Over the last decade, substantial scientific evidence suggests that the hospital environment is an important source of organisms that, when transmitted, can cause healthcare-associated infections for several reasons.Reference Weber, Rutala, Anderson, Chen, Sickbert-Bennett and Boyce 1 First, the hospital environment is commonly contaminated with epidemiologically important healthcare pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), multidrug-resistant (MDR) Acinetobacter, and Clostridium difficile.Reference Weber, Rutala, Anderson, Chen, Sickbert-Bennett and Boyce 1 , Reference Rutala and Weber 2 These pathogens share the following general characteristics: (1) devices and surfaces in the patient room are frequently contaminated; (2) an ability to survive for prolonged periods of time on environmental surfaces (eg, days to months); and (3) contact with surfaces contaminated with these results in hand or glove contamination, which may be transferred to patients. Finally, room disinfection reduces contamination with these organisms.Reference Weber, Rutala, Anderson, Chen, Sickbert-Bennett and Boyce 1 – Reference Stiefel, Cadnum, Eckstein, Guerrero, Tima and Donskey 3 Second, standard cleaning and disinfection methods are inadequate in most, if not all, hospitals. On average, only 50% of surfaces in hospital rooms are cleaned between patients.Reference Carling 4 As a result, patients admitted to the rooms previously occupied by patients with MDR organisms are at a 39%–353% increased risk of subsequent infection (a 120% increased risk on average).Reference Carling 4
An overhead light fixture technology, which continuously and safely disinfects the environment using light-emitting diodes (LEDs) by emitting a high-intensity, narrow-spectrum (HINS) light, has been proposed as an infection prevention strategy.Reference Maclean, Macgregor and Anderson 5 – Reference Bache, Maclean, MacGregor, Anderson, Gettinby, Coia and Taggart 7 This technology uses LEDs to create a narrow bandwidth of high-intensity visible violet light with a peak output of 405 nm. The wavelength of the LEDs is certified by the manufacturer to be 405 nm ± 3 nm. This light in turn reacts with porphyrin molecules to generate reactive oxygen species that kill microorganisms.Reference Maclean, Macgregor and Anderson 5 The purpose of this evaluation was to determine the effectiveness of HINS light for the reduction of epidemiologically important pathogens in the environment.
Methods
Light source and irradiance
An overhead, visible light disinfection technology (Indigo-Clean, Kenall Manufacturing, Kenosha, WI) was evaluated in 2 different clinical configurations. In phase 1 (“white” lights), two 61-cm × 61-cm (2-foot × 2-foot) blended-white, ceiling-mounted fixtures were used to provide both disinfection and ambient white illumination for use in normal clinical conditions in an occupied room. The measured surface irradiance of this “white” disinfecting light at the pathogen location was ~0.12–0.16 mW/cm2. In phase 2 (“blue” light), a higher-level of disinfection light was studied by adding a 61-cm × 122-cm (2-foot × 4-foot) overhead “blue” light fixture to the 2 preexisting 61-cm × 61-cm overhead, blended-white fixtures. The measured surface irradiance of disinfecting “blue” light at the pathogen location was ~0.34–0.44 mW/cm2. The surface irradiance measurements in the control area yielded values of 0.00 mW/cm2 (no measurable disinfecting light). These surface irradiance measurements were made using a National Institute of Standards and Technology (NIST)-calibrated spectroradiometer (model no. USB2000+, Ocean Optics, Wesley Chapel, FL).
Phase 1 and phase 2 testing were conducted in a 12.5 m2 (134 ft2) room. The room used did not have windows or external sources of light. Each of the 3 lights described above were connected via separate light switches and were simply switched “on” and “off” at the wall switch. Light placement was designed to treat the study room with intensity sufficient to cause inactivation of test bacteria. In both phases, the indicated lights were operated continuously (ie, 24 hours per day, 7 days per week) during the sampling period, and no other lights were present in the study room.
Study design
The vegetative bacteria were grown on sheep blood agar. Serial dilutions of inocula were made with trypticase soy broth (TSB, Remel, Lenexa, KS). The C. difficile spore preparation was stored in Dulbecco’s modified Eagle’s medium (HyClone, Logan, UT), and serial dilutions were similarly made using TSB. The 4 test organisms were C. difficile spores (BI strain), a MRSA strain (ATCC 43300), a VRE strain (ATCC strain 51299), and a clinical isolate of MDR Acinetobacter baumannii. Rodac plate templates were drawn on the Formica sheet and inoculated with 10–15 µL of a 104 dilution of test organisms suspended in TSB, producing an estimated inoculum of 100–500 test organisms. After inoculation, each surface was allowed to air dry for 10 minutes after inoculation. Once dry, the test Formica sheets were exposed to the disinfecting light and triplicate samples were collected with Rodac plates containing Dey-Engley Neutralizing Agar after 0, 1, 3, 5, 6, 7, 24, 48, and 72 hours. These plates were then incubated based on the test organism being studied (aerobically at 37oC for 48 hours for bacteria and anaerobically at 37oC for 48 hours for C. difficile) in an AnaeroPack anaerobic gas generator (Anaeropack, Mitsubishi Gas Chemical, Tokyo, Japan). After incubation, the colony-forming units (CFU) of the test organisms on each plate were quantified. Each template area was sampled only once. Surfaces were maintained at ambient room temperature and relative humidity. A control Formica sheet was placed in an adjacent area but not exposed to the HINS light to accommodate the expected natural in vitro die-off of vegetative bacteria. Triplicate samples were collected with Rodacs at the same test times as the test surfaces. Two experimental runs were conducted for all time points.
Statistical methods
We fit a mixed-effects negative binomial model to the data using the R statistical software 8 and the lme4 package.Reference Bates, Maechler, Bolker and Walker 9 We modeled the “blue” light as augmenting the “white” light. Both linear and squared time variables were included in the model to account for any nonlinear effects. The full model began with a 3-way interaction of treatment × bacteria × time, and hypotheses were tested using likelihood ratio tests of progressively nested models. A P value<.05 was considered significant.
Results
A 3-way interaction was significant (χ2=265.5; df=12; P<.001), indicating that the effect of the type of light treatment differed with different combinations of test organisms and time. The treatment (ie, both blue and white light) had significantly different rates of pathogen killing over time for all 4 organisms: Acinetobacter (χ2=117.2; df=4; P<.001), MRSA (χ2=80.5; df=4; P<.001), VRE (χ2=150.4; df=4; P<.001), and C. difficile (χ2=25.8; df=4; P<.001).
We also performed individual tests of the interactions between the white (vs the control) and time, and blue (vs white) and time. Both types of light treatments were associated with more rapid decreases in observed bacterial counts over time with all 4 organisms with 1 exception, the use of white light had no effect on C. difficile compared to control (Fig. 1). Specifically, the number of CFUs on test Rodac plates decreased over time for Acinetobacter with the white light (χ2=95.7; df=2; P<.001) and the blue light (χ2=16.6; df=2; P<.001); for MRSA, for both white (χ2=31.7; df=2; P<.001) and blue (χ2=29.9; df=2; P<.001); and for VRE, for both white (χ2=7.1; df=2; P<.029) and blue (χ2=138.5; df=2; P<.001). However, white was not superior to control for C. difficile (χ2=2.6; df=2; P=.20), but the use of blue light increased killing of C. difficile (χ2=23.9; df=2; P<.001).
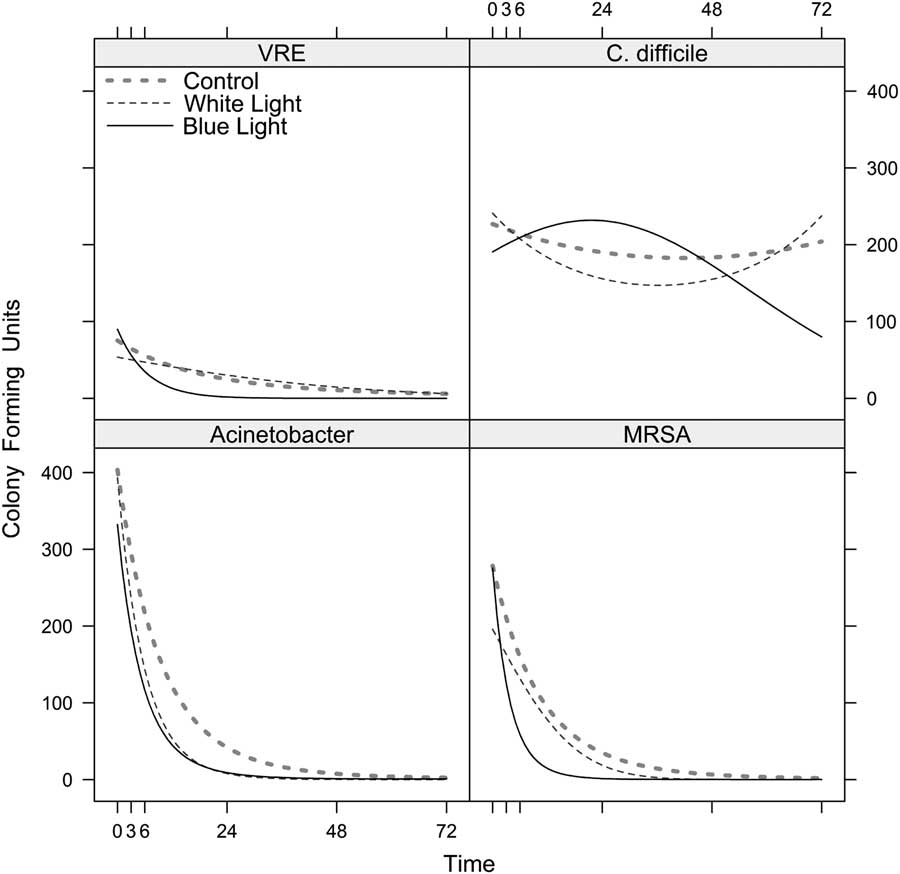
Fig. 1 Use of a continuous visible light disinfection system and predicted reduction (CFU/mL) in epidemiological important pathogens over time. Under the “blue,” “white,” and control lights, the models predicted the number of colony-forming units of (A) vancomycin-resistant Enterococcus-VRE, (B) C. difficile, (C) MDR-Acinetobacter, and (D) methicillin-resistant S. aureus-MRSA (see Methods). The curves are drawn continuously over the temporal interval from 0 to 72 hours. However, in the experiment, the actual time points when the CFUs were counted were at 0, 1, 3, 5, 6, 7, 24, 48, and 72 hours. Because the model treats time as continuous, we were able to calculate predicted values for any time point between 0 and 72.
Table 1 lists the earliest hour by which our statistical model predicted a sustained reduction in the number of CFUs by a given percentage. Overall, the model demonstrates enhanced inactivation of pathogens with the “blue” and “white” light.
Table 1 Use of a Continuous Visible Light Disinfection System and Predicted Reduction (%) of Common Environmental Pathogens over TimeFootnote a
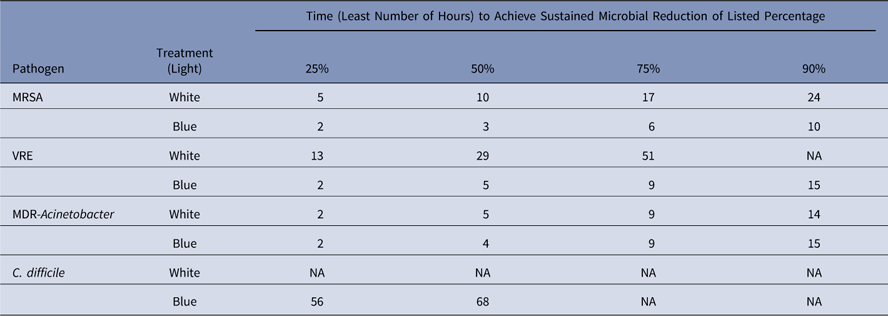
Note. MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus; MDR, multidrug-resistant; NA, sustained reduction not achieved.
a The earliest hour after which the model predicts a sustained reduction of CFUs by the stated percentage for epidemiologically-important pathogens with the white light and the blue light. The largest reduction listed is 90% because the model cannot predict a 100% reduction except after infinite hours have passed.
Discussion
The use of light disinfection technology for continuous disinfection of the healthcare environment has been proposed by various investigators.Reference Maclean, Macgregor and Anderson 5 – Reference Bache, Maclean, MacGregor, Anderson, Gettinby, Coia and Taggart 7 The use of disinfecting lights, if effective, could augment the episodic disinfection (eg, daily) that occurs in patient rooms or care areas by preventing or reducing the microbial regrowth on surfaces following disinfection, and by reducing the microbial level due to recontamination. These light sources are thought to be safe for surfaces and for humans,Reference Bache, Maclean, MacGregor, Anderson, Gettinby, Coia and Taggart 7 although there has been limited human experience.
We demonstrated that the “blue” and “white” light significantly reduced the 3 vegetative test bacteria; and “blue” light yielded lower counts of C. difficile spores after 72 hours. Whether the level of these reductions are sufficient to reduce healthcare-associated infections remains uncertain, and the question requires further study.
This study was a preliminary evaluation. Future studies will need to consider cost-effectiveness, multiple types of surfaces (eg, porous vs nonporous surfaces, stainless steel) with taxonomically diverse pathogens (eg, norovirus, Enterobacteriaceae) to include spores, use areas (eg, operating room), and the ability of the technology to continuously reduce the overall bioburden in inpatient and outpatient care areas and reduce HAIs. A separate issue is the acceptance of continuous light (ie, 24 hours) by patients and staff. If shorter durations of continuous light exposure are deemed necessary, the level of decontamination achieved by use during times when the patient is awake (eg, ~16 hours per day) needs further study. In addition, future studies should include rechallenging the surfaces with additional contamination (eg, every 4–6 hours). Given that environmental surfaces in a patient’s room are often not thoroughly disinfected and that recontamination occurs rapidly, it is important to develop either methods of continuous disinfection or a germicide with persistant antimicrobial effectiveness.
Acknowledgments
Kenall Manufacturing loaned UNC Hospitals the high-intensity, narrow spectrum light fixtures for the study period. They also allowed UNC Hospitals to use the NIST calibrated spectroradiometer to measure surface irradiance. Kenall had no role in the design, conduct, analysis, or publication of this study.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
Drs Rutala and Weber are consultants for PDI in 2017–2018 and consultants for Clorox in 2012–2016. Dr Weber is a consultant for Germitec. Dr Rutala has received an honorarium from Kenall.




