Introduction
Exposure to trauma during childhood is believed to be a major risk factor for lifelong psychiatric disorders, including schizophrenia, depression, post-traumatic stress disorder and eating disorders (EDs) (Dube et al. Reference Dube, Felitti, Dong, Giles and Anda2003; Chapman et al. Reference Chapman, Dube and Anda2007; Kessler et al. Reference Kessler, McLaughlin, Green, Gruber, Sampson, Zaslavsky, Aguilar-Gaxiola, Alhamzawi, Alonso, Angermeyer, Benjet, Bromet, Chatterji, de Girolamo, Demyttenaere, Fayyad, Florescu, Gal, Gureje, Haro, Hu, Karam, Kawakami, Lee, Lépine, Ormel, Posada-Villa, Sagar, Tsang, Ustün, Vassilev, Viana and Williams2010). The hypothalamic-pituitary adrenal (HPA) axis, via the secretion of cortisol, modulates endogenous responses to stressors, so its dysregulation has been proposed as one of the main mechanism underlying the early trauma-related risk for psychiatric disorders across the life span. Indeed, childhood life stress has been linked to both HPA axis hyper- and hypo-activity in the adulthood (Heim et al. Reference Heim, Newport, Mletzko, Miller and Nemeroff2008; MacMillan et al. Reference MacMillan, Georgiades, Duku, Shea, Steiner, Niec, Tanaka, Gensey, Spree, Vella, Walsh, De Bellis, Van der Meulen, Boyle and Schmidt2009; Peckins et al. Reference Peckins, Dockray, Eckenrode, Heaton and Susman2012). The mechanisms through which early life trauma can have such persistent detrimental effects are still poor understood, although epigenetic alterations have been suggested to be involved (Houtepen et al. Reference Houtepen, Vinkers, Carrillo-Roa, Hiemstra, van Lier, Meeus, Branje, Heim, Nemeroff, Mill, Schalkwyk, Creyghton, Kahn, Joëls, Binder and Boks2016).
It has been proposed that the opposite directions of HPA axis dysregulation observed across studies may depend upon the time passed since the trauma exposure with recent trauma exposure underlying an enhanced HPA axis activity and distant trauma exposure accounting for HPA axis hypoactivity (Steudte et al. Reference Steudte, Kolassa, Stalder, Pfeiffer, Kirschbaum and Elbert2011). However, different types of childhood trauma may also be responsible for different patterns of HPA axis dysregulation. In fact, Kuhlman et al. (Reference Kuhlman, Geiss, Vargas and Lopez-Duran2015) found heterogeneous relationships between different childhood trauma types and adolescent HPA axis functioning while cortisol reactivity to laboratory stress was enhanced in school-aged youth exposed to traumatic events (Ivanov et al. Reference Ivanov, Yehuda, Greenblatt, Davidow, Makotkine, Alfi and Newcorn2011) but reduced in youth exposed to abuse and neglect (MacMillan et al. Reference MacMillan, Georgiades, Duku, Shea, Steiner, Niec, Tanaka, Gensey, Spree, Vella, Walsh, De Bellis, Van der Meulen, Boyle and Schmidt2009; Trickett et al. Reference Trickett, Gordis, Peckins and Susman2014). Finally, a dose-dependent effect of traumatic experiences on different measures of HPA axis activity has been documented by some although not all the authors, suggesting a more dysregulated HPA axis functioning with increasing traumatic load (Gustafsson et al. Reference Gustafsson, Anckarsäter, Lichtenstein, Nelson and Gustafsson2010; Steudte et al. Reference Steudte, Kolassa, Stalder, Pfeiffer, Kirschbaum and Elbert2011; Michels et al. Reference Michels, Sioen, Huybrechts, Bammann, Vanaelst, De Vriendt, Iacoviello, Konstabel, Ahrens and De Henauw2012; Schalinski et al. Reference Schalinski, Elbert, Steudte-Schmiedgen and Kirschbaum2015).
Anorexia nervosa (AN) and bulimia nervosa (BN) are psychiatric disorders characterized by aberrant eating behaviours leading to chronic malnutrition, which may be responsible for several physical complications with potentially serious health consequences. Patients with EDs show a dysregulation of the HPA axis activity, which has been reported to be increased or decreased in relation to several clinical variables such as the presence of malnutrition, the type of the ED, the history of childhood trauma exposure (Lo Sauro et al. Reference Lo Sauro, Ravaldi, Cabras, Faravelli and Ricca2008; Monteleone et al. Reference Monteleone, Monteleone, Serino, Scognamiglio, Di Genio and Maj2015, Reference Monteleone, Monteleone, Marciello, Pellegrino, Castellini and Maj2017; Föcker et al. Reference Föcker, Stalder, Kirschbaum, Albrecht, Adams, de Zwaan, Hebebrand, Peters and Albayrak2016). Moreover, childhood trauma experiences have been found to occur more frequently in ED patients than in matched healthy controls (Caslini et al. Reference Caslini, Bartoli, Crocamo, Dakanalis, Clerici and Carrà2016), and we recently demonstrated that symptomatic AN and BN patients with a positive history of childhood maltreatment exhibit an attenuated salivary cortisol awakening response (CAR) compared with age-matched symptomatic AN and BN patients without childhood maltreatment (Monteleone et al. Reference Monteleone, Monteleone, Serino, Scognamiglio, Di Genio and Maj2015). However, because of the relatively small sample size, we were not able to investigate whether the effects of childhood trauma on the CAR differed according to the different subtype of trauma experienced in the childhood and/or whether there was a dose-dependent effect of traumatic experience on that HPA axis measure. Therefore, in the present explorative study we aimed to assess the impact of different types of childhood trauma and the effects of concomitant exposure to different childhood traumas on the CAR in a large sample of symptomatic patients with AN or BN compared with healthy controls.
Methods and materials
Participants
Subjects consecutively admitted to the outpatient unit of the EDs Center of the Department of Psychiatry of the University of Naples SUN were recruited from September 2014 to June 2016 if meeting the following inclusion criteria: (a) current diagnosis of AN or BN, according to DSM-IV, confirmed by the Structured Clinical Interview for DSM-IV Disorders (SCID-I)–Patient Edition (First et al. Reference First, Spitzer, Gibbon and Williams1995); (b) female gender; (c) age ⩾18; (d) willingness to cooperate to the experimental procedures and to sign a written informed consent; (e) no psychopharmacological treatment during the preceding 6 weeks; (f) no history of neurological or medical diseases; (g) no current comorbid Axis I psychiatric disorders. Forty-four women with AN (15 of the binge-purging subtype and 29 of the restrictive subtype) (age: mean ± s.d. = 26.5 ± 8.1 years) and 36 women with BN (age: mean ± s.d. = 28.9 ± 8.7 years) were enrolled. All of them were studied before entering specific treatments.
Forty-one healthy women (age: mean ± s.d. = 27.3 ± 5.7 years), who were regularly menstruating and in good physical and mental health, as assessed by physical examination, routine medical interview and SCID-I non-patient edition (First et al. Reference First, Gibbon, Spitzer and Williams1996), were also recruited. Inclusion criteria for healthy controls were as reported for patients in (b), (c), (d), (e), (f) and (g). They were from the same catchment area of enrolled patients. Twelve AN women, 8 BN women and 15 healthy women had participated into our previous study (Monteleone et al. Reference Monteleone, Monteleone, Serino, Scognamiglio, Di Genio and Maj2015).
The study was approved by the ethics committee of the University of Naples SUN, and all participants gave their written consent after being fully informed of the nature and procedures of the study.
Menstruating women were tested within the seventh day following start of menses to ensure a plasma estrogen milieu as close as possible to that of non-menstruating AN women.
Procedure
Childhood trauma was assessed by means of the short form of the Childhood Trauma Questionnaire (CTQ) (Bernstein et al. Reference Bernstein, Stein, Newcomb, Walker, Pogge, Ahluvalia, Stokes, Handelsman, Medrano, Desmond and Zule2003), a 28-item questionnaire yielding five subscales, which evaluate five specific forms of childhood trauma: sexual abuse, physical abuse, physical neglect, emotional abuse, and emotional neglect. Subjects scoring higher than the threshold of at least one subscale were classified as ‘maltreated participants’ (Mal); those who scored below the thresholds for all five subscales were classified as ‘non-maltreated participants’ (noMal). The previously validated cut-offs of each subscale were: sexual abuse ⩾8, physical abuse ⩾8, physical neglect ⩾8, emotional abuse ⩾10, emotional neglect ⩾15 (Walker et al. Reference Walker, Gelfand, Katon, Koss, Von Korff, Bernstein and Russo1999).
For CAR assessment saliva samples were collected by each participant at home on a working day, immediately upon awakening and after 15, 30 and 60 min. Participants were instructed to refrain from eating, drinking (except water), smoking and brushing teeth before completing sampling. Saliva samples were collected by placing a roll of cotton in their mouths, slightly chewing on it until it became saturated, and then returning it to salivette tubes (Sarstedt; Rommelsdorft, Germany). These were stored in home freezers before being returned to the laboratory, where saliva was separated by centrifugation and stored at −20 °C until assayed for cortisol levels. Subjects had to report the time they went to bed and the time they woke up in the morning of sampling. As suggested by Stalder et al. (Reference Stalder, Kirschbaum, Kudielka, Adam, Pruessner, Wüst, Dockray, Smyth, Evans, Hellhammer, Miller, Wetherell, Lupien and Clow2016), in order to maximize the participants’ adherence to the research protocol, we motivated them in an initial face-to-face meeting where they not only were instructed about the sampling procedure but were also engaged with research goals. Moreover, we provided them with take-home written instructions and, whenever possible, we asked a relative to supervise the whole sampling procedure.
Saliva cortisol concentrations were determined by an enzyme immunoassay method, using a commercially available ELISA kit (Biochem Immunosystem, Milan, Italy); intra- and inter-assay coefficients of variation were less than 8% and 8.7%, respectively. As measures of the CAR, according to Fekedulegn et al. (Reference Fekedulegn, Andrew, Burchfiel, Violanti, Hartley, Charles and Miller2007), the cortisol area under the curve relative to the ground (AUCg) and the AUC with respect to the increase (AUCi) were calculated.
Statistics
All statistical analyses were carried out by using the BMDP statistical software package (Dixon, Reference Dixon1985). Group differences in saliva cortisol levels were tested by a two-way analysis of variance (ANOVA) with repeated measure, followed by the post hoc Tukey's test. One-way ANOVA was used to assess differences in hormonal, demographic, clinical and anthropometric variables among groups. In the whole ED group correlations between cortisol AUCg, AUCi and patients’ clinical or demographic variables were examined using the Pearson's correlation test followed by a stepwise multiple regression. As recommended for exploratory studies, we did not perform multiple test adjustments (Bender & Lange, Reference Bender and Lange2001); the significance level was set at p < 0.05.
Results
Clinical and demographic data
No significant differences in age emerged among the three groups (F 2,118 = 1.04, p = 0.3); instead, as expected, mean BMI values significantly differed among the groups (F 2,118 = 39.56, p < 0.00001) with AN patients showing a significantly lower mean BMI than healthy participants (16.6 ± 1.4 v. 21.2 ± 2.5 kg/m2, F 1,83 = 102.22, p < 0.00001).
None of the healthy women reported childhood trauma experiences. According to the CTQ cut-off scores, 24 AN (17 of the ANR and 7 of the ANBP subtype) and 22 BN patients were classified as Mal participants. Clinical characteristics of Mal and noMal AN and BN patients are shown in Table 1.
Table 1. Clinical characteristics of the patient sample
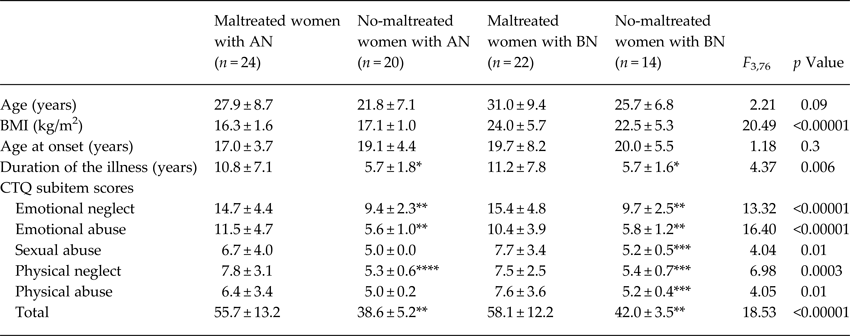
AN, anorexia nervosa; BN, bulimia nervosa; BMI, body mass index; CTQ, Childhood Trauma Questionnaire.
*p < 0.05 v. maltreated women with AN or BN, respectively; **p < 0.001 v. maltreated women with AN or BN, respectively; ***p < 0.05 v. maltreated women with BN; ****p < 0.005 v. maltreated women AN (Tukey's post-hoc test).
The whole patient sample was subdivided according to the number of childhood trauma types. A first group included 20 AN and 14 BN patients without trauma exposure; a second group included 10 AN and 6 BN patients with only one trauma type; a third group included 6 AN and 8 BN patients with two different trauma types; a fourth group included 5 AN and 5 BN patients with three different trauma types; and a fifth group included 3 AN and 3 BN patients with more than three different trauma types. No significant differences emerged among groups in mean BMI (F 4,75 = 0.85, p = 0.5) and mean age at onset of the disease (F 4,75 = 0.65, p = 0.6); instead, the five groups significantly differed in mean age (F 4,75 = 2.60, p = 0.04) and mean duration of the illness (F 4,75 = 5.68, p = 0.0005): ED patients with >3 trauma types were older (p < 0.025) than those without trauma exposure and showed a illness duration longer than those without trauma (p < 0.001) or those with two trauma types (p < 0.05); ED patients with one trauma type had a longer illness duration than those without trauma (p < 0.025).
The mean time of awakening and the mean duration of sleep the night before saliva sampling did not significantly differ between patients and controls and among patient groups.
CAR test
In a first analysis, Mal AN (n = 24), noMal AN (n = 20), Mal BN (n = 22) and noMal BN (n = 14) groups were compared with healthy participants. A five-group two-way ANOVA with repeated measures showed significant main effects for group (F 4,116 = 7.35, p < 0.0001) and time (F 3,348 = 57.18, p < 0.00001), and a significant group × time interaction (F 12,348 = 2.19, p = 0.01), indicating that saliva cortisol levels significantly changed after awakening and groups significantly differed in the magnitude and the timing of CAR. Indeed, noMal AN patients exhibited saliva cortisol levels after awakening significantly higher than healthy women and Mal AN participants (Fig. 1a ). NoMal BN patients, instead, exhibited a CAR not different from healthy participants, but significantly higher than that of Mal BN patients (Fig. 1a ). In this five-group comparison, the repeated-measure analysis of covariance controlling for BMI showed that this variable had no significant effects on CAR (F 1,115 = 0.01, p = 0.9).
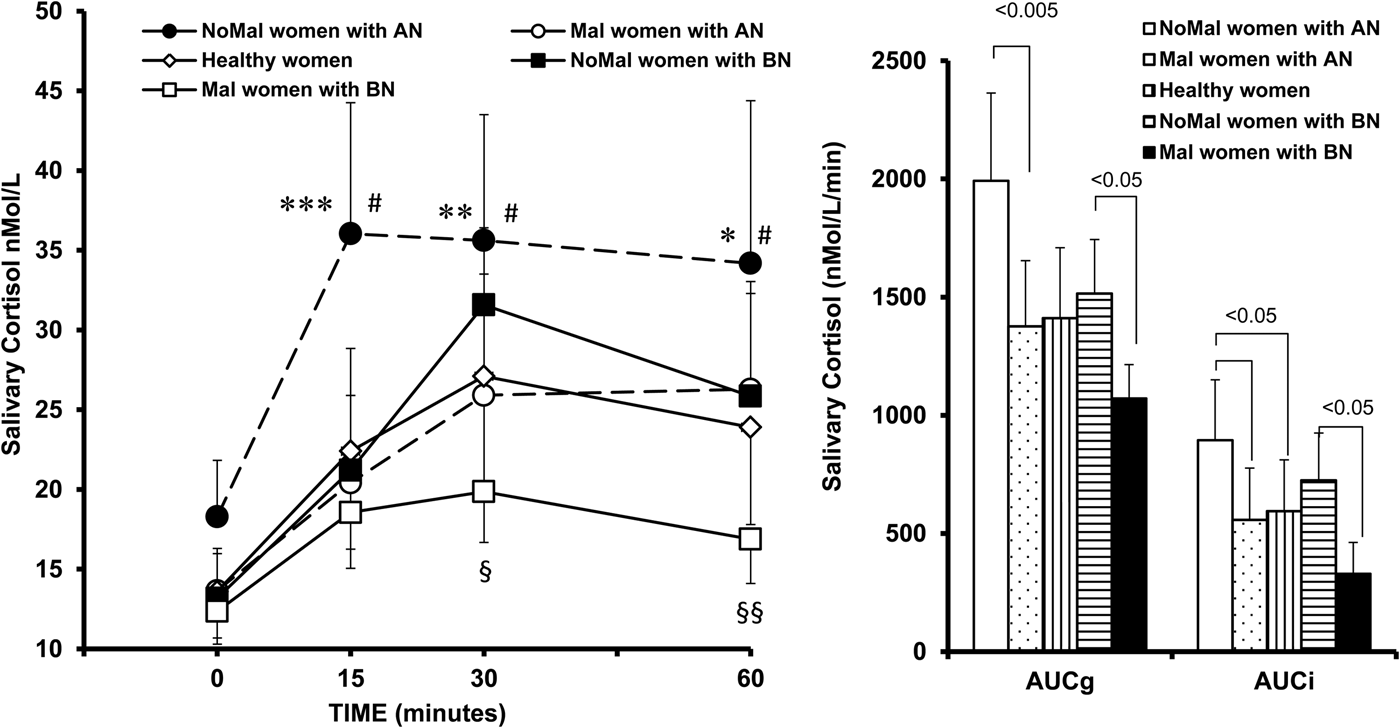
Fig. 1. Salivary cortisol awakening response (CAR) and areas under the curve with respect to the ground (AUCg) and to the increase (AUCi) in drug-free maltreated (Mal) and non-maltreated (NoMal) patients with anorexia nervosa (AN), bulimia nervosa (BN) and healthy controls. Data are expressed as mean ± SEM. # p < 0.001 v. correspondent time points of healthy women; *p < 0.05, **p < 0.005 and ***p < 0.001 v. correspondent time points of Mal AN women; § p < 0.005 and §§ p < 0.025 v. correspondent time points of noMal BN women (Tukey's post-hoc test).
Cortisol AUCg and AUCi significantly differed among the five groups (F 4,116 = 7.07, p < 0.0001 for AUCg and F 4,116 = 4.78, p = 0.001 for AUCi). Indeed, both cortisol AUCg and AUCi were significantly higher in noMal AN patients compared with Mal ones and healthy women, and in noMal BN participants compared with Mal BN ones (Fig. 1b ). After controlling for BMI, these differences remained significant (F 4,115 = 5.97, p = 0.0002 for AUCg and F 4,115 = 4.13, p = 0.003 for AUCi). Since Mal AN and Mal BN patients had a duration of illness significantly longer than respective noMal patients (Table 1), we conducted a 4-group ANOVA with illness duration as co-variate, and no significant effects of illness duration on the CAR AUCg (F 1,75 = 0.03, p = 0.8) and AUCi (F 1,75 = 1.11, p = 0.2) emerged.
In a second analysis, the CAR was evaluated in five ED subgroups clustered according to number of reported childhood trauma types (0, 1, 2, 3 and >3 trauma types). A five-group two-way ANOVA with repeated measures showed significant main effects for group (F 4,75 = 6.38, p = 0.0002) and time (F 3,225 = 18.73, p < 0.00001) but no significant group × time interaction (F 3,225 = 1.13, p = 0.3), indicating that the CAR significantly differed among the groups in the magnitude but not in the time pattern. Indeed, higher the number of trauma subtypes lower the CAR (Fig. 2a ). One-way ANOVA showed that cortisol AUCg and AUCi, significantly differed among the five groups (F 4,75 = 6.65, p = 0.0001 for AUCg and F 4,75 = 4.71, p = 0.001 for AUCi) (Fig. 2b ). Since the five ED groups significantly differed in both age and illness duration, these variables were introduced as covariates in the analysis. No significant effect on the CAR emerged for both age (F 1,74 = 0.01, p = 0.9 for AUCg and F 1,74 = 1.01, p = 0.3 for AUCi) and illness duration (F 1,74 = 0.03, p = 0.8 for AUCg and F 1,74 = 1.15, p = 0.2 for AUCi).
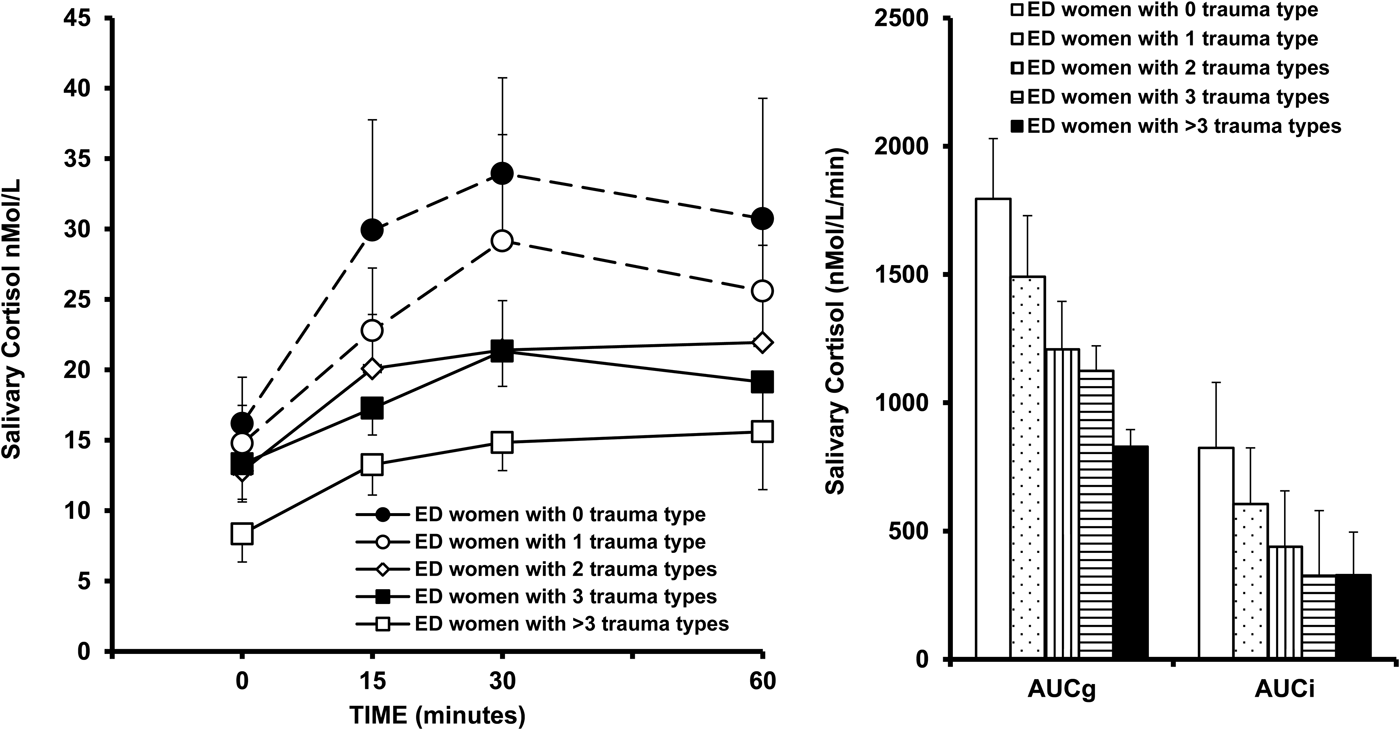
Fig. 2. Salivary cortisol awakening response (CAR) and areas under the curve with respect to the ground (AUCg) and to the increase (AUCi) in maltreated patients with eating disorders (ED) according to the different number of experienced childhood trauma types as assessed by Childhood Trauma Questionnaire. Data are expressed as mean ± s.e.m.
Correlation analyses
In the whole ED group both cortisol AUCg and AUCi resulted significantly and negatively correlated with all the CTQ subitem scores and the number of trauma types (Table 2 and Fig. 3); moreover, cortisol AUCg was negatively correlated with BMI while cortisol AUCi was negatively correlated with duration of illness (Table 2). A stepwise multiple regression analysis showed that the number of childhood trauma types was significantly associated with cortisol AUCg (F 1,78 = 26.77, p < 0.0001), explaining 25.5% of its variability (MR2 = 0.255). After removing this effect, BMI resulted still significantly associated with cortisol AUCg (F 2,77 = 17.34, p < 0.0001) and explained a further 5.5% of its variability (MR2 = 0.31). After removing this effect, no other variable was significantly associated with cortisol AUCg.
Table 2. Pearson's correlation coefficients between cortisol Area Under the Curve with respect to the ground (AUCg) or Area Under the Curve with respect to the increase (AUCi) and clinical variables or CTQ subitem scores in patients with eating disorders (n = 80)
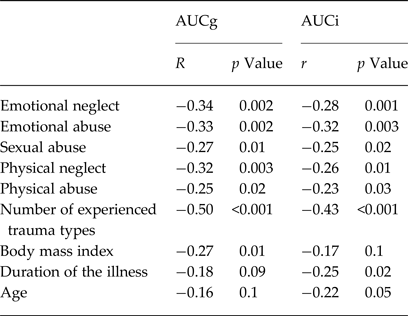
As for cortisol AUCi, the stepwise multiple regression showed that the number of childhood trauma types was significantly associated with cortisol AUCi (F 1,78 = 18.05, p < 0.001), explaining 18.7% of its variability (MR2 = 0.187). After removing this effect, no other variable was significantly associated to cortisol AUCi.
Discussion
In the present study the HPA axis activity, as measured by the CAR, was investigated in a relatively large sample of underweight patients with AN and symptomatic women with BN with respect to histories of childhood trauma exposure. In line with our previous studies (Monteleone et al. Reference Monteleone, Scognamiglio, Monteleone, Perillo and Maj2014; Monteleone et al. Reference Monteleone, Monteleone, Serino, Scognamiglio, Di Genio and Maj2015), we found that AN patients (but not BN ones) without childhood trauma exposure exhibited a CAR higher than that of healthy controls whereas AN and BN patients with childhood trauma history displayed a CAR lower than that of patients without childhood trauma exposure. These data confirm our previous suggestion that in patients with ED malnutrition (as documented by BMI) and childhood trauma exposure may have opposite effects on the HPA axis activity, at least as assessed by CAR (Monteleone et al. Reference Monteleone, Monteleone, Serino, Scognamiglio, Di Genio and Maj2015). This is in line with the widely acknowledged idea that malnutrition is the main cause of HPA axis dysfunctions in underweight patients with AN, although the direction of such dysfunctions is not consistent among the studies (Lo Sauro et al. Reference Lo Sauro, Ravaldi, Cabras, Faravelli and Ricca2008; Föcker et al. Reference Föcker, Stalder, Kirschbaum, Albrecht, Adams, de Zwaan, Hebebrand, Peters and Albayrak2016). Moreover, according to present results, a large body of literature shows that in both non-clinical and clinical populations childhood trauma exposure is significantly associated with adult HPA axis hyporeactivity (Bremner et al. Reference Bremner, Vythilingam, Anderson, Vermetten, McGlashan, Heninger, Rasmusson, Southwick and Charney2003; Meinlschmidt & Heim, Reference Meinlschmidt and Heim2005; Elzinga et al. Reference Elzinga, Roelofs, Tollenaar, Bakvis, van Pelt and Spinhoven2008; Mangold et al. Reference Mangold, Wand, Javors and Mintz2010) and other research groups also reported an impaired HPA axis activity in adult patients with AN and BN exposed to childhood trauma experiences (Steiger et al. Reference Steiger, Gauvin, Israël, Koerner, Ng Ying Kin, Paris and Young2001; Díaz-Marsá et al. Reference Díaz-Marsá, Carrasco, Basurte, Pastrana, Sáiz-Ruiz and López-Ibor2007).
The second and more innovative finding of our study was that in adult ED women significant negative associations emerged between the CAR and the CTQ subitem scores of emotional neglect or abuse, physical neglect or abuse and sexual abuse. However, those associations did not persist when the effect of the number of experienced trauma types was removed in the stepwise multiple regression analysis. Literature studies have reported various patterns of association between measures of HPA axis functioning and different types of childhood maltreatment such as emotional or physical neglect or abuse, sexual abuse or general traumatic experiences in both clinical and non-clinical adolescent or adult populations (Cicchetti & Rogosch, Reference Cicchetti and Rogosch2001; MacMillan et al. Reference MacMillan, Georgiades, Duku, Shea, Steiner, Niec, Tanaka, Gensey, Spree, Vella, Walsh, De Bellis, Van der Meulen, Boyle and Schmidt2009; Ivanov et al. Reference Ivanov, Yehuda, Greenblatt, Davidow, Makotkine, Alfi and Newcorn2011; Ouellet-Morin et al. Reference Ouellet-Morin, Odgers, Danese, Bowes, Shakoor, Papadopoulos, Caspi, Moffitt and Arseneault2011; Steudte et al. Reference Steudte, Kolassa, Stalder, Pfeiffer, Kirschbaum and Elbert2011; Kuhlman et al. Reference Kuhlman, Geiss, Vargas and Lopez-Duran2015), which support a connection between different HPA axis dysregulations and the type of childhood trauma. Our findings, instead, support the idea that in adults with ED the number of concomitant trauma types experienced in the childhood and not the type of trauma, at least as assessed by the CTQ, is the major determinant of the attenuated CAR, explaining 25.5% and 18.7% of variability of the CAR AUCg and AUCi, respectively. As a matter of fact, in our ED sample, the CAR progressively decreased as the number of experienced childhood trauma types rose, suggesting that childhood trauma exposure may result in a long-term dose-dependent effect on the CAR. This result parallels literature data showing negative correlations between the number of different lifetime traumatic events and hair cortisol concentrations (HCC) in patients with posttraumatic stress disorder as well as in traumatized healthy individuals (Steudte et al. Reference Steudte, Kirschbaum, Gao, Alexander, Schönfeld, Hoyer and Stalder2013; Steudte-Schmiedgen et al. Reference Steudte-Schmiedgen, Stalder, Schönfeld, Wittchen, Trautmann, Alexander, Miller and Kirschbaum2015). Similarly, lower saliva cortisol levels were found in a large sample of police officers and firefighters who experienced more negative life events in the past (>8 years earlier) (Witteveen et al. Reference Witteveen, Huizink, Slottje, Bramsen, Smid and van der Ploeg2010) and an attenuated salivary CAR was found in sheltered battered women who were exposed to a more chronic intimate partner violence as compared with women exposed to a less chronic abuse (Johnson et al. Reference Johnson, Delahanty and Pinna2008). All these data and our findings suggest a negative association between a lifetime traumatic load and CAR. In contrast, a reverse pattern of the relationship between the traumatic load and HCC was reported in severely traumatized Ugandan ex-combatants who showed higher levels of HCC with increasing traumatic load (Steudte et al. Reference Steudte, Kolassa, Stalder, Pfeiffer, Kirschbaum and Elbert2011). This discrepancy was explained by the time passed since the traumatic exposure as the Ugandan sample had been exposed more recently to traumatic stressors.

Fig. 3. Correlations between cortisol areas under the curve with respect to the ground (AUCg) and to the increase (AUCi) and number of experienced childhood trauma types in maltreated patients with eating disorders.
In the present study we found also that, in ED patients, BMI was negatively associated with the CAR, explaining 5.5% of the variability of the CAR AUCg but not of AUCi. According to Stalder et al. (Reference Stalder, Kirschbaum, Kudielka, Adam, Pruessner, Wüst, Dockray, Smyth, Evans, Hellhammer, Miller, Wetherell, Lupien and Clow2016), cortisol AUCg is a measure of the total hormone secretion at awakening, which is a sign of the HPA axis secretive capacity, while cortisol AUCi measures the pattern of change of the repeated measurements over time, reflecting the sensitivity of the HPA axis. Therefore, our data suggest that in ED patients malnutrition (at least as assed by BMI) may partially affect the overall cortisol output but not the sensitivity of the HPA axis to the phasic psychophysiological processes specific of the sleep-wake transition. As a matter of fact, we have repeatedly found that underweight AN patients but not normal-weight AN or BN women display an enhanced CAR (Monteleone et al. Reference Monteleone, Scognamiglio, Monteleone, Perillo and Maj2014; Monteleone et al. Reference Monteleone, Monteleone, Serino, Scognamiglio, Di Genio and Maj2015, Reference Monteleone, Monteleone, Serino, Amodio, Monaco and Maj2016, Reference Monteleone, Monteleone, Marciello, Pellegrino, Castellini and Maj2017).
A limitation of this study refers to the cortisol sampling procedure. Indeed, participants collected saliva samples at home and this could have not assured their full adherence to the protocol. However, we tried to minimize this drawback by motivating our participants to the sampling schedule, by providing them with take-home written instructions and, whenever possible, by asking a relative to supervise the whole sampling procedure. Moreover, we measured the CAR in a single work day, so state variations in day to day activities could have affected our results (Stalder et al. Reference Stalder, Kirschbaum, Kudielka, Adam, Pruessner, Wüst, Dockray, Smyth, Evans, Hellhammer, Miller, Wetherell, Lupien and Clow2016). We tried to control the influence of some state or trait variables, such as the duration of sleep the night before sampling, the time of awakening, the participants’ BMI, age and duration of the illness, by assessing possible differences among the groups and, when these emerged, by introducing the variable as a covariate in the statistical analyses. Third, to investigate the effects of the traumatic load on the CAR, we divided our patients according to the number of experienced trauma types and this resulted in subgroups not balanced in size. Indeed, especially the groups with higher number of childhood trauma types resulted relatively small and this may have partially influenced our results. Finally, present findings refers exclusively to trauma experienced in the childhood as assessed by the CTQ, so we cannot exclude that other types of traumas or traumas experienced later in life could have affected present findings. Moreover, the CTQ does not investigate the age of exposure to trauma, so we could not analyze potential correlates between time since the traumatic experience and CAR.
In conclusion, here we confirm that childhood trauma exposure impairs the CAR of adult patients with AN or BN and we report for the first time a negative dose-dependent effect of the traumatic load on this measure of the HPA axis activity. These findings, if confirmed in future studies, support the idea that childhood trauma experiences may have a role in the pathophysiology of EDs through their long-lasting influence on the functioning of the main endogenous stress response system and point to the need to implement such a knowledge in new pathophysiological models of EDs in order to develop more effective interventions for both the prevention and the treatment of these disorders.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Interest
None.







