Introduction
With the cultivation and large-scale planting of genetically modified (GM) crops, the transfer of Bacillus thuringiensis Berliner (Bacillaceae) (Bt) toxin from GM crops to higher trophic levels is of interest because the toxin may adversely affect the natural enemies of pest insects that feed on GM cultivars (Dutton et al. Reference Dutton, Klein, Romeis and Bigler2002; Torres and Ruberson Reference Torres and Ruberson2006; Zhang et al. Reference Zhang, Wan and Liu2006). Jiang et al. (Reference Jiang, Fu, Cheng, Zhu, Jiang and Ye2004) documented that the transfer of Cry1Ab protein from transgenic rice (KMD1) to larvae of the Asiatic rice borer (Chilo suppressalis (Walker); Lepidoptera: Crambidae) and the rice styrid (Mycalesis gotama Moore; Lepidoptera: Nymphalidae), and then to the spider, Pirata subpiraticus (Bösenberg and Strand) (Araneae: Lycosidae). Chen (Reference Chen2007) showed the transfer of Cry1A protein from transgenic cotton to the cotton bollworm (Helicoverpa armigera Hübner; Lepidoptera: Noctuidae) and then to the parasitoid, Microplitis mediator (Haliday) (Hymenoptera: Braconidae). Chen et al. (Reference Chen, Ye, Lu, Hu, Peng and Su2005) also detected Cry1Ab in the brown planthopper (Nilaparvata lugens (Stål; Hemiptera: Delphacidae) and a lycosid spider (P. subpiraticus Bösenberg and Strand; Araneae: Lycosidae), and found that the protein content in the predators did not increase with the prolongation of the feeding time. Tian et al. (Reference Tian, Liu, Yao, Ye and Peng2008) reported the presence of the Cry1Ab protein in the green rice leafhopper (Nephotettix cincticeps (Uhler) Hemiptera: Cicadellidae), and brown planthopper fed on transgenic rice and in several species of their parasitoids and predators. Recently, Shi et al. (Reference Shi, Yang, Cai, Zhang and Shi2009) also detected the Bt toxin in the bodies and excrement of the Asian corn borer larvae (Ostrinia furnacalis Guenée; Lepidoptera: Crambidae) fed with transgenic maize.
The beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) is an important leaf-feeding pest in the cotton field of China because of its increasing abundance and serious damage across cotton areas since 1999. As a result, this species was added into list of potential outbreak insect pests in 2001 (Xia et al. Reference Xia, Cui and Chang2000; Liu and Jiang Reference Liu and Jiang2002). Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) is an important predatory natural enemy and Microplitis pallidipes Szépligeti (Hymenoptera: Braconidae) is a key parasitic species in cotton fields, both of which play vital roles in controlling cotton pests such as S. exigua (Liu and Jiang Reference Liu and Jiang2002). While the transfer and accumulation of Bt toxin in transgenic cotton, pests, and their predators have been reported in several different systems or food chains, little is known about the transfer of Bt toxin in S. exigua and their natural enemies. Torres and Ruberson (Reference Torres and Ruberson2008) studied the migration of the toxin through the food chain of transgenic cotton (Cry1Ac), S. exigua and a big-eyed bug (Geocoris punctipes (Say); Hemiptera: Lygaeidae), and found that that 81% and 76% of Bt toxin in the leaves of two transgenic cotton varieties was transferred into the S. exigua larvae. However, Cry1Ac protein was not found in the natural enemy. In this study, we evaluated the transfer and accumulation of Bt toxin in the food chain of transgenic cotton, S. exigua, and their two natural enemies, C. carnea and M. pallidipes, and reported that Bt toxin can be transferred through the food chain and to natural enemies of various predatory habits.
Materials and methods
Cotton varieties
Four cultivars of cotton were obtained from the Institute of Plant Protection (IPP) of the Chinese Academy of Agricultural Sciences. Two cultivars carried transgenes for Bt; i.e., GK12 (Cry1Ac/Ab fusing gene) and New variety 33B (Cry1Ac). A third cultivar carried transgenes for Bt and cowpea trypsin inhibitor; i.e., SGK321 (Cry1Ac + CpTI). The fourth cultivar, Simian 3, was nontransgenic and served as a control. One plot (200 m2) of each cultivar was grown at Yangzhou University, Yangzhou, Jiangsu, China (32.4°N, 119.2°E). Plots were established using transplanted seedlings and maintained with conventional methods, but without the use of chemical pesticides.
Insect material tested
Spodoptera exigua and C. carnea were hatched from eggs provided by IPP. Larvae and adults were held at 27 ± 1 °C, 70 ± 10% relative humidity, and a photoperiod of 14:10 (light:dark). Larvae of S. exigua were reared on an artificial diet (Li et al. Reference Li, Li, Yang, He, Yang and Wang2001). The third set of leaves from the top were sampled to feed S. exigua and used for detection of Bt insecticidal protein expression by enzyme-linked immunosorbent assay. As has been reported by Zhang et al. (Reference Zhang, Chen, Chen and Yang2007), no significant difference in S. exigua susceptibility to Bt insecticidal protein was observed in this study among four cultivars of cotton (mortality rate <10%). Larvae of C. carnea were fed on aphids (Aphis gossypii Glover; Hemiptera: Aphididae) that were collected from conventional cotton varieties grown in the laboratory. Adults of C. carnea were fed on a mixture of dry yeast powder and sugar (Zhou et al. Reference Zhou, Liu, Chen and Qiu1980). The cocoons of M. pallidipes were provided by the Shanghai Academy of Agricultural Sciences (Shanghai, China) and were reared on S. exigua (Qu et al. Reference Qu, Wang and Zhu2005).
Spodoptera exigua
The neonates were placed into Petri dishes (15 cm in diameter) at a density of 25 larvae per dish. The larvae were fed with the top third set of leaves from the four cotton varieties. The petioles of the leaves were covered with a moist cotton swab as to extend the life of the leaf. The Petri dishes were covered with plastic wrap and white paper to prevent the escape of the larvae. Upon reaching second, third, fourth, and fifth instar stages, a subset of these larvae and their excrement were collected and held at −70 °C. Before the harvest, the larvae were starved for 12 hours to facilitate defecation.
Chrysoperla carnea
The second instars of S. exigua raised on the artificial diet were placed into cylindrical tubes (8 cm in length and 2 cm in diameter) at a density of 10 larvae per tube. The larvae were fed with the top third set of leaves from the four cotton varieties for 24 hours, and each variety was used to rear 100 larvae. One third instar of C. carnea was placed into each tube and was removed after 24 hours and kept at −70 °C. The experiment was repeated three times.
Microplitis pallidipes
The second instars of S. exigua raised on the artificial diet were placed into Petri dishes (15 cm in diameter) at a density of 25 larvae per dish. The larvae were fed with the top third set of leaves from the four cotton varieties. Two mated females of M. pallidipes were placed into Petri dishes for oviposition, and removed after 24 hours. The parasitised S. exigua larvae were collected after the wasps emerged from their cocoons without being fully hardened, and they were kept at −70 °C. The experiment was repeated three times.
Detection of Bt toxin
Sample pretreatment
To test for changes in Bt toxin expression with plant age, samples of cotton leaves from each variety (100 mg) were collected on nine different dates from 8 June 2011 to 28 August 2011 (Fig. 1). Samples of body and excrement of second instars (45 individuals), third instars (30 individuals), fourth instars (25 individuals), or fifth instars (20 individuals) of S. exigua were collected and placed into a 2 mL Eppendorf tube, respectively. Samples (100 mg) of the larvae of C. carnea (30 individuals) and cocoons of M. pallidipes (60 individuals) were collected and placed into a 2 mL Eppendorf tube. The samples were then treated with liquid nitrogen, ground, vortexed, and centrifuged. The resulting supernatant was transferred into a 1.5 mL Eppendorf tube containing 990 μL of PBST. Each sample was prepared in triplicate (Zhang et al. Reference Zhang, Wan, Guo and Hou2004).
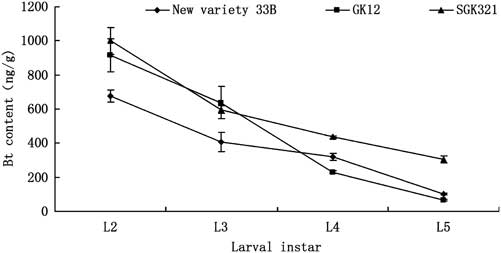
Fig. 1 Bt insecticidal protein expression in the third leaf of three cotton varieties during different growth stage. Bt, Bacillus thuringiensis.
Toxin detection
The toxin was detected using the Quantiplate™ Kit for Cry1Ab/Cry1Ac (EnviroLogix, Portland, Maine, United States of America), which includes antibody-coated 96-well microtiter plates, an enzyme conjugate, substrate, positive control, stop solution, and phosphate-buffered saline with Tween-20, and a washing buffer. The optical density (OD) values at 450 nm were measured using a Biotek Synergy 2 Multimode Plate Reader (BioTek Instruments, Winooski, Vermont, United States of America) and the OD values were translated into ng/g with the standard curve according to the kit's instructions.
Statistical analyses
All experiments were repeated three times. The Bt insecticidal protein level in the body and excrement of S. exigua larvae that fed on the different transgenic cottons, as well as the body of a predator (C. carnea) and a parasite (M. pallidipes) that predated/parasitised S. exigua were processed using Microsoft Excel 2003 software (Microsoft Corporation One Microsoft Way, Redmond, Washington, United States of America) and subjected to analysis of variance using Data Processing System v3.01 statistical software (Tang and Feng, Reference Tang and Feng2002), and means were separated (P < 0.05) by using Fisher Protected least significant difference test (Steel and Torrie Reference Steel and Torrie1980). Because of the absence of Bt protein in the control cultivar, only data for transgenic cultivars were included in the analyses.
Results and analysis
Bt toxin expressed in cotton leaves
The expression of Bt insecticidal protein in the third leaf from the top decreased with the development of cotton, that is, it was the seedling to squaring period > blooming period >bolling stage. For example, the squaring period (e.g., 28 June), the expression of Bt insecticidal protein in SGK321 (1630.0 ng/g) was higher than that in GK12 (1106.4 ng/g). Bt insecticidal protein expression in the three varieties was significantly different except before squaring stage (e.g., 8 June, 18 June) and mid-squaring period (e.g., 18 July) (6.8: F = 3.112, P = 0.074; 6.18: F = 1.391, P = 0.279; 6.28: F = 12.325, P < 0.001; 7.8: F = 18.560, P < 0.001; 7.18: F = 0.960, P = 0.405; 7.28: F = 6.357, P = 0.010; 8.8: F = 6.575, P = 0.009; 8.18: F = 5.926, P = 0.013; 8.28: F = 10.431, P < 0.001, df = 2, 24; Fig. 1).
Bt protein level in the body and excrement of S. exigua
Bt protein was detected in all S. exigua larvae fed leaves of transgenic cultivars (Fig. 2). No Bt protein was detected in larvae fed on the control cultivar. Protein concentrations declined with larval instar. Although generally lower levels of Bt protein were detected in New variety 33B (Fig. 1), no significant difference of Bt protein concentrations was observed among the same instar larvae fed on different transgenic cultivars (P > 0.05).
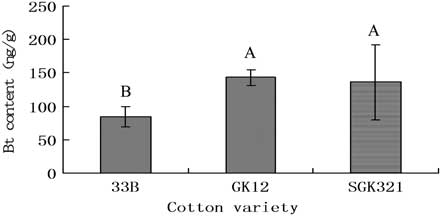
Fig. 2 Bt insecticidal protein levels (ng/g) detected in the body of Spodoptera exigua larvae fed with various transgenic cotton varieties. Bt, Bacillus thuringiensis.
Considerable levels of Bt protein were present in the excrement of the S. exigua larvae fed with the transgenic cotton varieties. In general, Bt toxin content in the excrement of the lower instar larvae was less than that of partially grown larvae. Bt toxin content in the excrement of the different instars of the larvae fed with New variety 33B exhibited the following pattern: L5 > L3 > L2 > L4. The difference of Bt content between the excrement of fifth instars and that of other instars was statistically significant. The Bt content in the excrement of the different instar larvae feeding on the GK12 transgenic cotton had the following pattern: L3 > L4 > L5 > L2. Bt content in the excrement of second instars was significantly lower than that of the other instars (Table 1).
Table 1 Bt insecticidal protein levels in the excrement of Spodoptera exigua larvae fed with transgenic cotton (ng/g).

All data are presented as mean ± standard error (the second instar [45 larvae], the third instar [about 30 larvae], fourth instar [about 25 larvae] and the fifth instar [about 20 larvae]).
The different letters in a column indicate a significant difference (P < 0.01).
Bt protein content in the natural enemies of S. exigua
Bt toxin was detected in C. carnea fed on S. exigua larvae that had consumed transgenic cotton. Bt levels (83.9 ± 15.4 ng/g) in C. carnea associated with consumption of New variety 33B were lower than those associated with consumption of cultivars GK12 (143.1 ± 12.1 ng/g) and SKG321 (136.4 ± 55.8 ng/g; F = 14.409, P = 0.001, df = 2, 6). Bt levels associated with the latter two cultivars did not differ (P > 0.05; Fig. 3).
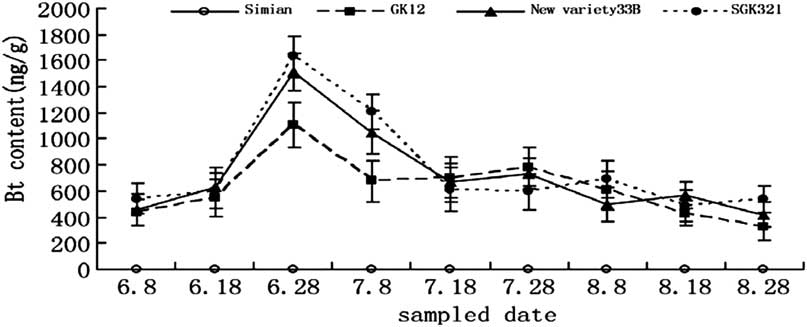
Fig. 3 Bt insecticidal protein levels in the body of Chrysoperla carnea after preying on Spodoptera exigua fed with different varieties of transgenic cotton.
Bt toxin was also detected in M. pallidipes, which parasitised the S. exigua larvae fed with transgenic cotton. Bt levels in M. pallidipes parasitised the S. exigua larvae fed with SKG321 was lower than those parasitised the S. exigua larvae fed with GK12 and New variety 33B (F = 17.632, P = 0.001, df = 3, 8; Fig. 4). No significant difference was observed in M. pallidipes parasitised the S. exigua larvae fed with the latter two cultivars (P > 0.05; Fig. 4).
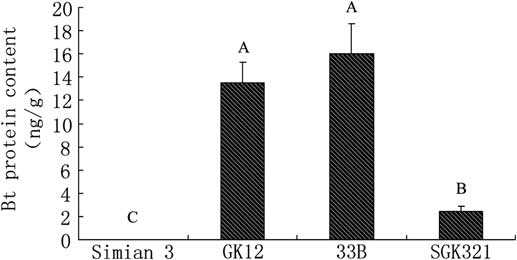
Fig. 4 Bt insecticidal protein levels in Microplitis pallidipes after parasitising Spodoptera exigua larvae fed with different transgenic cotton varieties. Bt, Bacillus thuringiensis.
Discussion
The transfer of the Bt protein in the food chains has been confirmed by many studies (Dutton et al. Reference Dutton, Klein, Romeis and Bigler2002; Torres and Ruberson Reference Torres and Ruberson2006; Zhang et al. Reference Zhang, Wan and Liu2006). As expected, Bt toxin was found in the bodies and excrement of the S. exigua larvae fed on any of the three transgenic cotton varieties. The results showed that the levels of Bt expression in the cotton decreased with the development (Fig. 1), and Bt protein concentrations declined with larval instar (Fig. 2). However, the Bt content in the body did not increase with development of the larvae and the ratio of Bt levels to larvae mass decreased. In contrast, the Bt content in the excrement increased with larval instar. In addition, the amount of Bt toxin in the excrement was not consistent with that in cotton leaves fed by S. exigua larvae. These discrepancies might be due to the enhanced metabolism of Bt toxin in late instar larvae. It has been reported that after feeding on transgenic cotton, lower instar S. exigua larvae have a higher mortality percent than that of higher instar larvae (Zhang et al. Reference Zhang, Chen, Chen and Yang2007), which suggests that a lower level of Bt toxin metabolism occurs in low instar stage larvae as compared with high instar stage larvae.
In the cotton growing region in the middle and lower reaches of Yangtze River, China the expression of Bt toxin in transgenic cotton leaves of GK12 and New variety 33B in July and August 2011 was 300–450 and 450–600 ng/g, respectively (Zhang et al. Reference Zhang, Li, Xu, Zhao and Zhao2000). Zhang et al. (Reference Zhang, Wan, Guo and Hou2004) found that Bt toxin (Cry1Ab) was 50.0 ng/g in GK12 leaves, and 138.2 ng/g in New variety 33B leaves. However, Bt toxin in the second larvae of Prodenia litura fed with either GK12 or New variety 33B was 978.0 and 720.0 ng/g, respectively. This indicates that Bt toxin was concentrated in the pest. In our study, Bt toxin content found in S. exigua larvae of each instar was not only equal or a little lower than that in cotton leaves, but also similar to that in different cotton varieties. However, Bt toxin in excrement of larvae and in its natural enemies was much lower than that in cotton leaves and in S. exigua, which indicates that changes of Bt toxin amount in pest and its natural enemies may be due to the metabolism of the protein.
The transfer of Bt protein to higher tropic levels via herbivorous insects has been demonstrated in several Bt transgenic crops, including maize (Harwood et al. Reference Harwood, Wallin and Obrycki2005; Vojtech et al. Reference Vojtech, Meissle and Poppy2005; Alvarez-Alfageme et al. Reference Álvarez-Alfageme, Ferry, Castañera, Ortego and Gatehouse2008), cotton (Torres and Ruberson Reference Torres and Ruberson2006, Reference Torres and Ruberson2008; Zhang et al. Reference Zhang, Wan and Liu2006; Chen et al. Reference Chen, Ye, Liu, Fang, Hu and Peng2009), rice (Jiang et al. Reference Jiang, Fu, Cheng, Zhu, Jiang and Ye2004; Li et al. Reference Li, Ye, Wu, Peng and Chen2007; Chen et al. Reference Chen, Ye, Liu, Fang, Hu and Peng2009), and canola (Ferry et al. Reference Ferry, Mulligan, Majerus, Stewart, Tabashnik and Port2006, Reference Ferry, Mulligan, Majerus and Gatehouse2007; Chen et al. Reference Chen, Zhao, Collins, Earle, Cao and Shelton2008). Bt protein was detected in C. carnea and M. pallidipes, two insects that preyed on or parasitised S. exigua larvae fed with each of the three transgenic cotton varieties. Interestingly, the Bt content in C. carnea was much higher than that in M. pallidipes (Figs. 3, 4), which may have been due to differences in species, feeding methods, transfer efficiency or Bt toxin metabolism rates and efficacy. Further studies are needed to address these questions and the final destination of the Bt protein present in C. carnea and M. pallidipes remains to be determined.
Acknowledgement
This study was funded by a grant from the Key Project for Breeding Genetically Modified Organisms (2013ZX08012004-005).







