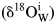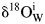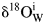During dynamic evolutions of the Earth, the interplays between the hydrosphere and lithosphere can considerably impact the mass and/or heat transfers, ore deposit formations, environmental changes, nuclear waste storage and/or disposal, life origin and/or extinction and much more (cf., extensive summaries therein by Henley et al. Reference Henley, Truesdell, Barton and Whitney1984; Walther & Wood Reference Walther and Wood1986; Hochella & White Reference Hochella and White1990; Spencer & Chou Reference Spencer and Chou1990; Hellmann & Wood Reference Hellmann and Wood2002; Oelkers & Schott Reference Oelkers and Schott2009). Since the water–rock interaction is essentially triggered by natural heat engines, hydrothermal processes accompanying magmatism and/or metamorphism are more realistic for thermodynamic and kinetic considerations. Previous studies have documented the magmatism and/or metamorphism driven water–rock interactions worldwide (Taylor Reference Taylor1977; Taylor & O'Neil Reference Taylor and O'Neil1977; Nabelek et al. Reference Nabelek, Labotka, O'Neil and Papike1984; Wickham & Taylor Reference Wickham and Taylor1985, Reference Wickham and Taylor1987; Valley et al. Reference Valley, Taylor and O'Neil1986; Taylor et al. Reference Taylor, O'Neil and Kaplan1991; Wickham et al. Reference Wickham, Peters, Fricke and O'Neil1993; Valley & Cole Reference Valley and Cole2001; Holk et al. Reference Holk, Taylor and Gromet2006; Holk & Taylor Reference Holk and Taylor2007). Because 18O/16O and 2H/1H ratios of meteoric water are usually far from equilibrium with most of common igneous rocks, a small influx of meteoric water could remarkably and/or effectively lower the oxygen and hydrogen isotopes of igneous rocks within continental settings. In this regard, much more efforts have been concentrated on the external infiltration of meteoric water with the continental igneous rock during past decades (Taylor Reference Taylor1971; Turi & Taylor Reference Turi and Taylor1971; Taylor & Forester Reference Taylor and Forester1979; Criss & Taylor Reference Criss and Taylor1986; Holt & Taylor Reference Holt and Taylor1998).
While diverse water–rock interactions were characterised through stable isotopes, there still exist uncertainties such as (1) forward modelling, which presumes initial isotopic compositions of water and rock as well as re-equilibration temperature, has been widely applied, but inverse modelling is less conducted; (2) the disequilibrium of oxygen isotopes between susceptible and resistant accessary minerals has been well recognised but the achievement of partial re-equilibration among constituent minerals is less constrained; (3) igneous rock externally infiltrated by meteoric water is well emphasised but external infiltration of other sourced water with metamorphic rock is less concerned.
It is well known that there are major sources of natural water with distinctive origin (i.e., meteoric, oceanic, magmatic and metamorphic water, Sheppard Reference Sheppard1981, Reference Sheppard1986) that could potentially interact with various rocks. As summarised in Table 1, a large span of water–rock interactions could occur with different combinations between water and rock. Among them, the interactions between meteoric water and igneous rock as well as magmatic water and metamorphic rock are certainly affiliated to the inventories of external water infiltration.
Table 1 Internal (I) vs. external (E) water interaction with rock

1 For terrestrial sedimentary rock, infiltration by late meteoric water is referred to as either internal or external interaction with some uncertainties. However, infiltration of meteoric water with early marine sedimentary rock is definitely external. Infiltration of a late seawater with terrestrial sedimentary rock is certainly external whereas its infiltration with early marine sedimentary rock is either internal or external.
2 These infiltrations of external water with rock are theoretically inverted in this study.
3 Tectonically, the continent–continent collision between the North and South China Blocks for the Dabie orogen was Triassic; there was no seawater thereafter to infiltrate with the high-temperature continental rocks.
4 Due to an over 100 Ma gap between the Triassic metamorphism and the early Cretaceous post-collisional magmatism in the Dabie orogen, it is geochronologically less likely that the early metamorphic water was externally infiltrated with late igneous rocks.
Compared with the sedimentary processes dominated by basinal settings, magmatism and/or metamorphism are important geological activities and ubiquitously developed within continental orogens all over the world. While continental deep subduction and collision can be petrogenetically archived by magmatic and metamorphic rocks through source tracing, age dating and temperature calculating, the late fluid evolution of continental orogen was not sufficiently constrained up to now. Being one of the integrated components of continental orogen, the externally infiltrated fossil hydrothermal system accompanying magmatism and/or metamorphism could fill this gap and advance our understanding of the thermal history of continental orogen. Therefore, the early Cretaceous post-collisional granitoids and intruded Triassic gneissic country rocks across the Dabie orogen in central-eastern China were studied as natural examples because some of them were evidently infiltrated by external water and partially re-equilibrated for oxygen isotopes among constituent minerals.
1. Geological settings and their geochemical characters
The Dabie-Sulu orogen is characterised by the largest occurrence of microdiamond- and/or coesite-bearing ultrahigh pressure (UHP) metamorphic rocks in the world (Wang et al. Reference Wang, Liou and Mao1989; Xu et al. Reference Xu, Okay, Ji, Sengor, Su, Liu and Jiang1992; Ye et al. Reference Ye, Cong and Ye2000; Zheng et al. Reference Zheng, Fu, Gong and Li2003; Zheng Reference Zheng2012). Triassic ages of 200 to 240 Ma were dated with different kinds of geochronometer for the eclogite-facies rocks and their cooling histories during exhumed processes were accordingly constrained (Li et al. Reference Li, Xiao, Liu, Chen, Ge, Zhang, Sun, Cong, Zhang, Hart and Wang1993, Reference Li, Jagoutz, Chen and Li2000; Ames et al. Reference Ames, Zhou and Xiong1996; Rowley et al. Reference Rowley, Xue, Tucker, Peng, Baker and Davis1997; Hacker et al. Reference Hacker, Ratschbacher, Webb, Ireland, Walker and Dong1998, Reference Hacker, Ratschbacher, Webb, McWilliams, Ireland, Calvert, Dong, Wenk and Chateigner2000; Liu et al. Reference Liu, Jian, Kroner and Xu2006; Cheng et al. Reference Cheng, Zhang, Vervoort, Wu, Zheng, Zheng and Zhou2011). Furthermore, the world record ultrahigh ɛNd(t) value up to +264 was measured for eclogites (Jahn et al. Reference Jahn, Conichet, Cong and Yui1996) and zircons with the lowest ever reported δ18O values down to about −11 ‰ were found (Rumble et al. Reference Rumble, Giorgis, Oreland, Zhang, Xu, Yui, Yang, Xu and Liou2002; Zheng et al. Reference Zheng, Wu, Chen, Gong, Li and Zhao2004; Fu et al. Reference Fu, Kita, Wilde, Liu, Cliff and Greig2013).
In contrast to the sporadically outcropped lenses or blocks of UHP eclogites, composite plutons and batholiths of the early Cretaceous post-collisional granitoid are the predominant igneous rocks, although a number of coeval small mafic to ultramafic plutons have been documented in the Dabie orogen (Xue et al. Reference Xue, Rowley, Tucker and Peng1997; Ma et al. Reference Ma, Li, Ehlers, Yang and Wang1998; Jahn et al. Reference Jahn, Wu, Lo and Tsai1999; Zhang et al. Reference Zhang, Gao, Zhong, Zhang, Zhang and Hu2002; Bryant et al. Reference Bryant, Ayers, Gao, Miller and Zhang2004; Zhao et al. Reference Zhao, Zheng, Wei and Wu2004, Reference Zhao, Zheng, Wei, Wu, Chen and Jahn2005, Reference Zhao, Zheng, Wei and Wu2007, Reference Zhao, Zheng, Wei and Wu2011; Xu et al. Reference Xu, Zhao, Zheng and Wei2005; Wang et al. Reference Wang, Wyman, Xu, Jian, Zhao, Li, Xu, Ma and He2007; Xu et al. Reference Xu, Ma, Zhang and Ye2012; He et al. Reference He, Li, Hoefs and Kleinhanns2013; Deng et al. Reference Deng, Yang, Polat, Kusky and Wu2014; Dai et al. Reference Dai, Zhao and Zheng2015). The early Cretaceous ages ranging from 125 to 135 Ma were dated for most of the plutons and batholiths using zircon U-Pb techniques. The upper intercept ages of Neoproterozoic for available zircon U-Pb dating indicated their affinities with the South China Block. Zircon Hf and whole-rock Nd-Sr isotopes suggested an old enriched source(s) was petrogenetically linked to the early Cretaceous post-collisional igneous rocks.
2. Sampling and analysing
The studied granitoids and their gneissic country rocks mainly occupy the northern and central-eastern lithotectonic units of the Dabie Block (DBB). From north to south, the Hepeng pluton (HP) outcrops in the utmost eastern tip of the Northern Huaiyang volcanic-sedimentary belt, whereas the Tianzhushan pluton (TZS) is adjacent to the Central Dabie UHP metamorphic belt (Fig. 1). The intrusive contact between granitoids and gneissic country rocks was unambiguously observed in the field. For comparison, two gneisses from the Sidaohe without being intruded by granitoids in the Hong'an Block were also studied.

Figure 1 Geological sketch of the Dabie orogen modified from Ma et al. (Reference Ma, Li, Ehlers, Yang and Wang1998), Zhang et al. (Reference Zhang, Gao, Zhong, Zhang, Zhang and Hu2002) and Ernst et al. (Reference Ernst, Tsujimori, Zhang and Liou2007). In terms of field relation and lithological assemblage, geological units bounded with faults were divided. Traditionally, the western portion beyond the Shang-Ma fault was termed Hong'an (or Xinxian) Block. The DBB was bounded by the Shang-Ma fault in the west and the Tan-Lu fault in the east. From north to south, the DBB was further subdivided into five belts: I. the Northern Huaiyang volcanic-sedimentary belt (flysch series); II. the Northern Dabie ultrahigh temperature gneissic and migmatitic belt; III. the Central Dabie UHP metamorphic belt; IV. the Southern-central Dabie high pressure metamorphic belt; and V. the Southern Dabie intermediate- to low-grade metamorphic belt. Bold italic capital letters denote the abbreviations of studied plutons, other details refer to supplementary Table 1. WSF = Wuhe-Shuihou fault; HMF = Hualiangting-Mituo fault; TMF = Taihua-Mamiao fault, respectively.
Fresh medium-grained granitoids and gneisses were collected from quarries and/or along road cuttings. Petrographically, quartz, alkali feldspar, biotite and sometimes amphibole are major rock-forming minerals, and accessory minerals include zircon and magnetite.
Conventional crushing, gravimetric, heavy liquid and magnetic techniques were applied to separate and concentrate zircon, quartz and alkali feldspar from whole rocks. In order to avoid metamict zircons and other impurities, the separated zircons were sequentially treated with concentrated HCl, HNO3 and HF acids under room conditions overnight. The purity of treated mineral separates is generally better than 98 % with optical microscope examination.
Oxygen isotopes were analysed with laser fluorination online techniques (Valley et al. Reference Valley, Kitchen, Kohn, Niendorf and Spicuzza1995; Wei et al. Reference Wei, Zhao and Spicuzza2008), and an airlock chamber was employed to avoid the ‘cross-talk’ of reactive alkali feldspar (Spicuzza et al. Reference Spicuzza, Valley and McConnell1998). The conventional δ18O notation in per mil (‰) relative to Vienna Standard Mean Ocean Water (VSMOW) is reported in supplementary Table 1 available at https://doi.org/10.1017/S1755691021000244.
In order to control the quality of δ18O analysis, the garnet standard, UWG-2, was routinely analysed. For 15 analytical days over 3 months, the daily average of measured δ18O values of UWG-2 varied from 5.54 to 5.89 ‰, and the daily analytical precision is better than ±0.11 ‰ (one standard deviation, 1 SD). Raw δ18O values of mineral separates were accordingly corrected in terms of the accepted UWG-2 value of 5.80 ‰. The international standard, NBS 28 quartz, was analysed and the corrected δ18O values for NBS 28 are from 9.31 to 9.69 ‰ during the course of this study.
The reproducibility of fresh crystalline zircon δ18O analysis is excellent throughout this study. As shown in supplementary Table 1, the 1 SD of most duplicate measurements with one triplicate is less than ±0.10 ‰, which is within the maximum routine analytical uncertainties demonstrated by daily UWG-2 garnet standard measurements.
3. Results
As shown in supplementary Table 1 and Fig. 2, zircon δ18O values of the gneisses scatter from −3.78 to 0.33 ‰, whereas zircon values of granitoids cluster around 5.16 ± 0.38 ‰ (1 SD, n = 25) and somehow overlap with mantle zircon. This suggests that the granitoids with uniform zircon δ18O values cannot petrogenetically link to those heterogeneous gneisses, which are almost threefold larger than zircon δ18O variability of the granitoids (4.11 vs. 1.42 ‰).
Some alkali feldspar δ18O values of the granitoids, however, steeply shift down to low values and evidently depart from isotherms (Fig. 2a), indicating infiltration by low δ18O water under post-magmatic conditions. While magmatic equilibrium fractionations are well preserved by most of the available zircon and quartz oxygen isotopes for the granitoids (Fig. 2b), further checks show that quartz oxygen isotopes were somehow lowered for sample 01HP05 from the Hepeng pluton and a disequilibrium with zircon accordingly occurred.

Figure 2 Diagrams of zircon vs. alkali feldspar (a) and quartz δ18O values (b) for the granitoids and gneisses from Dabie orogen. Lines labelled with temperatures are isotherms after Zheng (Reference Zheng1993), and two vertical lines in (b) denote the mantle zircon δ18O value for comparison (Valley et al. Reference Valley, Kinny, Schulze and Spicuzza1998). Arrowed lines denote the disequilibrated oxygen isotopes theoretically inverted in this study. The observed and initial δ18O values of constituent minerals refer to supplementary Tables 1 and 2, and the error bar is smaller than the symbol size throughout this study.
In contrast, the alkali feldspar and quartz oxygen isotopes were concurrently elevated for a gneissic country rock from the Tianzhushan pluton (labelled sample 01TZS05 in Fig. 2). This suggests that they were infiltrated by high δ18O water, and disequilibria with zircon were therefore resulted in. Another gneissic country rock from the same pluton, however, still retained equilibrium oxygen isotope fractionation between zircon and quartz (Fig. 2b), and accordingly yielded about 600°C of equilibrium temperature (Teq). Because this temperature is within the ranges from 585 to 655°C for the two gneisses which did not subsequently experience water disturbance from the Sidaohe, it is thus of geological significance and is applied to constrain the initial quartz and alkali feldspar δ18O values in the course of theoretical inversion of external water infiltration with gneissic country rock.
4. Discussion
Because the oxygen diffusivity of zircon is considerably slower than that of quartz and alkali feldspar within hydrothermal systems (Giletti et al. Reference Giletti, Semet and Yund1978; Giletti & Yund Reference Giletti and Yund1984; Fortier & Giletti Reference Fortier and Giletti1989; Watson & Cherniak Reference Watson and Cherniak1997; Zheng & Fu Reference Zheng and Fu1998), zircon is thus one of the most resistant accessory minerals to subsequent water disturbance and can maintain its original δ18O value well (Valley Reference Valley2003; Wei et al. Reference Wei, Zhao and Spicuzza2008). Disequilibria of oxygen isotopes therefore occur between the resistant and susceptible minerals during short-lived hydrothermal processes (Fig. 2), in good agreement with their kinetic behaviours. Since the discrepancy of oxygen exchange rates between quartz and alkali feldspar with water is less pronounced (Cole et al. Reference Cole, Ohmoto and Lasaga1983, Reference Cole, Ohmoto and Jacobs1992), re-equilibrations of rock-forming minerals were thus achieved for labelled samples during external water infiltrations (Figs 3–5). These make the following discussions possible and practicable.

Figure 3 The external infiltration of meteoric water with granitoid from the Hepeng pluton (sample 01HP05). (a) and (b) denote trajectories of alkali feldspar and quartz δ18O values along with W/R ratios, respectively, under an isothermal condition with varied ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values. (c) and (d) denote trajectories under a condition of constant
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values. (c) and (d) denote trajectories under a condition of constant ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value with varied temperatures. Symbol points connected by horizontal solid line illustrate the observed δ18O values of rock-forming minerals from supplementary Table 1, whereas their initial values refer to Table 2. Arrowed vertical lines denote W/R ratios required to reproduce the observed δ18O values, note that log10 scale of X axes is adopted for clarity.
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value with varied temperatures. Symbol points connected by horizontal solid line illustrate the observed δ18O values of rock-forming minerals from supplementary Table 1, whereas their initial values refer to Table 2. Arrowed vertical lines denote W/R ratios required to reproduce the observed δ18O values, note that log10 scale of X axes is adopted for clarity.

Figure 4 Diagrams of quartz vs. alkali feldspar δ18O values for the granitoid (a) and gneissic country rock (b). Dashed curves denote the trajectories of rock-forming mineral δ18O values infiltrated by the external meteoric or magmatic water theoretically inverted in Table 2, respectively, for open systems. Small ticks with numbers are (W/R)o ratios. The dotted curve in (b) demonstrates forward modelling with the internally buffered metamorphic water, calculated with the observed zircon δ18O value at 600°C for sample 01TZS05.

Figure 5 The relationship between temperature and ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of externally infiltrated water theoretically inverted from Dabie orogen in central-eastern China. Parameters refer to Table 2. Labelled solid and dashed curves in (a) and (b) denote
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of externally infiltrated water theoretically inverted from Dabie orogen in central-eastern China. Parameters refer to Table 2. Labelled solid and dashed curves in (a) and (b) denote ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values theoretically inverted for closed and open systems, respectively, along with magmatic or metamorphic temperatures varying from 500 to 850°C. The dependence of the
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values theoretically inverted for closed and open systems, respectively, along with magmatic or metamorphic temperatures varying from 500 to 850°C. The dependence of the ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value on re-equilibration temperature is illustrated in (c) and (d). Dashed curves are theoretically inverted with adjustment of quartz and alkali feldspar δ18O values to high or low ones for open systems. For other details see the text.
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value on re-equilibration temperature is illustrated in (c) and (d). Dashed curves are theoretically inverted with adjustment of quartz and alkali feldspar δ18O values to high or low ones for open systems. For other details see the text.
5. Forward and inverse modellings of the external infiltration of water
5.1. Non-unique constraint with the conventional forward modelling
The water–rock interaction was previously formulated for forward modelling with oxygen isotopes in terms of mass balance (Taylor Reference Taylor1977). For a closed system, the following relationship is satisfied:
 $$\left({\displaystyle{{\rm W} \over {\rm R}}} \right)_{\rm c}{\rm} = \left[{\displaystyle{{{\rm \delta }^{ 18}{\rm O}_{\rm R}^{\rm f} -{\rm \delta }^{ 18}{\rm O}_{\rm R}^{\rm i} } \over {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} -( {\rm \delta }^{ 18}{\rm O}_{\rm R}^{\rm f} -\Delta^{ 18}{\rm O}_{\rm W}^{\rm R} ) }}} \right]/\left[{\displaystyle{{{\rm n}_{\rm W}^{\rm O} } \over {{\rm n}_{\rm R}^{\rm O} }}} \right]\;$$
$$\left({\displaystyle{{\rm W} \over {\rm R}}} \right)_{\rm c}{\rm} = \left[{\displaystyle{{{\rm \delta }^{ 18}{\rm O}_{\rm R}^{\rm f} -{\rm \delta }^{ 18}{\rm O}_{\rm R}^{\rm i} } \over {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} -( {\rm \delta }^{ 18}{\rm O}_{\rm R}^{\rm f} -\Delta^{ 18}{\rm O}_{\rm W}^{\rm R} ) }}} \right]/\left[{\displaystyle{{{\rm n}_{\rm W}^{\rm O} } \over {{\rm n}_{\rm R}^{\rm O} }}} \right]\;$$And for an open-system, a simplified relationship is then held:
where W/R denotes the time-integrated water/rock ratio for a closed or open system. δ18O values with subscript W or R denote water and rock, and superscripts i and f denote initial and final oxygen isotopes of water or rock prior to and after water–rock interactions, respectively. ![]() $\Delta ^{18}{\rm O}_{\rm W}^{\rm R} $ denotes oxygen isotope fractionation between rock and water, and
$\Delta ^{18}{\rm O}_{\rm W}^{\rm R} $ denotes oxygen isotope fractionation between rock and water, and ![]() ${\rm n}_{\rm W}^{\rm O} /{\rm n}_{\rm R}^{\rm O} $ denotes the ratio of exchangeable oxygen content between water and rock.
${\rm n}_{\rm W}^{\rm O} /{\rm n}_{\rm R}^{\rm O} $ denotes the ratio of exchangeable oxygen content between water and rock.
Since most of the forward modellings for water–rock interaction were actually carried out for constituent minerals instead of whole rocks, the subscript R of δ18O values was accordingly replaced for an indicated mineral in Eqs 1 and 2. On the other hand, except for the observed final oxygen isotopes for a specified mineral (i.e., δ18Of value) and corresponding ![]() ${\rm n}_{\rm W}^{\rm O} /{\rm n}_{\rm R}^{\rm O} $ ratio, there are still three unknown variables to be solved. Among them, the δ18Oi values of constituent minerals prior to water–rock interaction can be confidently constrained in most cases. However, the
${\rm n}_{\rm W}^{\rm O} /{\rm n}_{\rm R}^{\rm O} $ ratio, there are still three unknown variables to be solved. Among them, the δ18Oi values of constituent minerals prior to water–rock interaction can be confidently constrained in most cases. However, the ![]() $ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ and Δ18O values (the latter is temperature dependent) are usually empirically assumed or estimated through a trial and error procedure during the forward modelling of water–rock interaction. In other words, the
$ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ and Δ18O values (the latter is temperature dependent) are usually empirically assumed or estimated through a trial and error procedure during the forward modelling of water–rock interaction. In other words, the ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ and Δ18O values were first assumed in order to fit the observed δ18Of value. When the observed δ18Of value was mathematically matched, the
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ and Δ18O values were first assumed in order to fit the observed δ18Of value. When the observed δ18Of value was mathematically matched, the ![]() $ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ and Δ18O values were then estimated. In principle, the two unknown variables of the
$ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ and Δ18O values were then estimated. In principle, the two unknown variables of the ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ and Δ18O values cannot be uniquely and/or simultaneously constrained with one observed δ18Of value by conventional forward modelling as shown below.
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ and Δ18O values cannot be uniquely and/or simultaneously constrained with one observed δ18Of value by conventional forward modelling as shown below.
Because alkali feldspar and quartz oxygen isotopes were lowered together and departed from isotherms for sample 01HP05 from the Hepeng granitoid pluton (Fig. 2), it could be externally infiltrated by low δ18O meteoric or oceanic water. Tectonically, the continent–continent collision between the North and South China Blocks for the Dabie orogen was Triassic (see section 1); there was no seawater thereafter to infiltrate with high-temperature continental rocks. Therefore, the concurrently lowered alkali feldspar and quartz oxygen isotopes and disequilibria with zircon observed for sample 01HP05 were ascribed to external infiltration by meteoric water, in good agreement with the following forward and inverse modellings.
Based on Eqs 1, 2 and parameters listed in Table 2, forward modelling is carried out to reproduce the observed δ18O values for sample 01HP05. Two end-member situations are considered, i.e., external infiltration by meteoric water under an isothermal and constant ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ conditions, respectively.
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ conditions, respectively.
Table 2 Parameters of inversion for the external water infiltration with rock-forming minerals

1 Calculated with the observed zircon δ18O value at 740°C, which is retrieved from the quartz-zircon pair of sample 01HP04 and assumed a similar Teq value on the pluton scale.
2 Re-equilibration temperature calculated with the observed δ18O values of the quartz–alkali feldspar pair.
3 Theoretically inverted with the observed δ18O values of quartz and alkali feldspar at corresponding re-equilibration temperature for an open system.
4 Calculated with observed zircon δ18O values at 600°C, which are retrieved from quartz–zircon pairs of samples 00DB63, 00DB64 and 01TZS07 and assumed a common thermal regime on the regional scale for gneissic country rocks.
5 Ratio of exchangeable oxygen content between water (89 % oxygen within H2O molecule) and mineral (53 % oxygen within SiO2 and 46 % oxygen within KAlSi3O8 molecules, respectively).
Under an isothermal condition of 140°C retrieved from the oxygen isotopes of a quartz–alkali feldspar pair for sample 01HP05 (Table 2), the upper limit of ![]() $ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values is estimated to be about −10.40 ‰ from the observed alkali feldspar δ18O value for a closed system with a very high (W/R)c ratio (labelled light solid curve in Fig. 3a). With the decrease in
$ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values is estimated to be about −10.40 ‰ from the observed alkali feldspar δ18O value for a closed system with a very high (W/R)c ratio (labelled light solid curve in Fig. 3a). With the decrease in ![]() $ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ to an extreme value of −20 ‰ for mid-latitude meteoric water, the (W/R)o ratio is systematically decreased to 0.21 for an open system (arrowed vertical light dashed line in Fig. 3a). Similar results obtain from the observed quartz δ18O value although the upper limit of
$ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ to an extreme value of −20 ‰ for mid-latitude meteoric water, the (W/R)o ratio is systematically decreased to 0.21 for an open system (arrowed vertical light dashed line in Fig. 3a). Similar results obtain from the observed quartz δ18O value although the upper limit of ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values for meteoric water is estimated to be a slightly low value around −10.70 ‰ (labelled light solid curve in Fig. 3b), and the (W/R)o ratio decreases to 0.10 accompanied by a decrease in the
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values for meteoric water is estimated to be a slightly low value around −10.70 ‰ (labelled light solid curve in Fig. 3b), and the (W/R)o ratio decreases to 0.10 accompanied by a decrease in the ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of meteoric water (arrowed vertical light dashed line in Fig. 3b), respectively.
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of meteoric water (arrowed vertical light dashed line in Fig. 3b), respectively.
With the constant ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of −11.01 ‰ for meteoric water theoretically inverted from sample 01HP05 (Table 2), the lower limit of the re-equilibration temperature is estimated as ca.130°C from the observed alkali feldspar δ18O value for a closed system (labelled light solid curve in Fig. 3c). With the re-equilibration temperature approaching the magmatic temperature of 740°C as the upper limit, the (W/R)o ratio is systematically decreased to 0.16 for an open system (arrowed vertical light dashed line in Fig. 3c). Forward modelling with the observed quartz δ18O value also yields similar results. A slightly high lower limit of re-equilibration temperature is estimated as about 135°C for a closed system (labelled light solid curve in Fig. 3d), whereas the (W/R)o ratio is systematically decreased to an extremely low value of around 0.06 for an open system along with an increase in re-equilibration temperature (arrowed vertical light dashed line in Fig. 3d).
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of −11.01 ‰ for meteoric water theoretically inverted from sample 01HP05 (Table 2), the lower limit of the re-equilibration temperature is estimated as ca.130°C from the observed alkali feldspar δ18O value for a closed system (labelled light solid curve in Fig. 3c). With the re-equilibration temperature approaching the magmatic temperature of 740°C as the upper limit, the (W/R)o ratio is systematically decreased to 0.16 for an open system (arrowed vertical light dashed line in Fig. 3c). Forward modelling with the observed quartz δ18O value also yields similar results. A slightly high lower limit of re-equilibration temperature is estimated as about 135°C for a closed system (labelled light solid curve in Fig. 3d), whereas the (W/R)o ratio is systematically decreased to an extremely low value of around 0.06 for an open system along with an increase in re-equilibration temperature (arrowed vertical light dashed line in Fig. 3d).
From the above discussions, it has been shown that forward modelling can only loosely estimate the upper limit of the ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value and lower limit of re-equilibration temperature for external infiltration of meteoric water. The observed δ18O values of different constituent minerals can be arbitrarily reproduced with noticeably varied
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value and lower limit of re-equilibration temperature for external infiltration of meteoric water. The observed δ18O values of different constituent minerals can be arbitrarily reproduced with noticeably varied ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values ranging from −20 to −10.40 ‰, re-equilibration temperatures from 130 to 740°C and W/R ratios from 0.06 up to 110 through forward modelling. In other words, a unique solution cannot be obtained by conventional forward modelling. On the contrary, the external infiltration of unique meteoric water is theoretically inverted from sample 01HP05 (heavy curves in Fig. 3 and dashed curve with ticks in Fig. 4a, respectively).
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values ranging from −20 to −10.40 ‰, re-equilibration temperatures from 130 to 740°C and W/R ratios from 0.06 up to 110 through forward modelling. In other words, a unique solution cannot be obtained by conventional forward modelling. On the contrary, the external infiltration of unique meteoric water is theoretically inverted from sample 01HP05 (heavy curves in Fig. 3 and dashed curve with ticks in Fig. 4a, respectively).
5.2. The unique constraint with theoretical inversion
For constituent minerals thermodynamically re-equilibrated with water, the ![]() $ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value and W/R ratio can be uniquely and robustly inverted (Wei & Zhao Reference Wei and Zhao2017). For a closed system, equations were derived for alkali feldspar (Ksp) and quartz (Qtz), respectively:
$ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value and W/R ratio can be uniquely and robustly inverted (Wei & Zhao Reference Wei and Zhao2017). For a closed system, equations were derived for alkali feldspar (Ksp) and quartz (Qtz), respectively:
 $${\rm \delta }^{ 18}{\rm O}_{{\rm Ksp}}^{\rm f} {\rm} = \displaystyle{{{\rm \delta }^{ 18}{\rm O}_{{\rm Ksp}}^{\rm i} {\rm} + [ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} + ( \Delta^{ 18}{\rm O}_{\rm W}^{{\rm Ksp}} ) _{\rm r}} ] \cdot {( {{\rm W}/{\rm R}} ) }_{\rm c}\cdot ( {{\rm n}_{\rm W}^{\rm O} /{\rm n}_{{\rm Ksp}}^{\rm O} } ) } \over { 1 + {( {{\rm W}/{\rm R}} ) }_{\rm c}\cdot ( {{\rm n}_{\rm W}^{\rm O} /{\rm n}_{{\rm Ksp}}^{\rm O} } ) }}$$
$${\rm \delta }^{ 18}{\rm O}_{{\rm Ksp}}^{\rm f} {\rm} = \displaystyle{{{\rm \delta }^{ 18}{\rm O}_{{\rm Ksp}}^{\rm i} {\rm} + [ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} + ( \Delta^{ 18}{\rm O}_{\rm W}^{{\rm Ksp}} ) _{\rm r}} ] \cdot {( {{\rm W}/{\rm R}} ) }_{\rm c}\cdot ( {{\rm n}_{\rm W}^{\rm O} /{\rm n}_{{\rm Ksp}}^{\rm O} } ) } \over { 1 + {( {{\rm W}/{\rm R}} ) }_{\rm c}\cdot ( {{\rm n}_{\rm W}^{\rm O} /{\rm n}_{{\rm Ksp}}^{\rm O} } ) }}$$ $${\rm \delta }^{ 18}{\rm O}_{{\rm Qtz}}^{\rm f} {\rm} = \displaystyle{{{\rm \delta }^{ 18}{\rm O}_{{\rm Qtz}}^{\rm i} {\rm} + [ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} + ( {\rm \Delta }^{ 18}{\rm O}_{\rm W}^{{\rm Qtz}} ) _{\rm r}} ] \cdot {( {{\rm W}/{\rm R}} ) }_{\rm c}\cdot ( {{\rm n}_{\rm W}^{\rm O} /{\rm n}_{{\rm Qtz}}^{\rm O} } ) } \over { 1 + {( {{\rm W}/{\rm R}} ) }_{\rm c}\cdot ( {{\rm n}_{\rm W}^{\rm O} /{\rm n}_{{\rm Qtz}}^{\rm O} } ) }}$$
$${\rm \delta }^{ 18}{\rm O}_{{\rm Qtz}}^{\rm f} {\rm} = \displaystyle{{{\rm \delta }^{ 18}{\rm O}_{{\rm Qtz}}^{\rm i} {\rm} + [ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} + ( {\rm \Delta }^{ 18}{\rm O}_{\rm W}^{{\rm Qtz}} ) _{\rm r}} ] \cdot {( {{\rm W}/{\rm R}} ) }_{\rm c}\cdot ( {{\rm n}_{\rm W}^{\rm O} /{\rm n}_{{\rm Qtz}}^{\rm O} } ) } \over { 1 + {( {{\rm W}/{\rm R}} ) }_{\rm c}\cdot ( {{\rm n}_{\rm W}^{\rm O} /{\rm n}_{{\rm Qtz}}^{\rm O} } ) }}$$and
Among all of the variables in Eqs 3–5, ![]() ${\rm \delta }^{ 18}{\rm O}_{{\rm Ksp}}^{\rm f} $ and
${\rm \delta }^{ 18}{\rm O}_{{\rm Ksp}}^{\rm f} $ and![]() ${\rm \;}{\rm \delta }^{ 18}{\rm O}_{{\rm Qtz}}^{\rm f} \;$are final values observed for a specified mineral;
${\rm \;}{\rm \delta }^{ 18}{\rm O}_{{\rm Qtz}}^{\rm f} \;$are final values observed for a specified mineral; ![]() ${\rm \delta }^{ 18}{\rm O}_{{\rm Ksp}}^{\rm i} $ and
${\rm \delta }^{ 18}{\rm O}_{{\rm Ksp}}^{\rm i} $ and![]() ${\rm \;}{\rm \delta }^{ 18}{\rm O}_{{\rm Qtz}}^{\rm i} \;$values can be calculated by Eq. 5 with the observed zircon (Zrc) δ18O value at magmatic or metamorphic temperature
${\rm \;}{\rm \delta }^{ 18}{\rm O}_{{\rm Qtz}}^{\rm i} \;$values can be calculated by Eq. 5 with the observed zircon (Zrc) δ18O value at magmatic or metamorphic temperature ![]() ${\rm ( i}.\;{\rm e}.{\rm , \;\;( }{\rm \Delta }^{ 18}{\rm O}_{{\rm Zrc}}^{{\rm Ksp}} ) _{\rm m}{\rm or}\;{\rm \;( }{\rm \Delta }^{ 18}{\rm O}_{{\rm Zrc}}^{{\rm Qtz}} ) _{\rm m}{\rm value) }$; and
${\rm ( i}.\;{\rm e}.{\rm , \;\;( }{\rm \Delta }^{ 18}{\rm O}_{{\rm Zrc}}^{{\rm Ksp}} ) _{\rm m}{\rm or}\;{\rm \;( }{\rm \Delta }^{ 18}{\rm O}_{{\rm Zrc}}^{{\rm Qtz}} ) _{\rm m}{\rm value) }$; and ![]() $( {\rm \Delta }^{ 18}{\rm O}_{\rm W}^{{\rm Ksp}} ) _{\rm r}\;$or
$( {\rm \Delta }^{ 18}{\rm O}_{\rm W}^{{\rm Ksp}} ) _{\rm r}\;$or ![]() $( {\rm \Delta }^{ 18}{\rm O}_{\rm W}^{{\rm Qtz}} ) _{\rm r}$value can be calculated with the re-equilibration temperature. Moreover,
$( {\rm \Delta }^{ 18}{\rm O}_{\rm W}^{{\rm Qtz}} ) _{\rm r}$value can be calculated with the re-equilibration temperature. Moreover, ![]() ${\rm n}_{\rm W}^{\rm O} /{\rm n}_{{\rm Ksp}}^{\rm O} {\rm and\ n}_{\rm W}^{\rm O} /{\rm n}_{{\rm Qtz}}^{\rm O} $ ratios are actually constants between water and alkali feldspar as well as quartz (last row in Table 2). In this case, both the
${\rm n}_{\rm W}^{\rm O} /{\rm n}_{{\rm Ksp}}^{\rm O} {\rm and\ n}_{\rm W}^{\rm O} /{\rm n}_{{\rm Qtz}}^{\rm O} $ ratios are actually constants between water and alkali feldspar as well as quartz (last row in Table 2). In this case, both the ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value and the (W/R)c ratio can thus be solved out by combining Eqs 3 and 4.
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value and the (W/R)c ratio can thus be solved out by combining Eqs 3 and 4.
In order to be self-consistent, theoretically calculated oxygen isotope fractionations are adopted throughout this study (Zheng Reference Zheng1993). Because the discrepancy between theoretical calculation and experimental calibration or empirical estimation is not notable for oxygen isotope fractionations of the studied constituent minerals, this will not significantly influence our results.
For an open-system, a similar inverse procedure can be applied. Owing to the natural logarithm (i.e., ln term in Eq. 2) or exponential function, an analytical expression cannot be obtained. Under this circumstance, a numerical reiteration with precision of at least 0.0001 is conducted to theoretically invert the ![]() $ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value and (W/R)o ratio.
$ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value and (W/R)o ratio.
5.2.1. Meteoric water with a low δ18OiW value theoretically inverted from granitoid
As shown in Table 2 and Figs 3, 4a, oxygen isotopes of quartz were re-equilibrated with alkali feldspar at 140°C for sample 01HP05 from the Hepeng granitoid pluton. In terms of Eqs 3–5, the ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value is theoretically inverted as −11.01 ‰ for an open system. It is certain that it was an externally infiltrated meteoric water.
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value is theoretically inverted as −11.01 ‰ for an open system. It is certain that it was an externally infiltrated meteoric water.
On the basis of parameters listed in Table 2, the lowered oxygen isotopes of rock-forming minerals are well reproduced (Figs 3, 4a). A minimum (W/R)o ratio of 1.10 is accordingly quantified. It is worth pointing out that a systematically high (W/R)c ratio is required if a closed system is adopted (arrowed heavy-solid vs. -dashed vertical lines in Fig. 3).
The external infiltration of cold meteoric water not only lowered the oxygen isotopes of the granitoid but also thermally enhanced its cooling. This is in good agreement with the low re-equilibration temperature of 140°C retrieved from sample 01HP05 in Table 2. Furthermore, this low re-equilibration temperature temporally implied that meteoric water was externally infiltrated during the post-magmatic stage and/or spatially localised within the upper portion of the granitoid pluton, which is geologically consistent with the high-level emplacement within the upper crust of the Northern Huaiyang volcanic-sedimentary belt (Fig. 1).
5.2.2. Magmatic water with a moderately high δ18OiW value theoretically inverted from gneissic country rock
For sample 01TZS05 from the gneissic country rock intruded by the Tianzhushan granitoid pluton, both alkali feldspar and quartz oxygen isotopes were evidently elevated and departed from the isotherms (Fig. 2). Hence, these observed disequilibria with zircon oxygen isotopes could be overprinted by high δ18O water.
Given that quartz oxygen isotopes were re-equilibrated with alkali feldspar at 340°C (Table 2; Fig. 4b), the ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value is theoretically inverted as 4.21 ‰ for an open system. The elevated oxygen isotopes of rock-forming minerals are satisfactorily reproduced (Fig. 4b, supplementary Fig. 1), and a minimum (W/R)o ratio of 1.23 is thus calculated.
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value is theoretically inverted as 4.21 ‰ for an open system. The elevated oxygen isotopes of rock-forming minerals are satisfactorily reproduced (Fig. 4b, supplementary Fig. 1), and a minimum (W/R)o ratio of 1.23 is thus calculated.
The ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of 4.21 ‰ somehow overlaps with that of either metamorphic or magmatic water (cf., Sheppard Reference Sheppard1986). Forward modelling with an internally buffered metamorphic water during the exhumed process, however, is too low to reproduce the observed δ18O values (dotted curve in Fig. 4b). Therefore, elevated oxygen isotopes of sample 01TZS05 were externally infiltrated by magmatic water. This is geochronologically consistent with the available zircon U-Pb dating result (Zhao et al. Reference Zhao, Zheng, Wei, Chen, Liu and Wu2008), that is, the studied Triassic gneissic country rock was subsequently superimposed by the early Cretaceous post-collisional magmatism of the adjacent Tianzhushan granitoid pluton. On the other hand, the moderately high
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of 4.21 ‰ somehow overlaps with that of either metamorphic or magmatic water (cf., Sheppard Reference Sheppard1986). Forward modelling with an internally buffered metamorphic water during the exhumed process, however, is too low to reproduce the observed δ18O values (dotted curve in Fig. 4b). Therefore, elevated oxygen isotopes of sample 01TZS05 were externally infiltrated by magmatic water. This is geochronologically consistent with the available zircon U-Pb dating result (Zhao et al. Reference Zhao, Zheng, Wei, Chen, Liu and Wu2008), that is, the studied Triassic gneissic country rock was subsequently superimposed by the early Cretaceous post-collisional magmatism of the adjacent Tianzhushan granitoid pluton. On the other hand, the moderately high ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of 4.21 ‰ could be the result of binary mixing between magmatic and meteoric water prior to external infiltration with gneissic country rock (Wei & Zhao Reference Wei and Zhao2017).
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of 4.21 ‰ could be the result of binary mixing between magmatic and meteoric water prior to external infiltration with gneissic country rock (Wei & Zhao Reference Wei and Zhao2017).
In contrast to external infiltration of cold meteoric water discussed in section 5.2.1., gneissic country rock was thermally re-equilibrated at 340°C with external infiltration of hot magmatic water for sample 01TZS05 (Table 2). This mildly warm re-equilibration temperature geologically suggests that the more deeply buried Triassic gneissic country rock was externally infiltrated by early Cretaceous magmatic water at least within the middle crustal settings, where the re-equilibration of oxygen isotopes between rock-forming minerals and magmatic water was kinetically facilitated (see section 7).
6. The reliability of  ${{\bf \delta }^{\bf 18}{\bf O}_{\bf W}^{\rm i} } $ values
${{\bf \delta }^{\bf 18}{\bf O}_{\bf W}^{\rm i} } $ values
In order to theoretically invert ![]() $ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $values, initial δ18O values of rock-forming minerals and re-equilibration temperature are prerequisites (Wei & Zhao Reference Wei and Zhao2017 and Eqs 3–5 in section 5.2). The initial δ18O values of rock-forming minerals prior to external water infiltration are calculated with the observed zircon oxygen isotopes at magmatic or metamorphic temperatures (Eq. 5), whereas the re-equilibration temperatures are calculated with the observed δ18O values of quartz–alkali feldspar pairs for corresponding samples (Table 2). In these aspects, the validity of
$ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $values, initial δ18O values of rock-forming minerals and re-equilibration temperature are prerequisites (Wei & Zhao Reference Wei and Zhao2017 and Eqs 3–5 in section 5.2). The initial δ18O values of rock-forming minerals prior to external water infiltration are calculated with the observed zircon oxygen isotopes at magmatic or metamorphic temperatures (Eq. 5), whereas the re-equilibration temperatures are calculated with the observed δ18O values of quartz–alkali feldspar pairs for corresponding samples (Table 2). In these aspects, the validity of ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values is evaluated as below.
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values is evaluated as below.
It can be seen that ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values of meteoric water theoretically inverted from the granitoid are gradually increased and converged when magmatic temperatures are increased from 500 to 850°C for sample 01HP05 (Fig. 5a). Moreover, subtle increase of
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values of meteoric water theoretically inverted from the granitoid are gradually increased and converged when magmatic temperatures are increased from 500 to 850°C for sample 01HP05 (Fig. 5a). Moreover, subtle increase of ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values is systematically inverted for the closed system all over the adopted temperature range (solid vs. dashed curve in Fig. 5a). The
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values is systematically inverted for the closed system all over the adopted temperature range (solid vs. dashed curve in Fig. 5a). The ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values of magmatic water theoretically inverted from the gneissic country rock, however, are steadily decreased with variation of metamorphic temperatures for sample 01TZS05 (Fig. 5b). Nevertheless, the variability of
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values of magmatic water theoretically inverted from the gneissic country rock, however, are steadily decreased with variation of metamorphic temperatures for sample 01TZS05 (Fig. 5b). Nevertheless, the variability of ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values is generally less than 0.28 ‰ along with the magmatic or metamorphic temperatures which varied for every 100°C. As the magmatic or metamorphic temperatures are well and tightly constrained (see Table 2 footnote), those temperatures adopted in this study cannot greatly influence the
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values is generally less than 0.28 ‰ along with the magmatic or metamorphic temperatures which varied for every 100°C. As the magmatic or metamorphic temperatures are well and tightly constrained (see Table 2 footnote), those temperatures adopted in this study cannot greatly influence the ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values theoretically inverted (Figs 5a, b) and their reliability is thus guaranteed.
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values theoretically inverted (Figs 5a, b) and their reliability is thus guaranteed.
Because of the susceptibility of rock-forming minerals (in particular feldspar) to subsequent water disturbance, the apparent temperature could be yielded in some cases. Adjusting the observed alkali feldspar and quartz δ18O values to high or low ones, hypothetical temperatures can be accordingly calculated. It can be seen that a large range of ![]() $ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values are theoretically inverted with hypothetical temperatures (labelled dashed curves in Figs 5c, d). A cross point, however, occurs for samples 01HP05 and 01TZS05. This means that both quartz and alkali feldspar oxygen isotopes were thermodynamically re-equilibrated with unique meteoric or magmatic water at the same temperature for each sample, which corresponded to the
$ {{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values are theoretically inverted with hypothetical temperatures (labelled dashed curves in Figs 5c, d). A cross point, however, occurs for samples 01HP05 and 01TZS05. This means that both quartz and alkali feldspar oxygen isotopes were thermodynamically re-equilibrated with unique meteoric or magmatic water at the same temperature for each sample, which corresponded to the ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values theoretically inverted with the observed alkali feldspar and quartz δ18O values (Table 2). In this regard, it suggests that true re-equilibration temperatures are retained and obtained for the studied samples and the
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values theoretically inverted with the observed alkali feldspar and quartz δ18O values (Table 2). In this regard, it suggests that true re-equilibration temperatures are retained and obtained for the studied samples and the ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values are therefore validated.
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values are therefore validated.
7. Kinetic considerations of oxygen exchange
As listed in Tables 2 and 3, the re-equilibration temperatures of the external infiltration of meteoric and magmatic water with rocks are overall less than 350°C. While the susceptible alkali feldspar from the gneissic country rock could re-equilibrate with the externally infiltrated hot magmatic water, an unreasonable timescale up to 450 Myr was required for quartz to diffusively exchange oxygen with water (arrowed line in supplementary Fig. 2). Given that re-equilibrations were achieved and/or reproduced between quartz and alkali feldspar oxygen isotopes for the studied samples (labelled data points in Figs 3–5), this probably suggests that surface reaction instead of volume diffusion actually controlled oxygen exchange during the post-magmatic stage and/or exhumed process of the retrograde metamorphism. Mechanisms such as dissolution, reprecipitation and exchange along micro-fractures and/or within networks were proposed to account for the variations of quartz and/or alkali feldspar δ18O values in the course of hydrothermal alterations (Cole et al. Reference Cole, Ohmoto and Lasaga1983, Reference Cole, Ohmoto and Jacobs1992; Matthews et al. Reference Matthews, Goldsmith and Clayton1983; Valley & Graham Reference Valley and Graham1996; King et al. Reference King, Barrie and Valley1997).
Table 3 Parameters of surface-reaction oxygen exchange for the infiltration of external water with rock-forming minerals

1 Re-equilibration temperature from Table 2.
2 (W/R)c ratio refers to Fig. 3, supplementary Fig. 1.
3 Mole fraction of mineral oxygen.
4 Mineral density from Anthony et al. (Reference Anthony, Bideaux, Bladh and Nichols2021).
5 Rate constant after Cole et al. (Reference Cole, Ohmoto and Lasaga1983, Reference Cole, Ohmoto and Jacobs1992).
6 Grain radius.
7 Time required to achieve 99 % oxygen exchange between mineral and water.
Based on parameters listed in Table 3, the time required to achieve 99 % oxygen exchange between mineral and water is calculated. As the original formulation was proposed for a closed system (Cole et al. Reference Cole, Ohmoto and Lasaga1983), the (W/R)c ratios are thus adopted in this study.
Owing to the slightly high re-equilibration temperature, a maximum timespan of about 12 Kyr is quantified for gneissic country rock externally infiltrated by hot magmatic water from the Tianzhushan granitoid pluton (italic bold number with underline in Table 3). An upper limit duration of ca.0.7 Myr, however, is constrained for the granitoid from the Hepeng pluton to achieve re-equilibration between quartz and alkali feldspar oxygen isotopes with externally infiltrated cold meteoric water. Compared with the short-lived magmatic hydrothermal system driven by the small Tianzhushan plutonism, the longer lifetime of the meteoric hydrothermal system could be sustained by the energetic budget of the Hepeng granitoid pluton of moderate size (Fig. 1).
8. Conclusions
Both forward and inverse modellings are conducted to constrain externally infiltrated fossil hydrothermal systems with oxygen isotopes in this study. Only the upper limit of ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values and lower limit of re-equilibration temperatures, however, can be loosely estimated by forward modelling for the meteoric hydrothermal system. In contrast, the
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values and lower limit of re-equilibration temperatures, however, can be loosely estimated by forward modelling for the meteoric hydrothermal system. In contrast, the ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values of externally infiltrated meteoric and magmatic water are uniquely inverted with oxygen isotopes of the re-equilibrated rock-forming minerals from the early Cretaceous post-collisional granitoid and intruded Triassic gneissic country rock across the Dabie orogen in central-eastern China, and their reliability is accordingly validated.
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ values of externally infiltrated meteoric and magmatic water are uniquely inverted with oxygen isotopes of the re-equilibrated rock-forming minerals from the early Cretaceous post-collisional granitoid and intruded Triassic gneissic country rock across the Dabie orogen in central-eastern China, and their reliability is accordingly validated.
A granitoid externally infiltrated by meteoric water with a low ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of −11.01 ‰ is theoretically inverted at 140°C, and the observed δ18O values of rock-forming minerals were thermodynamically re-equilibrated each other with a minimum (W/R)o ratio of 1.10. This meteoric hydrothermal system driven by the early Cretaceous post-collisional magmatism could be kinetically maintained up to 0.7 Myr. However, magmatic water with a moderately high
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of −11.01 ‰ is theoretically inverted at 140°C, and the observed δ18O values of rock-forming minerals were thermodynamically re-equilibrated each other with a minimum (W/R)o ratio of 1.10. This meteoric hydrothermal system driven by the early Cretaceous post-collisional magmatism could be kinetically maintained up to 0.7 Myr. However, magmatic water with a moderately high ![]() ${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of 4.21 ‰ was externally infiltrated with Triassic gneissic country rock at 340°C with a (W/R)o ratio of 1.23, and a duration no more than 12 Kyr was required to rapidly re-equilibrate between quartz and alkali feldspar oxygen isotopes for this magmatic hydrothermal system.
${{\rm \delta }^{ 18}{\rm O}_{\rm W}^{\rm i} } $ value of 4.21 ‰ was externally infiltrated with Triassic gneissic country rock at 340°C with a (W/R)o ratio of 1.23, and a duration no more than 12 Kyr was required to rapidly re-equilibrate between quartz and alkali feldspar oxygen isotopes for this magmatic hydrothermal system.
Thus, externally infiltrated fossil hydrothermal systems driven by magmatism or metamorphism can be theoretically inverted with oxygen isotopes, and their thermodynamic and kinetic processes can be quantified through the partial re-equilibration of constituent minerals. This study can also be applied beyond the Dabie orogen and strengthen our insights for late fluid evolution and thermal history of continental orogens from qualitative inference to quantitative inversion.
9. Acknowledgements
Yong-Fei Zheng is thanked for initiating this study. John W. Valley and Michael J. Spicuzza are acknowledged for hosting and assisting analytical aspects of this work during the senior author's sabbatical visit. This study was funded by the National Natural Science Foundation of China (40173008, 40033010 and 41888101), the Chinese Academy of Sciences (KZCX2-107 and XDB41000000) and the China Scholarship Council of Ministry of Education (20G05006). Thanks are due to two anonymous reviewers for their constructive comments; Chris Soulsby and Susie Cox are appreciated for their editorial handling; the senior author is responsible for any errors if present.
10. Supplementary material
Supplementary material is available online at https://doi.org/10.1017/S1755691021000244