INTRODUCTION
The sequencing of 2 G. lamblia assemblages (Morrison et al. Reference Morrison, Mcarthur, Gillin, Aley, Adam, Olsen, Best, Cande, Chen, Cipriano, Davids, Dawson, Elmendorf, Hehl, Holder, Huse, Kim, Lasek-Nesselquist, Manning, Nigam, Nixon, Palm, Passamaneck, Prabhu, Reich, Reiner, Samuelson, Svard and Sogin2007; Franzen et al. Reference Franzen, Jerlstrom-Hultqvist, Castro, Sherwood, Ankarklev, Reiner, Palm, Andersson, Andersson and Svard2009) opens new opportunities for research on one of the most prevalent human parasites. The identification of thousands of open reading frames for which the function is unknown has highlighted the unrealized potential of the genome sequence for advancing our understanding of this parasite. Genes whose function is unknown have either diverged from a common ancestor to a point where homology is no longer apparent, or are unique to this species. This uniqueness makes them particularly relevant for uncovering metabolic pathways that are not present in other eukaryotes. Insight into unique or highly diverged pathways may lead to new drug targets which, given the apparent emergence of resistance to metronidazole, is of particular interest.
Converting sequence information into biologically relevant information has become a priority of ‘post-genomic’ research. The application of genome-wide analyses has the potential to complement and accelerate gene-focused methods, which for many years have been a mainstay of the research effort. Methods that are independent of prior knowledge are particularly relevant to study an organism like G. lamblia, which has diverged from other eukaryotes and has adapted to a parasitic life style in a low-oxygen environment.
Compounds that inhibit trophozoite growth in culture have been known for some time (Morgan et al. Reference Morgan, Reynoldson and Thompson1993; Nohynkova et al. Reference Nohynkova, Draber, Reischig and Kulda2000; Dawson et al. Reference Dawson, Sagolla, Mancuso, Woessner, House, Fritz-Laylin and Cande2007; Poxleitner et al. Reference Poxleitner, Dawson and Cande2008) and have been used to study mitosis. Several of these inhibitors, and many previously unknown inhibitory compounds, were detected in a cell-based high-throughput screen designed to identify compounds that perturb trophozoite multiplication (Bonilla Santiago et al. Reference Bonilla Santiago, Wu, Zhang and Widmer2008). In this initial screen all hits were inhibitors. The absence of agonists could indicate that the rate of trophozoite multiplication in TYI-S-33 medium cannot be shortened, or that no compound capable of accelerating the process was present in the libraries. To further explore the presence of growth agonists, we screened the same libraries with a modified assay in which trophozoites were grown in diluted medium for 2 days. Here we present results from this modified screen and discuss the mode of action of confirmed agonists.
MATERIALS AND METHODS
High-throughput screens
High-throughput (HT) screening of a collection of 1520 compounds from the BIOMOL ICCB Known Bioactives 2 and the NINDS Custom Collection 2 was performed essentially as described (Bonilla Santiago et al. Reference Bonilla Santiago, Wu, Zhang and Widmer2008) except that trophozoites were grown in medium diluted with water to 66% of the original formulation (Keister, Reference Keister1983). Briefly, G. lamblia (WB strain, ATCC 50583 (Smith et al. Reference Smith, Gillin, Spira and Nash1982)) was maintained by serial passage in TYI-S-33 medium in 4 ml screw-cap glass tubes. A volume of 45 μl of medium was dispensed into each well of flat-bottom black 384-well plates (cat # 3712, Corning, Corning, New York, USA) with a Matrix WellMate liquid handling robot (Thermo Fisher Scientific, Hudson, NH, USA). Portions of 100 nl of compounds (typical stock concentration is 10 mm) were pin-transferred to a duplicate set of 6 plates and 45 μl of a suspension of 2×104 trophozoites/ml added to each well using the WellMate dispenser. Plates were incubated in a humidified 37°C/5% CO2 cell-culture incubator. One set of 6 plates was incubated for 24 h, the other for 48 h. Trophozoites were exposed to compound for the entire incubation period. Following incubation, trophozoites were fixed in situ in 8% glutaraldehyde as described (Bonilla Santiago et al. Reference Bonilla Santiago, Wu, Zhang and Widmer2008). Trophozoites were stained with 20 μg/ml propidium iodide (PI) and the plates imaged using the procedure described by Bonilla Santiago et al. (Reference Bonilla Santiago, Wu, Zhang and Widmer2008). The number of trophozoites in each image was determined using CellProfiler (Carpenter et al. Reference Carpenter, Jones, Lamprecht, Clarke, Kang, Friman, Guertin, Chang, Lindquist, Moffat, Golland and Sabatini2006). Four non-overlapping images together covering about 90% of each well's surface were acquired at 100x magnification. The raw cell count for each well was obtained by averaging the 4 counts from each well.
Secondary screens
Cultures of confluent trophozoite monolayers were incubated on ice for 15 min to release the attached trophozoites. Suspended trophozoites were counted using a haemocytometer (Fisher Scientific) and 1·2×104 or 3·6×104 trophozoites/ml were inoculated into TYI-S-33 medium in 2 ml or 4 ml screw-cap glass vials in the presence of one of the following compounds: 10 μ m nerol, 10 μ m strychnine (Sigma), 13 μ m N-acetyl-S-farnesyl-L-cysteine (AFC), 16 μ m farnesyl thioacetic acid (FAT) (BIOMOL), or DMSO. In some experiments the medium was diluted to 66% with phosphate-buffered saline (PBS). The vials were incubated at 37°C and trophozoites were counted every day up to 4 days from separate cultures for each day. To eliminate the possibility that growth may be affected by unintended differences in the initial trophozoite concentration, a separate culture was counted each day and discarded.
To microscopically examine the effect of strychnine and nerol on dividing trophozoites, 106 trophozoites were collected by centrifugation from a treated and a control 96 h culture, washed in PBS, fixed in absolute methanol, washed once again in PBS and incubated for 30 min in 40 μg/ml RNase A at room temperature. The trophozoite nuclei were then stained for 30 min at 37°C in 50 μg/ml PI. Excess PI was removed by washing once in PBS, and the parasites examined under an epifluorescent microscope fitted with an Olympus MSWG filter cube. To quantify the number of dividing trophozoites, fluorescence emitted by stained trophozoites cultured for 4 days in the presence of 10 μ m strychnine were compared to that of trophozoites from untreated cultures using flow cytometry. A total of 10 000 events were acquired for each sample with an Accuri C6 flow cytometer. Propidium iodide fluorescence was measured in the FL3 channel. Doublet discrimination was used to exclude cell agrregates (Wresto et al. Reference Wresto, Chrest, Leary, Morris, Stetler-Stevenson and Gabrielson2001).
Data analysis
We adjusted for positional effects within each plate using the median polish, a robust method to remove any row and column bias (Malo et al. Reference Malo, Hanley, Cerquozzi, Pelletier and Nadon2006). This was necessary because trophozoite growth in edge rows and columns was inferior to that observed in interior wells. The corrected values were centred and standardized by subtracting the plate mean and dividing by the standard deviation.
RESULTS
A previous screen of bioactive compound libraries using a similar G. lamblia trophozoite growth assay identified numerous inhibitors but failed to identify compounds that promote trophozoite multiplication. Given the diversity of the compounds screened, this outcome was unlikely to be due to the absence of compounds with such activity. Assuming TYI-S-33 medium supports optimal growth, we wished to investigate whether trophozoite cultures grown in suboptimal conditions could be used to identify agonists of proliferation. To assess the feasibility of modulating trophozoite multiplication, an experiment in which trophozoites were cultured in TYI-S-33 medium diluted with water to 100%, 75%, 50% and 25% of the original concentration was performed. As expected, increasing dilutions correlated with a deterioration of the culture (Fig. 1). This effect was manifested in lower growth rates and lower peak trophozoite concentrations. Based on this observation we chose 66% TYI-S-33 for HT screening. Although the dilution experiment indicated that some growth still occurs at this concentration, cultures in multi-well plates were not expected to do as well as in the sealed cultures used to evaluate the different dilutions. On the basis of these results we expected that 66% medium would allow the parasite to survive but would not support growth.

Fig. 1. Trophozoite growth in various dilutions of TYI-S-33 medium. Giardia lamblia strain WB was used. Each measurement was obtained from a separate culture in 2 ml glass vials.
HT screening in 66% medium was performed with a duplicate set of plates. One set of plates was incubated in the presence of compounds for 24 h, the second set for 48 h. This approach was used to increase the likelihood of detecting agonists as the time-period required to reveal enhanced growth was unknown. Consistent with the evaluation of medium dilutions, random microscopic inspection of the plates showed little parasite growth and diminishing numbers of motile parasites over the course of incubation. Trophozoites were enumerated using Cell Profiler and normalized 24 h counts plotted vs the 48 h counts from the duplicated plate. The scatter plots show that the majority of counts clustered between −2 SD and +2 SD. In the 24 h dimension, except for some low counts and a few wells above 4 SD, little spread was observed. Among the high counts we also observed control wells, indicating that this amount of variability reflects experimental noise. Low counts included most previously identified inhibitors. The vast majority of 48 h counts clustered around the plate mean, but a few counts deviating by more than 6 SD were identified on 2 plates (Fig. 2). The compounds present in these wells include strychnine and nerol on plate 1921, and farnesyl thioacetic acid (FTA) and N-acetyl-S-farnesyl-L-cysteine (AFC) on plate 1791. Wells F6 and K19 on plate 1791 both contain AFC, further confirming the validity of the hits.

Fig. 2. High-throughput screen for compounds promoting trophozoite multiplication. The screen was conducted in duplicate in TYI-S-33 medium diluted to 66%. One set of plates was counted 24 h after the compounds were added (x axis), the other set was counted after 48 h (y axis). Normalized means of 4 counts/well for plate 1791 (left, BIOMOL ICCB-Longwood Known Bioactives 2) and 1921 (right, NINDS Custom Collection) are shown. X symbol (n=64) represents wells without compound, circles (n=320) wells with compound. Full circles represent wells previously found to contain inhibitors (Bonilla Santiago et al. Reference Bonilla Santiago, Wu, Zhang and Widmer2008). Compounds without inhibitory activity in previous screen are shown with empty circles. Compounds from 5 wells with >6 SD above plate mean were tested in secondary assays in regular culture tubes: K19 and F6, N-acetyl-S-farnesyl-L-cysteine (same compound); C16, farnesyl thioacetic acid; O15, nerol; H17, strychnine. The codes indicate the position on the plate where the letter indicates the row and the number the column. X and Y axes are drawn on the same scale spanning 12 SD units.
Consistent with a genuine compound effect, none of the wells deviating by more than 6 SD on the 48 h plate were control wells. Microscopic examination of random wells in 48 h plates showed that few trophozoites were motile, which is consistent with the unfavourable growth conditions resulting from a combination of the diluted medium and exposure to air. This outcome is reflected in the relative tightly clustered distribution of the counts in the Y dimension, and the absence of counts significantly lower than the mean.
Compounds which were identified as promoting growth of trophozoites were tested in secondary assays in sealed glass tubes in complete medium and medium diluted to 66%. In both conditions strychnine and nerol exerted a measurable agonistic effect, and growth in the presence of these compounds resulted in higher peak trophozoite concentrations (Fig. 3). The growth curves indicate that the compounds actually promoted growth, and suggest that the elevated plate counts are not only the result of increased trophozoite survival. Similar observations were made with the 2 hits from plate 1791, AFC and FTA (not shown). The response of trophozoite cultures to nerol was further investigated by testing the effect of 3 concentrations of this compound i.e. 5 μ m, 10 μ m and 20 μ m (Fig. 4). This experiment showed that the 10 μ m concentration used in HT screening did not induce maximal growth, as growth in the presence of 20 μ m nerol was superior. This observation indicates that additional agonists could be present in the plates but are not at a concentration that induces a measurable phenotype. Thus, the growth-enhancing effect of all 4 compounds identified in HT screening was confirmed in secondary assays in complete and diluted medium. Compounds which generated counts less than 6 SD above plate mean were not tested.

Fig. 3. Enhanced growth of Giardia lamblia trophozoites in the presence of nerol and strychnine. Growth was measured in 66% TYI-S-33 medium (A) and complete TYI-S-33 medium (B).

Fig. 4. Effect of different nerol concentrations on trophozoite growth. Trophozoites were cultured for 96 h in complete TYI-S-33 medium in the presence of 3 concentrations of nerol. The 10 μ m concentration to which trophozoites were exposed during HT screening does not induce maximal growth.
Trophozoites grown for 96 h in the presence of 10 μ m strychnine and trophozoites from an untreated control culture were stained with PI to visualize the nuclei and observed under a fluorescent microscope. In the treated culture many forms resembling dividing trophozoites with more than 4 nuclei were observed (Fig. 5). This unusual morphology was not seen in the control culture. The effect of strychnine was quantified with flow cytometry. In 2 replicate experiments, 28·1% and 20·8% of trophozoites emitted more PI fluorescence than the 4n peak, whereas the corresponding proportion in untreated cultures was 3·9% and 3·3%. As suggested by the growth curves, this observation indicates that the drug directly affects mitosis and that the high trophozoite counts observed in HT screening are not only a result of differential survival. In contrast to strychnine, the agonist nerol did not visibly affect trophozoite morphology. Only bi-nucleated trophozoites were observed in 96 h cultures (Fig. 6), indicating that these two agonists have a different mode of action.
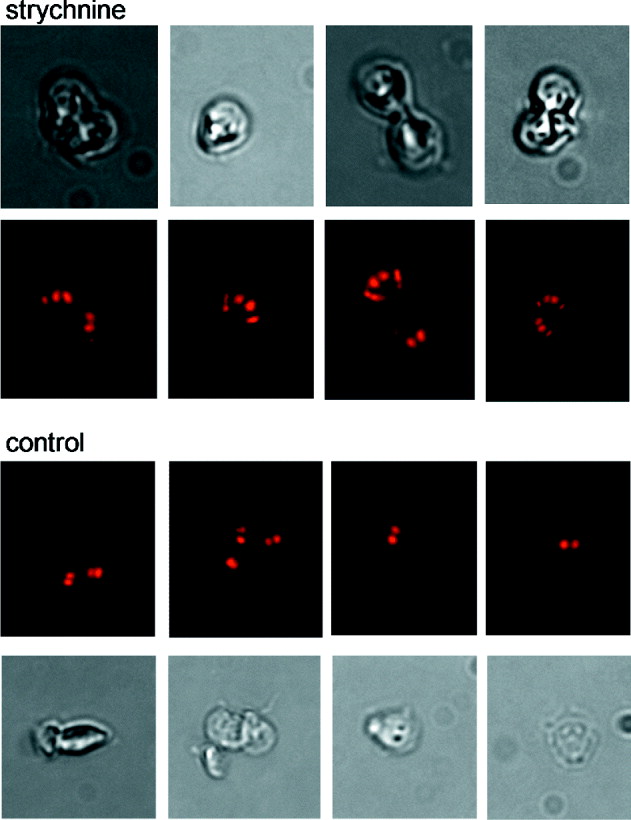
Fig. 5. Multinucleated trophozoites in cultures grown in the presence of strychnine. Matching phase-contrast and fluorescent images of WB trophozoites grown in the presence or absence of 10 μ m strychnine. Cultures were examined at 96 h. Trophozoites are approximately 12 μm in length.

Fig. 6. Nerol enhances growth without affecting trophozoite morphology. Matching phase-contrast and fluorescent images of WB cultures grown in the presence of 10 μ m and 20 μ m nerol or in the absence of compound. Note absence of cells with more than 2 nuclei.
DISCUSSION
Multiplication of G. lamblia trophozoites in the gastro-intestinal tract leads to the colonization of the intestinal mucosa. In the absence of known G. lamblia toxins, the rate and extent of colonization of the intestine is thought to be the main determinant of virulence. Division of G. lamblia trophozoites is a complicated process involving 2 nuclei and a complex cytoskeleton (Nohynkova et al. Reference Nohynkova, Draber, Reischig and Kulda2000; Bernander et al. Reference Bernander, Palm and Svard2001; Ghosh et al. Reference Ghosh, Frisardi, Rogers and Samuelson2001; Elmendorf et al. Reference Elmendorf, Dawson and Mccaffery2003; Sagolla et al. Reference Sagolla, Dawson, Mancuso and Cande2006). G. lamblia proteins which regulate mitosis have been described (Dawson et al. Reference Dawson, Sagolla, Mancuso, Woessner, House, Fritz-Laylin and Cande2007; Davids et al. Reference Davids, Williams, Lauwaet, Palanca and Gillin2008) but our understanding of this process is incomplete. To complement research focused on specific proteins, we are investigating the feasibility of applying system-wide methods to identify genes controlling specific phenotypes and eventually construct networks of interacting proteins. From what is known about the mode of action of active compounds we will be able to infer regulatory mechanisms that can subsequently be experimentally tested.
A previous screen of 2 libraries of bioactive compounds using a similar cell-based trophozoite growth assay identified no compounds which promoted trophozoite multiplication (Bonilla Santiago et al. Reference Bonilla Santiago, Wu, Zhang and Widmer2008). This outcome was originally attributed to a limited dynamic range of the assay, but the present results indicate that this may have been caused by the fact that plates were cultured for 24 h. The present observation indicates that the effect of agonists only becomes apparent after a 48 h incubation period.
Elucidating the mode of action of the agonists identified here is of primary interest as this information can lead to the formulations of testable hypotheses on mechanisms that regulate trophozoite multiplication. The search for plausible mechanisms is not easy because library compounds are typically known for their inhibitory properties, rather than activities which promote a biological process. An example is strychnine which is widely known as a potent toxin. It exerts its lethal effect by inducing respiratory paralysis resulting from the inhibition of post-synaptic glycine receptors in the central nervous system (Young and Cepko, Reference Young and Cepko2004). The annotation of the G. lamblia genome currently does not feature any glycine receptors, making it difficult to speculate whether strychnine acts by binding to such a currently unidentified receptor in G. lamblia, or has an entirely different mode of action in this parasite. An exploratory experiment in which subconfluent monolayers of human HCT-8 cells were exposed to 10 μ m of strychnine showed no obvious agonistic or toxic drug effect, indicating that strychnine may target a pathway not present in higher eukaryotes.
Nerol is a 154 molecular weight monoterpene alcohol used for its fragrance. Related molecules such as limonene and carvone have been shown to have anti-cancer activity (Crowell, Reference Crowell1999). This activity includes cancer blocking and suppression which is exerted through a variety of mechanisms such as promotion of apoptosis (Mills et al. Reference Mills, Chari, Boyer, Gould and Jirtle1995) or induction of enzymes which metabolize certain carcinogens (Ariyoshi et al. Reference Ariyoshi, Arakaki, Ideguchi, Ishizuka and Noda1975; Austin et al. Reference Austin, Shephard, Pike, Rabin and Phillips1988). Inhibition of protein isoprenylation, a post-translational modification involving the attachment of a farnesyl or geranygeranyl group, is another activity of monoterpenes that is of particular interest here (Gelb et al. Reference Gelb, Tamanoi, Yokoyama, Ghomashchi, Esson and Gould1995). In fact, our screen for agonists uncovered 2 other, chemically unrelated farnesylation inhibitors on plate 1791 (AFC and FTA), suggesting that post-translational modification may be a target shared by these trophozoite agonists. The genome of G. lamblia encodes a farnesyl diphosphate synthase, indicating a possible target for nerol and other inhibitors of isoprenylation. Significantly, Luján and co-workers demonstrated many years ago a protein isoprenylation activity in G. lamblia by metabolically labelling trophozoites with radio-isotope labelled mevalonate, a farnesyl precursor (Lujan et al. Reference Lujan, Mowatt, Chen and Nash1995). These authors also tested the effect of the 2 isoprenylation inhibitors limonene, a monoterpene related to nerol, and AFC for their effect of trophozoite growth. Contrary to our observations these compounds were inhibitory, albeit at concentrations (25–100 μ m) which are higher than used in our assay. Consistent with the importance of protein and lipid isoprenylation in controlling the cell cycle (Tamanoi et al. Reference Tamanoi, Gau, Jiang, Edamatsu and Kato-Stankiewicz2001) these observations suggest that isoprenylation, and perhaps protein post-translational modification in general, may be important for regulating G. lamblia growth.
For 35 years giardiasis has been treated with metronidazole. Reports of resistance to this drug highlight the need for alternative treatments. The cell-based HT assay described here and elsewhere (Bonilla Santiago et al. Reference Bonilla Santiago, Wu, Zhang and Widmer2008) offers an alternative to research focused on known pathways and on previously tested compounds and their derivatives (Upcroft et al. Reference Upcroft, Dunn, Wright, Benakli, Upcroft and Vanelle2006; Valdez et al. Reference Valdez, Tripp, Miyamoto, Kalisiak, Hruz, Andersen, Brown, Kangas, Arzu, Davids, Gillin, Upcroft, Upcroft, Fokin, Smith, Sharpless and Eckmann2009). The results presented here suggesting that protein isoprenylation may regulate trophozoite multiplication illustrate the power of chemical genetics and its potential for uncovering unknown regulatory mechanisms. The surprising agonistic effect of strychnine indicates that other regulators remain to be discovered. Together with previously indentified antagonists, the compounds identified here will enable the diversified perturbation of trophozoites in culture to identify genes with similar expression profiles. Information on co-regulated genes will assist in improving the functional annotation of the G. lamblia genome.
ACKNOWLEDGMENTS
We thank Su Chiang (NSRB/NERCE) for supporting the Giardia high-throughput screening and facilitating access to compound libraries and the screening facility. Eric London and Spurthi Patil provided technical assistance.
FINANCIAL SUPPORT
Financial support from the National Institute of Allergy and Infectious Diseases (grant R21AI083719) is gratefully acknowledged. Facilities and compound libraries for high-throughput screening were made available through the NSRB/NERCE program and the ICCB-Longwood at Harvard Medical School.








