree of prefrontal gray matter atrophy has been linked to reduced working memory performance among a-MCI patients (Zheng et al., Reference Zheng, Sun, Dong, Liu, Xu, Chen and Wang2014). Such changes could account for reduced performance in various executive functions (including working memory) even when their severity does not warrant diagnosis of amnestic, multiple-domain MCI (Economou et al., Reference Economou, Papageorgiou, Karageorgiou and Vassilopoulos2007; Zheng et al., Reference Zheng, Dong, Sun, Xu, Ma and Wang2012), as with the patients included in the a-MCI group in the current study. Despite, however, the absence of clinically significant deficits in working memory, we did find significant moderation of the association between working memory and AVLT delayed recognition performance by clinical Group. Pending replication by future studies, this finding suggests that a-MCI patients relied more heavily on working memory capacity to form resistant episodic traces of newly encoded verbal material than age- and education-matched controls. This tentative conclusion is in line with the compensation hypothesis of age-related cognitive changes (Park & Reuter-Lorenz, Reference Park and Reuter-Lorenz2009) and predicts that sub-clinical working memory difficulties may serve as independent predictors of disease progression (Belleville, Gauthier, Lepage, Kergoat, & Gilbert, Reference Belleville, Gauthier, Lepage, Kergoat and Gilbert2014; DeCarli et al., Reference DeCarli, Mungas, Harvey, Reed, Weiner, Chui and Jagust2004; Summers & Saunders, Reference Summers and Saunders2012).
CONCLUSIONS, LIMITATIONS, AND FUTURE DIRECTIONS
The current data support the notion that age-related decline in supraspan word-list recall and recognition indices continues across the lifespan. The decline is mediated by age-related decline in verbal working memory, and varies in degree with educational history and clinical diagnosis of a-MCI. Conversely, the ability to encode and briefly maintain verbal information in consciousness without further processing (attention span) did not emerge as a significant limiting factor for verbal episodic memory capacity. Further studies are needed to identify which of the several component processes involved in performing short-term memory tasks such as Digits Reverse are more susceptible to aging and significantly contribute to episodic verbal memory deficits in a-MCI—notably a condition not typically accompanied by significant impairments in non-episodic memory functions. Results based on the limited set of clinical measures used in the present study are consistent with the cognitive load theory favoring a key role of updating and monitoring of working memory representations for long term memory (Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000; Sweller, Merrienboer, & Paas, Reference Sweller, Van Merrienboer and Paas1988) over and beyond considerations of working memory performance as mere measures of processing capacity restrictions. Processing speed (Facal, Juncos-Rabadán, Pereiro, & Lojo-Seoane, Reference Facal, Juncos-Rabadán, Pereiro and Lojo-Seoane2014) and sustained attention (Klekociuk & Summers, Reference Klekociuk and Summers2014), which were not measured independently in the current study, have all been implicated as further factors accounting for the significant links between WM and long-term memory measures in patients with a-MCI. The potential role of these and other related functions await further experimental investigation (see for instance Altmeyer, Schweizer, Reiss, Ren, & Schreiner, Reference Altmeyer, Schweizer, Reiss, Ren and Schreiner2013), which is far from the limited clinical scope of the present study. Despite the aforementioned limitation, our findings support the view that cognitive enhancement programs targeting working memory could facilitate verbal episodic memory performance.
While adults at risk for dementia (a-MCI) demonstrate higher rates of episodic memory decline across time than age-matched neurologically intact controls, they are also expected to decline more rapidly if they also display relatively poor verbal working memory capacity. Therefore, profiles of attention span, working memory, and verbal episodic memory should be taken into consideration during clinical assessment and in making prognostic decisions regarding the expected pattern of cognitive decline in patients with a-MCI as suggested by many groups (Belleville et al., Reference Belleville, Gauthier, Lepage, Kergoat and Gilbert2014; DeCarli et al., Reference DeCarli, Mungas, Harvey, Reed, Weiner, Chui and Jagust2004; Summers & Saunders, Reference Summers and Saunders2012).
The present study focused on two demographic variables: education and gender. However, continued cognitive engagement during adulthood may influence performance on memory measures. Therefore, future research may want to incorporate additional variables such as lifetime achievement and leisure activities in the model of aging. The use of slope analysis across ages is an important contribution to the literature as compared to the more conventional approach of between group mean comparisons. The present study incorporated a continuum of ages to determine the moderation/mediation effects of important variables such as age, education, and presence of pathology. While the cross-sectional approach used in this study was practical, a longitudinal design would have been the optimal study design. As our data base continues to grow and as we continue to re-test participants at regular intervals, future research will be able to test the stability of the proposed moderation/mediation approach in the study of cognitive aging.
ACKNOWLEDGMENTS
This work was supported by the Cyprus Research Promotion Foundation though a grant co-funded by the Cyprus Government and the European Regional Development Fund (F.C., ΑΝΘΡΩΠΙΣΤΙΚΕΣ/ΚΟΙΝΩ/0308(ΒΕ)/07 and ΝΕΑΥΠΟΔΟΜΗ/ΣΤΡΑΤΗ/0309/37), and the “IRAKLITOS II - University of Crete” of the Operational Programme for Education and Lifelong Learning 2007-2013 (D.K). The authors would like to thank the study volunteers who participated in theproject and the researchers who contributed in the data collection process.














