Introduction
Catechol-O-methyltransferase (COMT) provides the main mechanism for the degradation of released cortical dopamine (DA) (Karoum et al. Reference Karoum, Chrapusta, Brinjak, Hitri and Wyatt1994; Gogos et al. Reference Gogos, Morgan, Luine, Santha, Ogawa, Pfaff and Karayiorgou1998). Its gene contains a common functional polymorphism at codon 158/108 which results in the substitution of valine (Val) for methionine (Met) in the COMT peptide sequence (Lotta et al. Reference Lotta, Vidgren, Tilgmann, Ulmanen, Melen, Julkunen and Taskinen1995; Lachman et al. Reference Lachman, Papolos, Saito, Yu, Szumlanski and Weinshilboum1996). As the Val158 isoform is more active than the Met158 in degrading DA (Lotta et al. Reference Lotta, Vidgren, Tilgmann, Ulmanen, Melen, Julkunen and Taskinen1995; Lachman et al. Reference Lachman, Papolos, Saito, Yu, Szumlanski and Weinshilboum1996; Mannisto & Kaakkola, Reference Mannisto and Kaakkola1999) this polymorphism has important ramifications for functions involving DA neurotransmission, such as cognition and affect regulation.
Carriers of the Val158 allele show impaired performance (Egan et al. Reference Egan, Goldberg, Kolachana, Callicott, Mazzanti, Straub, Goldman and Weinberger2001; Joober et al. Reference Joober, Gauthier, Lal, Bloom, Lalonde, Rouleau, Benkelfat and Labelle2002; Bosia et al. Reference Bosia, Bechi, Marino, Anselmetti, Poletti, Cocchi, Smeraldi and Cavallaro2007) coupled with increased dorsal prefrontal cortical (PFC) activation during executive function tasks (Mattay et al. Reference Mattay, Goldberg, Fera, Hariri, Tessitore, Egan, Kolachana, Callicott and Weinberger2003; Blasi et al. Reference Blasi, Mattay, Bertolino, Elvevag, Callicott, Das, Kolachana, Egan, Goldberg and Weinberger2005; Bertolino et al. Reference Bertolino, Blasi, Latorre, Rubino, Rampino, Sinibaldi, Caforio, Petruzzella, Pizzuti, Scarabino, Nardini, Weinberger and Dallapiccola2006a, Reference Bertolino, Rubino, Sambataro, Blasi, Latorre, Fazio, Caforio, Petruzzella, Kolachana, Hariri, Meyer-Lindenberg, Nardini, Weinberger and Scarabinob; Schott et al. Reference Schott, Seidenbecher, Fenker, Lauer, Bunzeck, Bernstein, Tischmeyer, Gundelfinger, Heinze and Duzel2006; Winterer et al. Reference Winterer, Musso, Vucurevic, Stoeter, Konrad, Seker, Gallinat, Dahmen and Weinberger2006; Mier et al. Reference Mier, Kirsch and Meyer-Lindenberg2009). In contrast the Met158 allele is associated with greater reactivity to emotionally negative stimuli as evidenced by increased activation in the ventral PFC and associated limbic regions (Smolka et al. Reference Smolka, Schumann, Wrase, Grusser, Flor, Mann, Braus, Goldman, Buchel and Heinz2005; Drabant et al. Reference Drabant, Hariri, Meyer-Lindenberg, Munoz, Mattay, Kolachana, Egan and Weinberger2006; Mier et al. Reference Mier, Kirsch and Meyer-Lindenberg2009). Based on these observations it is thought that optimal function of the dorsal and ventral PFC is respectively associated with the Met158 and Val158 alleles; this confers an advantage in executive function for Met carriers and in emotional resilience for Val carriers (Mier et al. Reference Mier, Kirsch and Meyer-Lindenberg2009).
Because of its effect on cognitive and emotional function the COMT gene has been considered in many psychiatric disorders. Key associations have been noted between the Val158 allele and schizophrenia (Glatt et al. Reference Glatt, Faraone and Tsuang2003) and between the Met158 allele and disorders of affective morbidity such as anxiety disorders (Hamilton et al. Reference Hamilton, Slager, Heiman, Deng, Haghighi, Klein, Hodge, Weissman, Fyer and Knowles2002; Woo et al. Reference Woo, Yoon and Yu2002), bipolar disorder (BD) (Craddock et al. Reference Craddock, Owen and O'Donovan2006) and possibly major depressive disorder (MDD) (Ohara et al. Reference Ohara, Nagai, Suzuki and Ohara1998; Massat et al. Reference Massat, Souery, Del-Favero, Nothen, Blackwood, Muir, Kaneva, Serretti, Lorenzi, Rietschel, Milanova, Papadimitriou, Dikeos, Van Broekhoven and Mendlewicz2005). As the disease risk conferred is very small and not consistently detected, the COMT Val158Met may primarily act as a modifier locus, having an impact on disease-associated traits such as brain function or cognition, rather than clinical diagnosis.
Here we used functional magnetic resonance imaging (fMRI) to examine the relationship between the COMT Val158Met polymorphism and clinical diagnosis in families from the Vulnerability to Bipolar Disorders Study (VIBES; Frangou, Reference Frangou2009) where more than one member had an affective disorder. Detecting the effect of modifier genes is difficult in human studies because environmental factors cannot be standardized and because participants differ in many genes in addition to the gene of interest. By restricting the study to individuals that share a genetic predisposition to BD we might be able to reduce genetic variability relevant to the influence of the COMT polymorphism on brain processes. Apart from patients with BD, a significant proportion of relatives from the VIBES sample have MDD or anxiety disorders. This allowed us to investigate whether the role of the COMT genotype on neural networks relating to affective processing is consistent across a number of clinical diagnoses characterized by affective disturbance. Additionally, the sample includes psychiatrically healthy relatives and unrelated healthy controls that could inform on clinically silent effects of the genotype on neural circuitry.
We chose a sad affect categorization task as a probe for two reasons. First, emerging evidence suggests a relative specificity for abnormal sad facial affect processing in mood disorders (Keedwell et al. Reference Keedwell, Drapier, Surguladze, Giampietro, Brammer and Phillips2009). Second, the task engages a widespread neural network believed to underlie emotional processing (Adolphs, Reference Adolphs2002; Phan et al. Reference Phan, Wager, Taylor and Liberzon2002; Murphy et al. Reference Murphy, Nimmo-Smith and Lawrence2003). This network involves a number of anatomically and functionally interconnected regions primarily encompassing the cingulate cortex and the dorsal and ventral PFC, the amygdala and insula (Amaral & Price, Reference Amaral and Price1984; LeDoux, Reference LeDoux2000; Dolan, Reference Dolan2007; Fairhall & Ishai, Reference Fairhall and Ishai2007). In this study we were particularly interested in the amygdala and in the ventromedial and ventrolateral PFC based on three lines of inquiry: (a) previously published data of abnormal engagement of these regions during facial affect processing in patients with affective morbidity (Domschke et al. Reference Domschke, Ohrmann, Braun, Suslow, Bauer, Hohoff, Kersting, Engelien, Arolt, Heindel, Deckert and Kugel2008; Foland et al. Reference Foland, Altshuler, Bookheimer, Eisenberger, Townsend and Thompson2008; Lee et al. Reference Lee, Seok, Lee, Cho, Yoon, Lee, Chae, Choi and Ham2008); (b) evidence that activity within these regions during negative emotional processing is modulated by the COMT Val158Met polymorphism (Kempton et al. Reference Kempton, Haldane, Jogia, Christodoulou, Powell, Collier, Williams and Frangou2009; Mier et al. Reference Mier, Kirsch and Meyer-Lindenberg2009); and (c) the explicit processing of facial affect during categorization tasks consistently engages ventral, and particularly ventrolateral, PFC regions which are thought to represent the neural basis of modulatory control of emotional experiences (Hariri et al. Reference Hariri, Bookheimer and Mazziotta2000; Foland et al. Reference Foland, Altshuler, Bookheimer, Eisenberger, Townsend and Thompson2008). Additionally, our regions of interest (ROI) within the amygdala and the PFC overlap with those implicated in current models for mood disorders that emphasize reduced inhibitory control by the PFC of the amygdala and associated subcortical regions during emotional processing (Haldane & Frangou, Reference Haldane and Frangou2006).
Therefore we tested the hypotheses that during the processing of sad facial stimuli: (a) the Met158 allele will be associated with ventral PFC inefficiency evidenced by enhanced activation in these regions in Met158 carriers; and (b) this effect would be amplified in participants with affective morbidity.
Method
Participants
Patients were identified by clinicians' referrals and were included if they: (a) were aged between 17 and 65 years; (b) fulfilled DSM-IV-R criteria (APA, 1994) for BD, type I; (c) had at least one first-degree relative unaffected by BD; and (d) no family history (up to second degree) of schizophrenia or schizophrenia spectrum disorders. Their relatives were invited to participate, with the patients' consent, if aged between 17 and 65 years and without a personal history of bipolar spectrum disorders.
Healthy volunteers were recruited through advertisement in the local press and were enrolled if they were: (a) aged between 17 and 65 years and (b) had no personal or family history of any Axis-I or -II DSM-IV disorder. Healthy volunteers were selected so that they matched both patients and relatives in gender and level of education. Level of education was rated on a 5-point scale ranging from 1 (no educational qualification) to 5 (postgraduate university level qualifications).
Exclusion criteria for the entire sample (patients, relatives and controls) included: (a) head trauma resulting in loss of consciousness; (b) personal history of neurological or medical disorders; (c) family history of hereditary neurological disorders; and (d) fulfilling DSM-IV criteria for lifetime drug or alcohol dependence and drug or alcohol abuse in the preceding 6 months.
All participants were enrolled in the VIBES (Frangou, Reference Frangou2009; Kempton et al. Reference Kempton, Haldane, Jogia, Christodoulou, Powell, Collier, Williams and Frangou2009; Walterfang et al. Reference Walterfang, Wood, Barton, Velakoulis, Chen, Pantelis, Reutens, Kempton, Haldane and Frangou2009). The study was approved by the Joint South London and Maudsley and the Institute of Psychiatry NHS Research Ethics Committee and written informed consent was obtained from all participants.
Clinical assessment
All participants were interviewed personally by trained psychiatrists, who were initially blind to diagnostic but not family status (BD family member or unrelated control), using the Structured Clinical Interview for DSM-IV (SCID) for Axis I (patient and non-patient version) (First et al. Reference First, Spitzer, Gibbon and Williams2002a, Reference First, Spitzer, Gibbon and Williamsb) and the SCID-II Personality Questionnaire for Axis II diagnoses (First et al. Reference First, Spitzer, Gibbon and Williams1997). Inter-rater reliability was κ >0.91 for all instruments. Where applicable, further information about age of onset, number and polarity of previous episodes, number of hospital admissions and current medication (type, dose and duration) was collected from medical notes. Family history of psychiatric disorders was assessed using the Family Interview for Genetic Studies (Maxwell, Reference Maxwell1992) supplemented by medical notes as necessary. All participants were rated using the Hamilton Depression Rating Scale (HAMD; Hamilton, Reference Hamilton1960), the Young Mania Rating Scale (YMRS; Young et al. Reference Young, Biggs, Zieglar and Meyer1978) and the Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, Reference Overall and Gorham1962). Prior to scanning, all participants with an Axis I disorder were assessed weekly over a minimum period of 1 month to ensure that at each evaluation they (a) scored <7 in the HAMD and YMRS and (b) there had been no change to their medication status. For all participants an estimate of current full-scale IQ was obtained on the day of scanning using the Wechsler Adult Intelligence Scale – Revised (Wechsler, Reference Wechsler1981).
A total of 92 BD patients from an equal number of families were screened by telephone interview for eligibility by a trained psychiatrist. Families of 53 BD patients were enrolled in the study; these yielded 47 BD patients and 75 first-degree relatives. The sample included in this analysis consisted of 40 BD type I patients, 25 healthy relatives, 22 relatives with another Axis I diagnosis (15 with MDD and seven with an anxiety disorder) and 50 healthy controls. Details are shown in Fig. 1.

Fig. 1. Vulnerability to Bipolar Disorders Study (VIBES) sample: identification and recruitment of bipolar disorder (BD) patients and relatives.
DNA extraction and genotyping
DNA was obtained from buccal swabs using established procedures (Freeman et al. Reference Freeman, Smith, Curtis, Huckett, Mill and Craig2003). The COMT Met158Val (rs4680) genotype was determined by a TaqMan genotyping assay (Assay ID C_25746809_50; Applied Biosystems, USA) using the manufacturer's instructions. Endpoint analysis was performed on an ABI7900 DNA analyser and genotypes called with the sds package (ABI, USA) with a probability >95%. To improve genotyping reliability sufficient additional samples of similar DNA quality and concentration were used to fill a complete plate for genotyping (382 samples and two controls). Allele frequencies in unrelated individuals did not violate the Hardy–Weinberg principle (χ2=0.40, df=1, p=0.51). In total, 38 individuals (11 BD patients, seven healthy relatives, five relatives with an Axis I diagnosis and 15 controls) were Val158 homozygotes, 65 (18 BD patients, 11 healthy relatives, 15 relatives with an Axis I diagnosis and 21 controls) were Met158Val heterozygotes and 35 (11 BD patients, seven healthy relatives, three relatives with an Axis I diagnosis and 14 controls) were Met158 homozygotes.
Neuroimaging
Sad facial affect discrimination task
This comprised a 5-min event-related task. Ten different facial identities (six female, four male; http://www.paulekman.com) depicting either 150% intensity of a sad expression or a neutral expression were presented in a pseudo-random order interspersed with a fixation cross. The 150% level of sadness intensity was chosen to minimize ambiguity and uncertainty about the nature of the stimuli (Phillips et al. Reference Phillips, Young, Senior, Brammer, Andrew, Calder, Bullmore, Perrett, Rowland, Williams, Gray and David1997; Calder et al. Reference Calder, Young, Rowland and Perrett1997). Each image was displayed for 2 s and the interstimulus interval followed a Poisson distribution and was varied between 3 s and 9 s (mean interval 5 s). In all, 60 images were displayed; the fixation cross, faces with neutral expression, and faces expressing sadness were each presented 20 times. Participants were instructed to indicate if the facial affect displayed was sad or neutral by pressing the right or left button respectively on an MRI-compatible response box. No response was required when subjects viewed the fixation cross. Response time and accuracy data were collected.
Image acquisition
Gradient echo planar MR images were acquired using a 1.5 T GE Sigma MR system (General Electric, USA) fitted with 40 mT/m highspeed gradients. Foam padding and a forehead strap were used to limit head motion, and a quadrature birdcage head coil was used for radio frequency transmission and reception. In each of the 16 non-contiguous planes parallel to the inter-commissural [anterior commissure–posterior commissure (AC–PC)] plane, T2*-weighted MR images depicting blood-oxygenation level-dependent (BOLD) contrast were acquired [repetition time (TR)=2000 ms, echo time (TE)=40 ms, flip angle=70°, slice thickness=7 mm, slice skip=0.7 mm, matrix size 64×64, voxel dimensions 3.75×3.75×7.7 mm]. During the facial affect discrimination task 150 images were acquired. During the same session, a T1-weighted three-dimensional inversion recovery prepared spoiled gradient recalled acquisition sequence was used to acquire structural images in 124 axial planes [TR=18 ms, TE=5.1 ms, inversion time (TI)=450 ms, flip angle=20°, slice thickness=1.5 mm, matrix size 256×192, field of view 240×180 mm, voxel dimensions 0.9375×0.9375×1.5 mm, number of excitations=1] for subsequent Talairach mapping (Talairach & Tourneaux, Reference Talairach and Tourneaux1988).
Image analysis
Statistical analysis of the fMRI data was performed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm) analysis software, in a Matlab (version 7.01, The Mathworks Inc., USA) environment. Images were qualitatively assessed using the display function. Images were realigned to the first image in the dataset to correct for movement and normalized into Montreal Neurological Institute (MNI) space using the participant's structural MRI image. The transformed dataset for each subject was smoothed with an isotropic Gaussian filter (full width half maximum=8 mm) to compensate for normal variation in structural and functional anatomy across subjects. Images demonstrating acquisition or anatomical abnormalities were excluded, as were individual scans showing translation >4 mm or rotation >4°. On this basis, six BD patients were excluded.
First-level analysis
Vectors of onset representing the correctly identified sad faces and correctly identified neutral faces were convolved with the haemodynamic response function, global signal changes were removed and a high-pass filter (128 s) was applied to remove low-frequency artefacts for each subject. In the design matrix we also modelled the fixation cross, incorrect responses, and subject button response, although contrasts comparing these conditions were not used in the second-level analysis. An explicit mask was used to ensure that only voxels within the brain were included in the analysis. We entered six movement parameters as nuisance covariates and contrast images of brain activations associated with correct recognition of sad faces compared with neutral faces were produced for each participant.
Second-level analysis
For the second-level analysis, a random-effects model was implemented. Weighted parameter estimates were extracted from contrast images produced at the first level using MarsBaR (Brett et al. Reference Brett, Anton, Valabregue and Poline2002) from a priori ROIs. The selection of ROIs was based on known anatomical and functional connectivity of the amygdala and the ventral PFC (Ramnani & Owen, Reference Ramnani and Owen2004; Dolan, Reference Dolan2007) and on the effect of the COMT genotype in these regions during emotional processing tasks (Mier et al. Reference Mier, Kirsch and Meyer-Lindenberg2009). ROIs were defined bilaterally in the amygdala, the ventromedial PFC [Brodmann area (BA) 11] and ventrolateral PFC (BA 47) using WFU PickAtlas (Maldjian et al. Reference Maldjian, Laurienti, Kraft and Burdette2003). To examine the effect of affection status and genotype on activations in each region, an analysis of covariance was performed in SPSS version 15.0 (SPSS Inc., USA). Affection status (BD, relatives with MDD and anxiety disorders, healthy relatives, and healthy controls) and COMT genotype were entered as factors with age, BPRS scores and reaction time as covariates. Significant main effects or interactions were further examined using post-hoc, Bonferroni-corrected, pairwise comparisons. Only findings below p<0.01 are reported.
Although we had data on HAMD and YMRS scores from the entire sample, these scales are not validated in non-clinical populations. Since all three scales were highly correlated (r>0.733, p<0.0001) we chose the BPRS as a covariate as it allows for meaningful comparisons between clinical and non-clinical groups.
We used t tests to compare mean weighted parameter estimates from each of the ROIs between unmedicated and medicated BD patients in order to explore the possible contribution of medication to our findings.
Results
Detailed information on sample characteristics and behavioural performance is shown in Table 1. All participants were of self-reported white British ancestry.
Table 1. Demographic, clinical and behavioural data

BD, Bipolar disorder; IQ, intelligence quotient; BPRS, Brief Psychiatric Rating Scale; HAMD, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale; GAF, Global Assessment of Functioning.
Values are given as mean (standard deviation).
Mean value was significantly greater than those of all the other groups
* p<0.04, ** p<0.003 (no other group differences).
a Mean value was significantly lower than those of all the other groups (p<0.02).
b Mean value was significantly greater than that of the relatives with an Axis I diagnosis (p<0.006).
Behavioural data
Group differences were identified in reaction time [F(3, 138)=3.39, p=0.02]; BD patients had longer mean reaction time compared with controls and healthy relatives (both p<0.04). There was no effect of genotype [F(2, 138)=0.44, p=0.64] nor an interaction between group and genotype [F(6, 138)=1.08, p=0.37] in reaction time. There was no effect of group [F(3, 138)=2.76, p=0.06] or genotype [F(2, 138)=1.33, p=0.26] in accuracy; their interaction was also not significant [F(6, 138)=2.03, p=0.07].
Functional imaging
There was no difference in brain activation between medicated and unmedicated BD patients in any of the ROIs examined (p>0.65) and therefore medication status was not considered in further analyses.
There was a significant effect of genotype on amygdala activation [left: F(2, 138)=5.76, p=0.04; right: F(2, 138)=2.73, p=0.07], with Val158 homozygotes having the highest percentage BOLD signal change and Met158 homozygotes having the lowest on both sides. There was no effect of group (both p>0.13) or genotype×group interaction (both p>0.29). Similarly, none of the covariates was significant (all p>0.39).
There was a significant effect of genotype on ventromedial PFC (BA 11) activation [left: F(2, 138)=3.07, p=0.05; right: F(2, 138)=6.71, p=0.002], with Met homozygotes having higher percentage BOLD signal change on both sides compared with Val carriers, but no effect of group (both p>0.10) or genotype×group interaction (both p>0.15). Likewise, none of the covariates showed a significant effect (all p>0.36).
In the ventrolateral PFC, a significant effect of group [F(3, 138)=3.76, p=0.01] and a group×genotype interaction [F(6, 138)=2.21, p=0.01] was observed on the right (Fig. 2). There was no significant effect of genotype (p>0.9) or of group (p>0.9), or of group×genotype interaction (p>0.37) on left BA 47. In participants with an Axis I diagnosis (BD, MDD or anxiety disorders) the Met158 allele was associated with the greatest and the Val158 allele with the lowest activation (p=0.03). The effect size of the difference compared with controls was greater for relatives with MDD and anxiety disorder (Cohen's d=1.5) than for BD patients (d=0.49) and healthy relatives (d=22). It was not possible to compare the effect of the COMT genotype between relatives with MDD and those with anxiety disorders because the number of subjects in each group was too small.
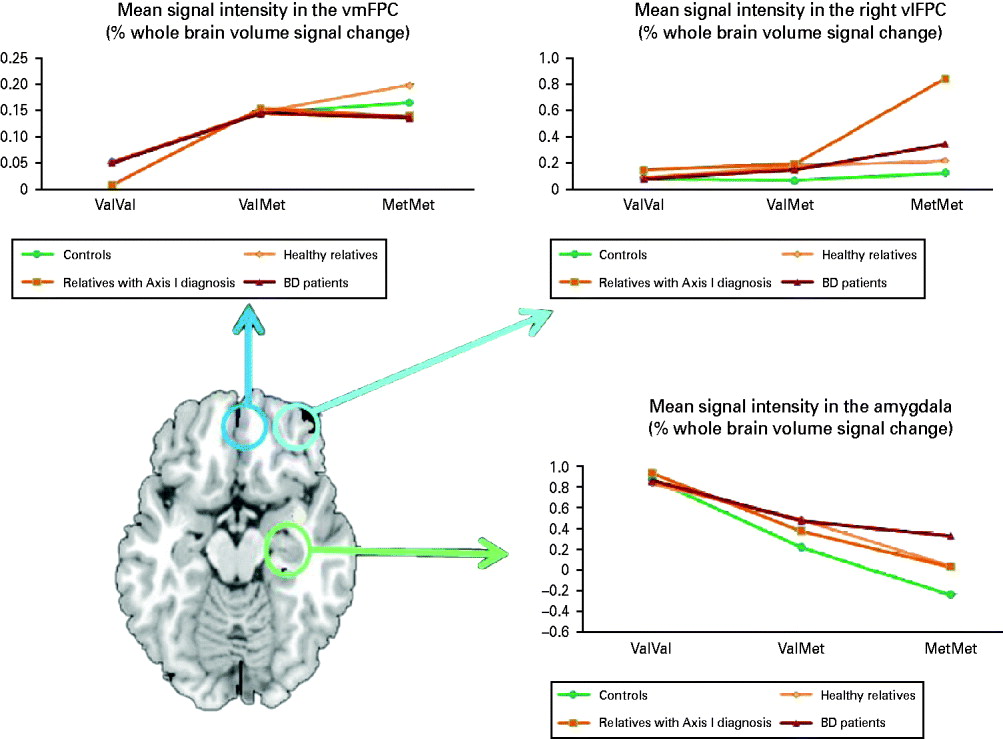
Fig. 2. Effect of catechol-O-methyltransferase (COMT) Val158Met genotype on brain activation in bipolar disorder (BD) patients and their relatives. Axial section at axial z=−11 showing the regions of interest examined: ventromedial prefrontal cortex (vmPFC) (blue), ventrolateral prefrontal cortex (vlPFC) (turquoise) and the amygdala (green). Graphs show the mean weighted estimates of neural responses in regions of interest defined using WFU PickAtlas extracted using MarsBaR: (i) bilateral vmPFC, (ii) right vlPFC and (iii) bilateral amygdala, by affection status and COMT Val158Met genotype.
Discussion
We found a pleiotropic effect of COMT Val158Met genotype on PFC and amygdala activation during sad facial affect processing. Specifically we found an effect of genotype but not clinical diagnosis or their interaction in the amygdala and ventromedial PFC in BA 11. The Val158 allele was associated with greater amygdala activation while in the ventromedial PFC signal change was greater for the Met158 allele. In the right ventrolateral PFC the Met158 allele was not only associated with increased signal change but also with disease expression.
According to the model proposed by Bilder et al. (Reference Bilder, Volavka, Lachman and Grace2004), DA regulation within limbic regions depends on the interplay between high-amplitude ‘phasic’ DA release in response to salient environmental stimuli and low-level, ‘tonic’ DA concentration regulated by PFC afferents. DA catabolism in the amygdala is mainly mediated by the DA transporter (Revay et al. Reference Revay, Vaughan, Grant and Kuhar1996), as the COMT concentration in this region is very low (Hong et al. Reference Hong, Shu-Leong, Tao and Lap-Ping1998). Our results are in complete agreement with this model since lower tonic DA concentration in the amygdala, associated with the high-activity COMT Val158 allele, is predicted to enhance the amplitude of phasic DA release (Rosenkranz & Grace, Reference Rosenkranz and Grace1999). Our results also confirm the previously reported (Mier et al. Reference Mier, Kirsch and Meyer-Lindenberg2009) association between the Met158 allele and a greater (i.e. more inefficient) activation in the ventral PFC.
Ventromedial PFC activation has repeatedly been found during externally triggered or internally generated sadness (George et al. Reference George, Ketter, Parekh, Horwitz, Herscovitch and Post1995; Lane et al. Reference Lane, Reiman, Ahern, Schwartz and Davidson1997; Reiman et al. Reference Reiman, Lane, Ahern, Schwartz, Davidson, Friston, Yun and Chen1997; Damasio et al. Reference Damasio, Grabowski, Bechara, Damasio, Ponto, Parvizi and Hichwa2000) and during imitation of sad faces (Lee et al. Reference Lee, Seok, Lee, Cho, Yoon, Lee, Chae, Choi and Ham2008). In healthy subjects, medial PFC activation has been associated with the appraisal of emotions (Lane et al. Reference Lane, Reiman, Ahern, Schwartz and Davidson1997; Teasdale et al. Reference Teasdale, Howard, Cox, Ha, Brammer, Williams and Checkley1999) and with self-referential evaluation (Kircher et al. Reference Kircher, Senior, Phillips, Benson, Bullmore, Brammer, Simmons, Williams, Bartels and David2000; Kelley et al. Reference Kelley, Macrae, Wyland, Caglar, Inati and Heatherton2002). In this context, the Met158 allele is likely to decrease cortical efficiency in the processing and appraising of sad affect relative to the Val158 allele although our data suggest that this association is not related to disease expression.
In the ventrolateral PFC (BA 47), the Met158 allele was associated with greater activation in BD patients and their relatives with MDD and anxiety disorders. It is also noteworthy that the effect size of the impact of the COMT Val158Met genotype was greater in those individuals with MDD and anxiety disorders than in those with BD. Abnormal ventrolateral PFC engagement has been reported in BD, MDD and anxiety disorders across a number of paradigms involving elements of inhibitory control (Blumberg et al. Reference Blumberg, Leung, Skudlarski, Lacadie, Fredericks, Harris, Charney, Gore, Krystal and Peterson2003; Altshuler et al. Reference Altshuler, Bookheimer, Townsend, Proenza, Eisenberger, Sabb, Mintz and Cohen2005; McClure et al. Reference McClure, Monk, Nelson, Parrish, Adler, Blair, Fromm, Charney, Leibenluft, Ernst and Pine2007; Foland et al. Reference Foland, Altshuler, Bookheimer, Eisenberger, Townsend and Thompson2008). It is generally accepted that the ventrolateral PFC regulates stimulus-driven action by modulating the influence of emotional stimuli on cognition with respect to appropriate behaviour (Quirk & Beer, Reference Quirk and Beer2006). Our results suggest that the Met158 allele reduces inhibitory capacity by decreasing cortical efficiency in the ventrolateral PFC and that this effect is associated with disease expression.
Although medication effects cannot be excluded they are unlikely to have contributed to our findings. Similarly, differences in brain activation cannot be attributed to underlying structural volumetric group differences as we have already shown in a previous report from the VIBES sample (Kempton et al. Reference Kempton, Haldane, Jogia, Christodoulou, Powell, Collier, Williams and Frangou2009). Although an effect of the COMT genotype on brain structure has been reported in some studies (Honea et al. Reference Honea, Verchinski, Pezawas, Kolachana, Callicott, Mattay, Weinberger and Meyer-Lindenberg2009), this is likely to be small (Dutt et al. Reference Dutt, McDonald, Dempster, Prata, Shaikh, Williams, Schulze, Marshall, Walshe, Allin, Collier, Murray and Bramon2009).
In summary, our study supports the notion that the COMT Val158Met polymorphism is relevant to mood disorders where increased limbic activation and reduced PFC inhibitory control are considered key pathophysiological features (Mayberg, Reference Mayberg1997; Phillips, Reference Phillips2003). Our results are consistent with previous literature suggesting that the Met158 allele may confer increased risk of affective morbidity but also underscore the pleiotropic effect of the polymorphism in different brain regions during emotional processing. Additionally, our findings highlight the importance for developing strategies to elucidate the gene interactions that contribute to variation in penetrance, severity and subtype of disorders of affective dysregulation.
Acknowledgements
We thank the patients and their families for their support and Dr Tessa Christodoulou, Dr Andrea Galea and Dr Morgan Haldane for their help in data collection and preliminary processing. The authors acknowledge financial support from the National Institute for Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health award to the South London and Maudsley NHS Foundation Trust and the Institute of Psychiatry, King's College London. M.J.K. was also supported by a Wellcome Trust Value in People Fellowship.
Declaration of Interest
None.





