INTRODUCTION
The genus Leishmania (Kinetoplastida: Trypanosomatidae) includes species that are causative agents of leishmaniases, which are widely spread anthropozoonotic diseases in tropical and subtropical regions. In Brazil, Leishmania (Viannia) braziliensis is the main species involved in the cutaneous and mucocutaneous forms of the disease (Reithinger et al. Reference Reithinger, Dujardin, Louzir, Pirmez, Alexander and Brooker2007). In the southeast and northeast regions of Brazil, this species is mainly transmitted by the sand flies Lutzomyia (Nyssomyia) intermedia and Lutzomyia (Nyssomyia) whitmani (Rangel and Lainson, Reference Rangel, Lainson, Rangel and Lainson2003).
During its life cycle, Leishmania parasites alternate between promastigote and amastigote forms within the invertebrate and vertebrate hosts, respectively. The former forms survive in the sand fly digestive tract lumen, whereas the latter are found in the parasitophorous vacuoles of macrophages (Bates and Rogers, Reference Bates and Rogers2004). In the case of L. (V.) braziliensis, a peripylarian parasite, its development begins in the hindgut of the insect followed by migration to the anterior midgut and mouth parts (Lainson and Shaw, Reference Lainson, Shaw, Peters and Killick-Kendrick1987).
The cell surfaces of Leishmania spp. present molecules that are related to specific activities during the life cycle of the parasite and contribute to survival in harsh surroundings (Descoteaux and Turco, Reference Descoteaux and Turco1999). These surface molecules not only afford protection to the parasite within the insect vectors and vertebrate hosts but also provide specificity for their interactions with cells in the sand fly gut and with mononuclear phagocytic cells of mammals. The most intensely studied molecules located on the surfaces of promastigotes and amastigotes are the glycoconjugate lipophosphoglycan (LPG) and the metallo-proteinases (Moody, Reference Moody1993). Leishmania spp. also produce a number of less-abundant surface molecules: glycosylinositol phospholipids (McConville et al. Reference McConville, Homans, Thomas-Oates, Dell and Bacic1990), a promastigote surface antigen-2 complex of glycoproteins (Murray et al. Reference Murray, Spithill and Handman1989; Symons et al. Reference Symons, Murray, Ji, Simpson, Osborn and Cappai1994), a glycoprotein of approximately 46 kDa (Kahl and McMahon-Pratt, Reference Kahl and McMahon-Pratt1987) and 2 cysteine-proteinases of 63 and 43 kDa (Rebello et al. Reference Rebello, Côrtes, Pereira, Pascarelli, Côrte-Real, Finkelstein, Pinho, d'Avila-Levy and Alves2009). These molecules share the common trait of attaching to the plasma membrane via glycosylphosphatidylinositol lipid anchors.
The activity of these metallo-proteinase surface components has been associated with the hydrolysis and inactivation of immunoglobulin G, the inactivation of factor C3b of the complement C3bi in the mammalian host (Brittingham et al. Reference Brittingham, Morrison, McMaster, McGwire, Chang and Mosser1995), and the protection of promastigotes from trypsin and chymotrypsin in the gut of the insect vector (Pimenta et al. Reference Pimenta, Modi, Pereira, Shahabuddin and Sacks1997). Although LPGs have commonly been identified as having a life cycle-maintaining role, they can also act on the vertebrate host (i.e., influencing the innate and acquired immune responses and subverting the functions of the macrophage – Becker et al. Reference Becker, Salaiza, Aguirre, Delgado, Carrillo-Carrasco, Kobeh, Ruiz, Cervantes, Torres, Cabrera, González, Maldonado and Isibasi2003; Brandonisio et al. Reference Brandonisio, Spinelli and Pepe2004; Lodge and Descoteaux, Reference Lodge and Descoteaux2008; Soong, Reference Soong2008) and on the insect vector (i.e., protecting the parasite from the proteolytic activities of the blood-digesting midgut, participating in the attachment to the gut wall, inducing the production of chitinases that degrade the stomodeal valve of the sand fly and influencing the secretion of aneuropeptide that arrests midgut and hindgut peristalsis – Sacks and Kamhawi, Reference Sacks and Kamhawi2001; Kamhawi, Reference Kamhawi2006).
Metacyclic forms of L. (V.) braziliensis LPGs have also been reported to display differential attachment to the midgut of Lutomyia (Nyssomyia) whitmani and Lutzomyia (Nyssomyia) intermedia sand flies (Soares et al. Reference Soares, Margonari, Secundino, Macedo, da Costa, Rangel, Pimenta and Turco2010). This observation has been related to an upregulation of β-glucose residues in the LPG repeated units during metacyclogenesis of this parasite (Soares et al. Reference Soares, Cardoso, Barron, Araújo, Pimenta and Turco2005).
There is evidence that glycosaminoglycans, such as heparin, influence the development of L. (L.) major in the gut of the vector, increasing the parasitic load in experimentally infected insects (Volf et al. Reference Volf, Svobodova and Dvorakova2001). It has also been reported that specific receptors on the surface of Leishmania spp. are able to bind glycosaminoglycans with structures similar to heparin present in host tissues. Such receptors are known as heparin-binding proteins (HBPs), and these molecules can influence the parasite-host interactions during infection.
Experiments performed using promastigotes of Leishmania (Leishmania) donovani, for example, have shown that HBPs can be found on the surface of the parasite and are related to the inhibition of protein kinase C (Mukhopadhyay et al. Reference Mukhopadhyay, Shome, Saha, Hassell and Glew1989). HBPs have been associated with the infective forms of L. (L.) donovani, as they predominate on the surface of stationary-phase promastigotes from recently isolated samples. However, subsequent passages of parasites in culture have led to the loss of the heparin-binding abilities of the parasites (Butcher et al. Reference Butcher, Sklar, Seamer and Glew1992; Kock et al. Reference Kock, Gabius, Schmitz and Schotteliu1997).
HBPs have also been described in other Leishmania species, specifically Leishmania (Leishmania) amazonensis and Leishmania (Leishmania) major; however, contrary to what has been observed for L. (L.) donovani, in these species those proteins appear to be predominant in amastigotes (Love et al. Reference Love, Esko and Mosser1993).
In addition, HBPs can influence the parasitic attachment to the gut of the insect vector because hydrophobic HBPs from L. (V.) braziliensis have been shown to possess the physicochemical potential to bind to L. (N.) whitmani and L. (N.) intermedia gut cells (Azevedo-Pereira et al. Reference Azevedo-Pereira, Pereira, Oliveira, Brazil, Côrtes, Madeira, Santos, Toma and Alves2007).
Due to the lack of information on HBPs in L. (V.) braziliensis, we present new data regarding the subcellular distribution, proteolytic activity and binding kinetics of HBPs from this parasite.
MATERIALS AND METHODS
Chemicals
Detergents {Tween 20, Triton X-100 (TX-100), and 3-[(3-cholamidopropyl)-dimethylammonium]-1-propanesulfonate (CHAPS)}, heparin sodium salt, biotinylated heparin, gelatin, bovine serum albumin (BSA), penicillin, proteinase inhibitors [trans-epoxysuccinyl-L-leucylamido-(4-guanidino)butane (E-64), phenylmethanesulfonyl fluoride (PMSF), 1,10-phenanthroline (o-phe), pepstatin A (Pep A), and p-phenylenediamine], reducing agents [dithiothreitol (DTT) and β-mercaptoethanol (β-ME)] were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, USA). A heparin-Sepharose column (HiTrap-Heparin; 1·5×2·5 cm) was purchased from GE Healthcare. Fetal calf serum (FCS) was purchased from Cultilab S/A (Brazil). Brain heart infusion (BHI) medium was purchased from Difco (Detroit, USA). Epon resin was purchased from Hexion Specialty Chemicals, Inc. (US). The electrophoresis reagents were purchased from Bio-Rad Laboratories Inc. (US). Pre-Stained™ Plus Protein Ladder was purchased from Fermentas Life Sciences (US). Amicon Centriprep YM-10 filter devices were purchased from Millipore (Billerica Inc, MA, USA). Chemiluminescence luminol reagent-ECL kit was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). All other reagents were of analytical grade or better.
Parasite strain and culture
Infective promastigotes of L. (V.) braziliensis (strain MCAN/BR/1998/619) were maintained at 28°C as a stock culture in Novy, MacNeal and Nicolle medium and subcultured every 4 days. Promastigote cultures were grown in Brain Heart infusion medium supplemented with 10% heat-inactivated FCS until a density of 1×108 cells/ml was obtained.
Subcellular fractionation
Subcellular fractions enriched for flagella or surface membranes were obtained by centrifugal fractionation as previously described (Morgado-Diaz et al. Reference Morgado-Diaz, Silva-Lopez, Alves, Soares, Corte-Real and Giovanni-De-Simoni2005). Briefly, after 4 days of culturing, promastigotes were washed twice (3800 g, 10 min, 4°C) in phosphate-buffered saline, pH 7·2, (PBS). The remaining pellet was then washed twice with 10 mm Tris-HCl (pH 7·2) buffer containing 1 m NaCl, 0·2 m K2HPO4 and 0·5 m MgCl2. The pellet was then resuspended in 10 mm Tris-HCl, pH 7·5, containing 0·05 m sucrose (S buffer; 10 ml/g of cells) and disrupted in 1% CHAPS with 40–80 strokes of a Dounce-type homogenizer. Isotonic conditions were restored by adding sucrose to reach a final concentration of 0·25 m. Cell lysates were centrifuged (10 min, 4300 g, 4°C), and the supernatant was collected and centrifuged (15 min, 12 000 g, 4°C). The pellet from the final centrifugation constituted the flagellar fraction (Ff), whereas the supernatant was centrifuged again (45 min, 35 000 g, 4°C) to obtain the pellet that comprised the membrane fraction (Mf).
Electron microscopy
The ultrastructural composition of subcellular fractions was determined as previously described (Morgado-Diaz et al. Reference Morgado-Diaz, Silva-Lopez, Alves, Soares, Corte-Real and Giovanni-De-Simoni2005). Briefly, subcellular fractions were fixed with 2·5% glutaraldehyde in a 0·1 m sodium cacodylate buffer, pH 7·2, for 1 h at 4 °C and post-fixed with a 1% osmium tetroxide (OsO4) solution for 1 h at 4°C. Samples were dehydrated in a graded series of acetone and embedded in PolyBed 812 resin. Ultrathin sections were stained with uranyl acetate and lead citrate and observed using a JEOL-1011 (JEOL UK – Welwyn Garden City, Hertfordshire, UK) transmission electron microscope.
Affinity chromatography
The soluble proteins from Ff and Mf were dialysed against an equilibrium buffer (10 mm sodium phosphate, pH 7·0, and then passed through a HiTrap-Heparin column previously equilibrated in the same buffer. The column was washed with the equilibrium buffer adjusted with 0·5 m NaCl, and the bound proteins were eluted using the equilibrium buffer adjusted with 2·0 m NaCl at a flow rate of 2 ml/min. The eluted fractions were concentrated using a Centriprep filter device, and the protein concentration was determined as previously described (Lowry et al. Reference Lowry, Rosebrough, Farr and Randall1951).
Electrophoresis and Western blot analysis
Soluble proteins (∼10 μg) were resolved using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Laemmli, 1970) and silver staining, as previously described. Electrophoresis was performed at 25°C in a Mini Protean II system (Bio-Rad Laboratories, USA). For Western blot assays, proteins were resolved using SDS-PAGE and then transferred onto 0·22 μm nitrocellulose membranes, as previously described (Azevedo-Pereira et al. Reference Azevedo-Pereira, Pereira, Oliveira, Brazil, Côrtes, Madeira, Santos, Toma and Alves2007). Non-specific binding sites were blocked (4°C for 16 h) using a solution of 5% skimmed milk (w/v) in PBS containing 0·5% Tween 20. The blots were washed 3 times with PBS containing 0·05% Tween 20 (PBST) and incubated (37°C, 1 h) with biotinylated heparin diluted 1:500 in PBST. After 6 washes with PBST, the blots were incubated (37°C, 1 h) with 1:1000 streptavidin conjugated to horseradish peroxidase in PBST. After 6 washes with PBST, the complex was revealed by chemiluminescence.
Zymographic assays
Protease activities of the HBPs (in samples of 5 μg) were determined using a gelatin substrate (0·1%) in 12% polyacrylamide gels (Heussen and Dowdle, Reference Heussen and Dowdle1980; Alves et al. Reference Alves, Marzochi and Giovanni-de-Simone1993). Electrophoresis under denaturing conditions (using SDS) was performed at 4°C in a Mini Protean II system and, subsequently, the gels were washed with 2·5% Triton X-100 under agitation (60 min, 25°C) and incubated (16 h, 37°C) in the appropriate buffer for each proteinase class (aspartic-proteinase: 10 mm sodium acetate buffer, pH 3·0; cysteine-proteinase: 10 mm sodium acetate buffer, pH 5·5, containing 1 mm DTT; serine-proteinase: 10 mm Tris-HCl, pH 7·5; metallo-proteinase: 10 mm Tris-HCl, pH 8·0). Incubations were performed in the presence or absence of inhibitors for each proteinase class (aspartic-proteinase: 1·0 μ m Pep A; cysteine-proteinase: 10·0 μ m E-64; serine-proteinase: 1·0 mm PMSF; and metallo-proteinase: 1·0 mm o-phe). The hydrolysis of gelatin was detected by staining the gels with 0·1% (w/v) Coomassie blue prepared in a methanol:acetic acid:water (3:1:6, v/v/v) solution.
Surface plasmon resonance (SPR) assays
SPR assays were performed using sensor chips that presented a carboxyl surface (COOH) coated with immobilized neutravidin (Biocap; Nomadics, USA). The sensor chip surface was further covered with biotinylated heparin (0·5 μg) and used to detect the presence of HBPs in each fraction (2 μg), BSA (1·0–0·01 μg) or in whole promatigotes (1·4×105 cells). Alternatively, promastigotes were pre-incubated (2 h, 4°C) with different concentrations of heparin (1·0 μg/ml, 0·1 μg/ml and 0·01 μg/ml) in PBS to assess specific binding inhibition. Prior to interaction with the sensor chip surfaces the promastigotes were fixed (1 h, 4°C) with 1% paraformaldehyde and washed 3 times by centrifugation (800 g, 10 min, 4°C) in PBS. SPR assays were performed at 25°C with 100 μl of material injected under a flow rate of 10 μl/min. The binding assays were performed in PBS and registered in real time using a sensorgram, where changes in the SPR angle (θspr) were measured as resonance units (RU). Such changes were proportional to the concentration of bound proteins or the number of cells attached to the sensor chip surfaces. Resonance signals of the samples were analysed after subtraction of the RU values from the reference channel. Association (Ka) and dissociation (Kd) constants were measured and used to calculate the affinity constant of the fraction samples (KD). SPR experiments were conducted in an optical biosensor SensiQ Pioneer (Icx Nomadics, USA), and the data were analysed using Qdat software (Icx Nomadics, USA).
RESULTS
Ultrastructural characterization of the subcellular fractions of promastigotes
Subcellular fractions were obtained from promastigotes in the early stationary growth phase. Differential centrifugation was used for the enrichment of flagella (Ff) and membrane (Mf) fractions (Fig. 1A and B, respectively). Ff was assessed by the presence of typical flagellum fragments and flagellar membranes, whereas Mf was assessed by the predominance of spherical membrane-bound vesicles, using transmission electron microscopy.

Fig. 1. Ultrastructural analysis of flagellar – Ff – (A) and membrane – Mf – (B) subcellular fractions of Leishmania (V.) braziliensis promastigotes obtained after differential centrifugation. The Ff was recognized by flagellum fragments (![]() ) and flagellar membrane (►), and the Mf by spherical membrane-bound vesicles (
) and flagellar membrane (►), and the Mf by spherical membrane-bound vesicles (![]() ). These images are representative of 3 independent experiments.
). These images are representative of 3 independent experiments.
Heparin-binding proteins from Ff and Mf fractions of promastigotes
We assessed the heparin-binding properties in proteins from both aforementioned isolated subcellular fractions. These assays yielded an estimative value of 0·04±0·001 mg of HBPs in Ff, corresponding to 13·3% of the total fraction of proteins (0·3±0·1 mg), whereas HBPs in M f yielded 0·05±0·001 mg of HBPs, corresponding to 8·3% of the total Mf proteins (0·6±0·02 mg).
The protein profiles of Ff and Mf (and their correspondent heparin-binding fractions) were analysed by SDS-PAGE. Western blot analysis with Ff and Mf indicated that these fractions present complex and distinct band profiles ranging from 65·0 to 17·0 kDa. Surprisingly, denaturant electrophoresis assays with samples from both subcellular fractions after elution from the heparin-affinity columns showed 2 main protein bands with relative molecular masses of approximately 65·0 and 55 kDa, as revealed by silver staining (Fig. 2).

Fig. 2. Electrophoresis assays of samples from Leishmania (V.) braziliensis. Samples from flagellar (Ff) and membrane (Mf) fractions collected prior to (1 and 4, respectively) or after heparin-Sepharose column purification (HBP Ff (3) and HBP Mf (6)) were applied into each slot, submitted to SDS-PAGE and revealed by silver staining. In addition, Western blotting using biotinylated heparin as marker and revelation by chemiluminescence was performed with Ff (2) and Mf (5) to detect HBPs. Molecular mass markers (in kDa) are indicated on the left. These results are representative of 5 independent experiments.
Proteinase properties of HBPs from Mf and Ff fractions
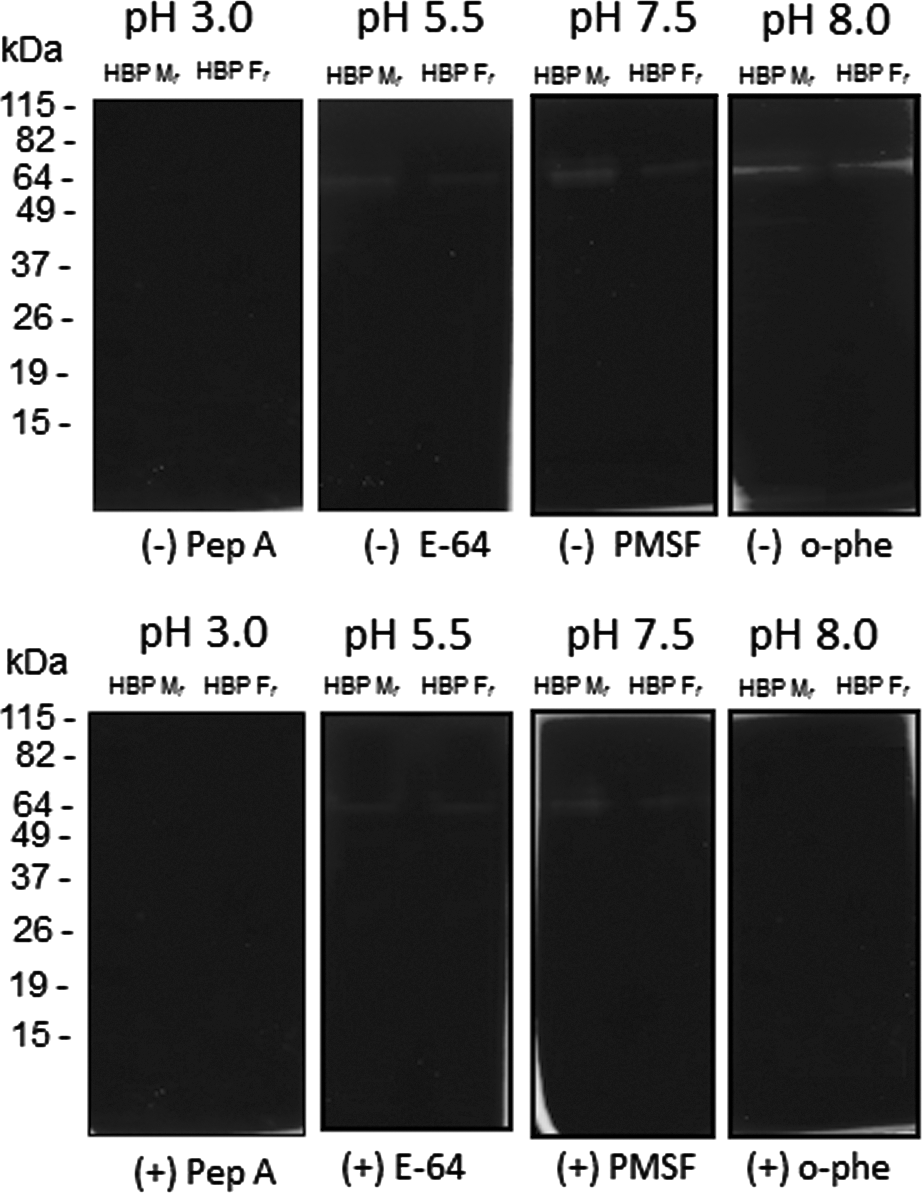
Our results indicate that the 65 kDa-protein band, from both HBP fractions, has a major gelatinolytic activity within a pH range between 5·5 and 8·0. A band with a minor gelatinolytic activity was also observed around 49 kDa in HBP Mf. The activity of these bands was sensitive to inhibition by 1,10-phenanthroline, and no inhibition was evident in gels incubated with other proteinase inhibitors, thus suggesting metallo-proteinase-like activity (Fig. 3).
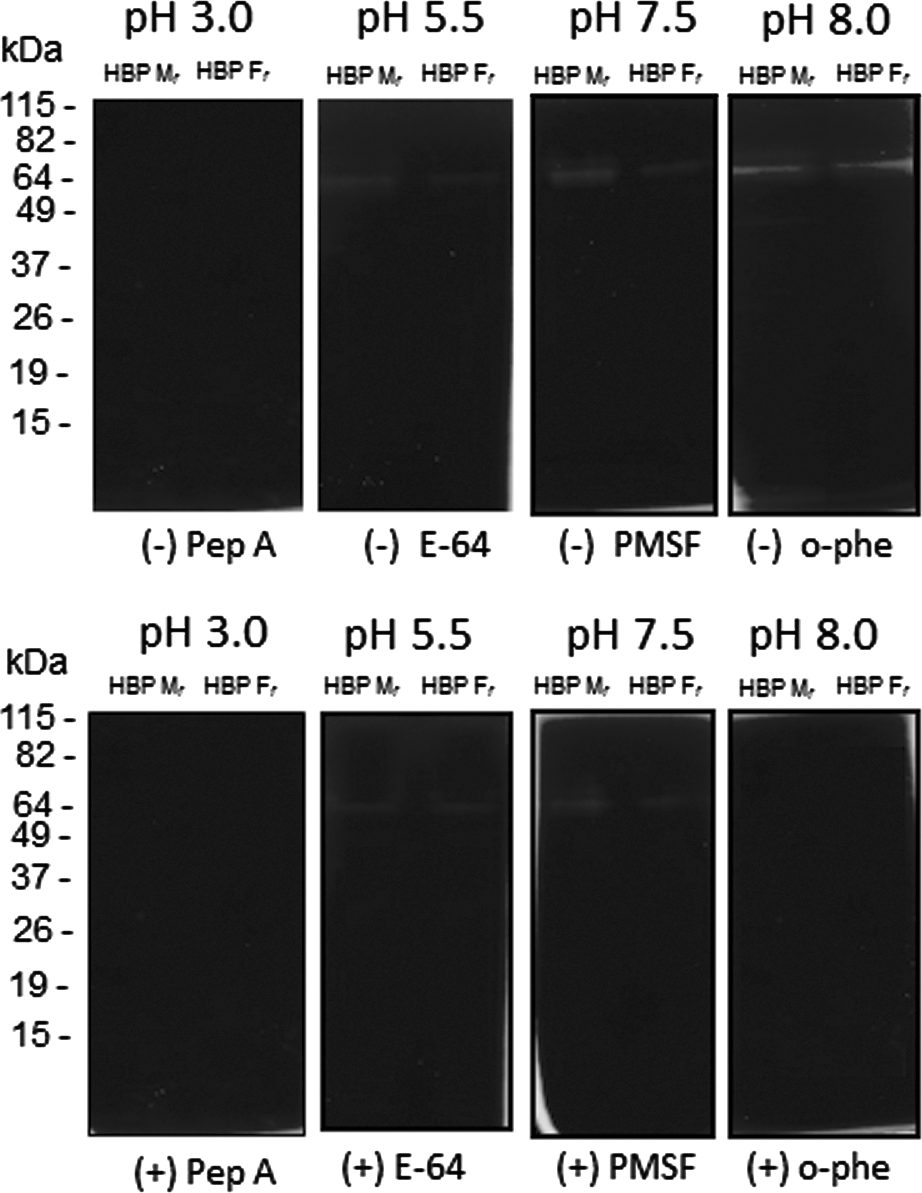
Fig. 3. Measurement of proteinase activity in Leishmania (V.) braziliensis HBPs by gelatin-SDS-PAGE. After electrophoresis of samples eluted from a heparin-Sepharose column (membrane proteins – HBP Mf; flagellum proteins – HBP Ff), gels were incubated with different buffers (pH 3·0, pH 5·5, pH 7·5 and pH 8·0) in the absence (−) or presence (+) of specific inhibitors for different classes of proteinases: Pep A, E-64, PMSF and o-phe. The gelatinolytic bands were detected by negative staining with a Coomassie blue solution. Molecular mass markers are indicated (kDa). These results are representative of 4 independent experiments.
Heparin-binding assays with Leishmania (V.) braziliensis proteins
SPR assays were designed to assess the presence of heparin ligands on L. (V.) braziliensis promastigote surfaces and to directly quantify these ligands in parasite subcellular fractions obtained by affinity chromatography. Kinetic constants obtained during the binding of biotinylated heparin onto a sensor chip surface characterized the specificity of this immobilization (Ka=2·4×105 M−1 s−1; Kd=0·079 s−1; KD=320 nm).
The presence of HBPs on the surface of promastigotes was confirmed by the sensorgram display of association and dissociation values of 33·0± 2·0 RU and 14±1·0 RU, respectively, following the injection of parasites onto the sensor chip surface. These values of association and dissociation are 1·6-fold higher than those observed after the injection of heparin (baseline), thus indicating binding of parasites to the immobilized heparin on the sensor chip surface (Fig. 4).

Fig. 4. Analysis of HBPs on the surfaces of Leishmania (V.) braziliensis promastigotes by surface plasmon resonance biosensing. Sensor chips were covered with biotinylated heparin, and promastigotes were passed onto the chip surface. The parasites were assayed without pre-incubation with heparin (A) or with pre-incubation with 0·01 μg (B), 0·1 μg (C) or 1·0 μg (D) of heparin. The data are presented as resonance units (RU) and are representative of 2 independent experiments.
The specificity of parasite binding to heparin was confirmed by additional assays in which the promastigotes were incubated with increasing concentrations of heparin prior to being injected on the sensor chip surface. In these assays, it was possible to detect a dose-dependent inhibition of promastigotes binding to immobilized heparin with decreasing RU values of 48%, 39%, and 33% inhibition when parasites were incubated with 1·0, 0·1 and 0·01 μg/ml of heparin, respectively, as shown in Fig. 4.
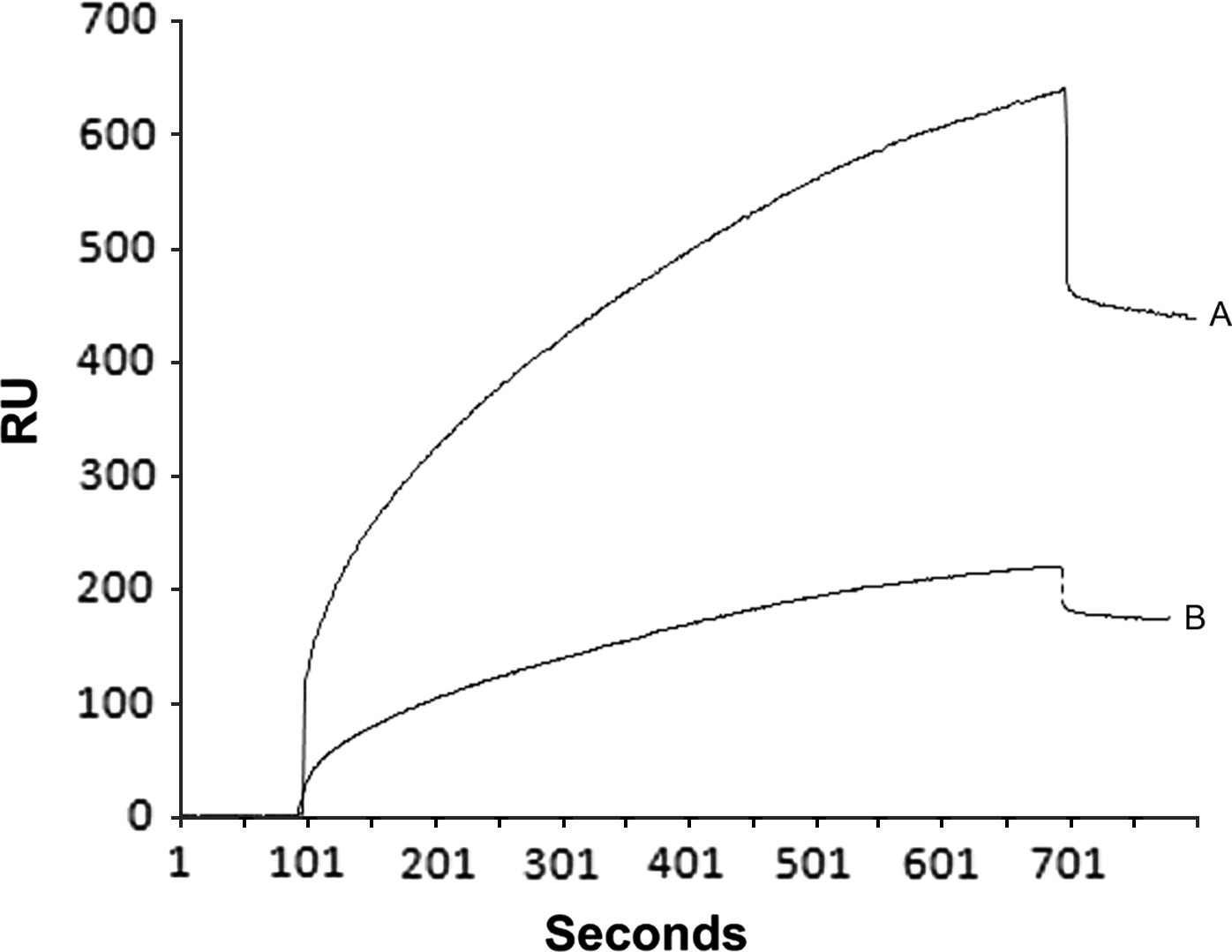
We also analysed the capacity of proteins from HBP Ff and HBP Mf to bind to heparin at neutral pH. The analysis of the dissociation RU values indicated that HBP Ff contains more HBPs (445±30 RU, equivalent to 0·4 ng/mm2 of proteins on the sensor chip surface) than HBP Mf (175±20 RU, equivalent to 0·1 ng/mm2). The RU values observed for the HBP Ff and Mf fractions were 53-fold and 20-fold higher, respectively, than the RU value observed after the immobilization of heparin (baseline). The binding of HBP Ff samples to immobilized heparin was also observed to be stronger than that of HBP Mf with lower dissociation rates (Fig. 5). As expected, the negative controls using BSA showed very low dissociation RU values at 3 different concentrations (1·0 μg=2·0±0·3 RU, 0·1 μg=1·2±0·1 RU and 0·01 μg=0·4±0·3 RU).
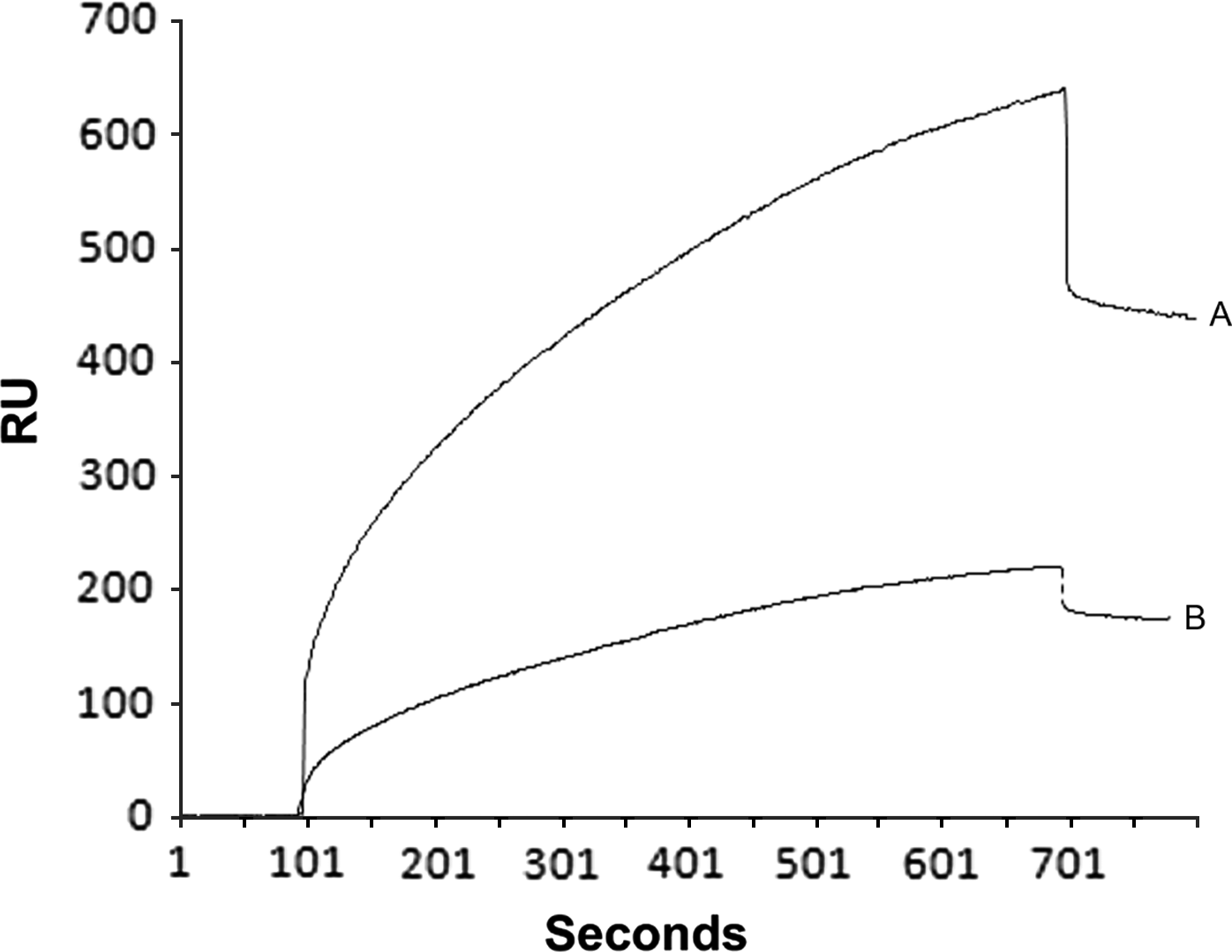
Fig. 5. Analysis of the interactions between heparin and HBP-enriched fractions from Leishmania (V.) braziliensis promastigotes by surface plasmon resonance biosensing. The interaction kinetics of HBP Ff (A) and HBP Mf (B) with biotinylated heparin immobilized on a sensor chip were assessed. The data are presented as resonance units (RU) and are representative of 2 independent experiments.
DISCUSSION
The surfaces of protozoan parasites, such as those belonging to the genus Leishmania, regulate interactions with the extracellular environment and are responsible for the uptake of nutrients and signalling pathways. In this context, we have previously proposed the potential of HBPs from L. (V) braziliensis promastigotes to mediate parasitic adhesion to proteins from the guts of Lutzomyia sand flies (Azevedo-Pereira et al. Reference Azevedo-Pereira, Pereira, Oliveira, Brazil, Côrtes, Madeira, Santos, Toma and Alves2007). Herein, we have confirmed that these proteins are components of surface membranes and flagella of L. (V) braziliensis promastigotes, reinforcing their potential roles in the parasite-vector interaction.
Cell fractionation paired with morphological and/or biochemical methodology has increasingly been used for parasite analyses (de Souza et al. Reference de Souza and da Cunha-e- Silva2003). In this study, this approach was applied to investigate HBPs from L. (V) braziliensis promastigotes to indicate that cellular membranes and flagella contain two main HBP bands (65 and 55 kDa) after elution from affinity chromatography, as previously described (Azevedo-Pereira et al. Reference Azevedo-Pereira, Pereira, Oliveira, Brazil, Côrtes, Madeira, Santos, Toma and Alves2007).
The complex structural organization of heparin receptors has been previously described in other organisms. The mollusk Anadara granosa presents an HBP that has a native molecular mass of 300 kDa and is composed of several identical 60 kDa subunits (Dam et al. Reference Dam, Bandyopadhyay, Sarkar, Ghosal, Bhattacharya and Choudhury1994). In Trypanosoma cruzi, a 60 kDa HBP was detected in trypomastigote membranes (Ortega-Barria and Pereira, Reference Ortega-Barria and Pereira1991), and these receptors appear to be involved in the interaction of this parasite with cardiomyocytes (Oliveira Jr et al. Reference Oliveira, Alves, Calvet, Toma, Bouças, Nader, Castro Côrtes, Krieger, Meirelles and Souza Pereira2008). Based on this evidence, it is possible that the heparin receptor detected in the present study also presents a complex structural organization corresponding to its functionality in L. (V) braziliensis promastigotes.
The cell surfaces of Leishmania parasites are the first sites of contact between these organisms and different microenvironments; thus, the regulation of surface macromolecule expression provides an important mechanism for adaptation during their life cycle. The surfaces of Leishmania spp. have an outer layer consisting of a latticework of carbohydrates covalently linked to lipids and proteins, known as the glycocalyx. This structure is rich in glycoconjugates with complex oligosaccharide structures that may be incorporated into the extracellular matrix or attached to secreted glycoproteins (Novozhilova and Bovin, Reference Novozhilova and Bovin2010). Many components of the glycocalyx play roles in the interactions between protozoa and host cells (Chava et al. Reference Chava, Bandyopadhyay, Chatterjee and Mandal2004; Naderer et al. Reference Naderer, Vince and McConville2004).
Glycosaminoglycans, such as heparin and heparan sulfate, are covalently attached to core proteins and have different cellular localizations in cells of animal species. Although heparin is found inside mastocytes (Nader et al. Reference Nader, Chavante, dos-Santos, Oliveira, de-Paiva, Jerônimo, Medeiros, de-Abreu, Leite, de-Sousa-Filho, Castro, Toma, Tersariol, Porcionatto and Dietrich1999; Strauss et al. Reference Strauss, Nader, Takahashi and Dietrich1982), heparan sulfate is found on the cell surface of vertebrates and invertebrates (Cassaro and Dietrich, Reference Cassaro and Dietrich1977; Dietrich et al. Reference Dietrich, Sampaio, Montes, OCA and Nader1980; Nader et al. Reference Nader, Ferreira, Paiva, Medeiros, Jeronimo, Paiva and Dietrich1984). It is important to note that heparan sulfate is the actual glycosaminoglycan that acts in the interaction between promastigotes and the sand fly gut epithelium; however, because heparin and heparan sulfate are both glycosaminoglycans that share a common chemical structure (Dreyfuss et al. Reference Dreyfuss, Regatieri, Jarrouge, Cavalheiro, Sampaio and Nader2009), the use of heparin in the binding assays of this study is an acceptable adaptation.
Our analyses show that HBPs from L. (V.) braziliensis promastigotes surface have biochemical properties of metallo-proteinases, which are well-known glycoproteins present on the surface and flagellar pockets of Leishmania spp. and other trypanosomatids (Yao, Reference Yao2010). In recent years, it has been shown that the molecular weights of metallo-proteinases of Leishmania spp. are not homogeneous, and enzymes of this class with molecular weights ranging from 50 kDa to values above 63 kDa have previously been detected (Alves et al. Reference Alves, Mendonca-Lima and Alves2004; Cuervo et al. Reference Cuervo, Saboia-Vahia, Costa Silva-Filho, Fernandes, Cupolillo and dE Jesus2006).
For the first time, the interaction of HBPs from L. (V.) braziliensis promastigotes with heparin was assessed in real time using SPR. The use of this technology is a new trend in identifying cell surface proteins using a biosensing system (Velasco-Garcia, Reference Velasco-Garcia2009) due to its flexible and powerful capacity for detecting biomolecular interactions (Tanious et al. Reference Tanious, Nguyen and Wilson2008). SPR analysis enabled the real-time assessment of the interaction between HBPs from the promastigote surface and immobilized heparin (without specific markers). This method was performed in a similar manner to a previously proposed technique for the detection of ligands in mammalian cells (Quinn et al. Reference Quinn, O'Neill, Doyle, McAtamney, Diamond, McCraith and O'Kennedy2000; Hide et al. Reference Hide, Tsutsui, Sato, Nishimura, Morimoto, Yamnoto and Yoshizato2002) and pathogenic microorganisms in the environment or in food (Bergwerff and van Knapen, Reference Bergwerff and van Knapen2006).
Biosensing surface technologies have been useful in elucidating the adhesion and invasion processes in parasite-host interactions that involve parasite proteins and binding with glycosaminoglycans. For example, biosensing surface methods have been used to assess the interactions between heparin and a malarian circumsporozoite protein and demonstrate the role of parasitic glycosaminoglycan receptors in the invasion of liver cells (Rathore et al. Reference Rathore, McCutchan, Garboczi, Toida, Hernáiz, LeBrun, Lang and Linhardt2001). In addition, the direct measurement of the binding between the purified measles virus and heparin proved that this interaction prevents the infection of SLAM-negative cells lines (Terao-Muto et al. Reference Terao-Muto, Yoneda, Seki, Watanabe, Tsukiyama-Kohara, Fujita and Kai2008). Therefore, our results that indicated the binding of promastigotes to immobilized heparin onto a biosensing surface confirm the presence of heparin receptors in the surface of the parasites and also support the effectiveness of this methodology.
It was also observed that the HBPs present in flagella are more efficient at binding to heparin than HBPs from the plasma membranes of promastigotes. However, further studies are necessary to clarify the reasons for this difference and to define the nature of the glycosaminoglycan binding site involved in this interaction while considering the quantity of saccharides of the different glycosaminoglycans.
Collectively, our results provide evidence that HBPs, which are the heparin receptors from the surface of L. (V.) braziliensis promastigotes, present characteristics of metallo-proteinases and are capable of forming stable complexes with glycosaminoglycans (similar to the activity of heparin). It is possible that HBPs modulate signalling activity in the cellular environment and play specific roles in parasite-host interactions.
ACKNOWLEDGMENTS
We are grateful for the transmission electron microscopy facility of the Oswaldo Cruz Institute. Luzia M. C. Côrtes, M.Sc. is a doctoral student at the Fiocruz institution; Francisco O. R. O. Jr, M.Sc. is a doctoral student at the Fiocruz/CAPES institution, and Franklin S. Silva, M.Sc. is doctoral study fellow at the Fiocruz/CNPq institution. Bernardo A. S. Pereira, Ph.D. is a postdoctoral researcher fellow at the CAPES/FAPERJ. Carlos R. Alves, PhD and Mirian C. S. Pereira, Ph.D. are fellow researchers at the CNPq institution.
FINANCIAL SUPPORT
This work is supported by the Brazilian research agencies PAPES V – CNPq/Fiocruz (300731/2010-8), CNPq (509737/2010-2), CAPES (EDITAL – 11/2009) and FAPERJ (E-26/103.060/2008, E-26/110.257/2010).