Introduction
Major depressive disorder (MDD) is characterised by a variety of predominately negatively valenced emotional symptoms such as sadness, anhedonia, guilt hopelessness and worthlessness (Reference Rimes and Watkins1–Reference Grimm, Boesiger and Beck3). These affective symptoms are often accompanied by cognitive impairments in working memory, attention and problem solving Reference Levin, Heller and Mohanty(4).
It has been shown that cognitive and emotional processing are not separated but, in contrast, they strongly depend on each other Reference Damasio(5). Thus, it has to be assumed that these two aspects of MDD, the altered emotional processing and the cognitive impairments, are closely related to each other. In addition, Pessoa Reference Pessoa(6) suggested that emotion and cognition are often integrated and jointly contribute to behaviour. He argued that complex cognitive–emotional behaviours are related to common networks of brain areas that are neither specifically cognitive nor emotional. Moreover, functional imaging studies showed emotional–cognitive interaction using various experimental paradigms (Reference Baker, Frith and Dolan7–Reference Ochsner and Gross12). This interaction might reflect our ability to weight, integrate and reciprocally adjust emotional and cognitive demands within a task Reference Northoff, Heinzel and Bermpohl(9,Reference Bartolic, Basso and Schefft13). Disturbances of this interaction might account for co-occurrence of certain emotional and cognitive symptoms in psychiatric disorders such as major depression Reference Northoff, Heinzel and Bermpohl(9).
Functional imaging studies during emotional stimulation and resting state in MDD showed abnormally high signal intensities in the ventral prefrontal cortex (VPFC), the anterior cingulate cortex (ACC) and the right dorsolateral prefrontal cortex (DLPFC), while cerebral blood flow and metabolism in the left DLPFC seem to be reduced (Reference Grimm, Boesiger and Beck3,Reference Elliott, Rubinsztein and Sahakian14–Reference Lawrence, Williams and Surguladze20).
The results of the imaging studies are complemented by neuropsychological investigations on the cognitive deficits of MDD patients. These studies show neuropsychological deficits in many cognitive domains, especially the so-called executive functions are impaired (Reference Goodwin21–Reference Grant, Thase and Sweeney25). The executive functions include, among others, visual discrimination as well as maintenance, shifting and flexibility of attention. These specific functions can be probed by applying attentional set-shifting tasks. Several studies described depressive subjects to be prominently impaired in such tasks (Reference Austin, Ross and Murray23,Reference Austin, Mitchell and Wilhelm26–Reference Purcell, Maruff and Kyrios29).
The impaired executive functions have been predominately associated with altered activity in the prefrontal cortex Reference Levin, Heller and Mohanty(4). Moreover, it has been suggested that the cognitive symptoms of MDD are related to dorsal frontal regions Reference Dolan, Bench and Liddle(30). This assumption was corroborated by other studies on patients with depression applying cognitive task such as a complex planning task Reference Elliott, Baker and Rogers(31), and a verbal fluency task Reference Okada, Okamoto and Morinobu(32). However, because of lack of studies using consistent methods a recent meta-analysis could not confirm these findings by qualitative analysis Reference Fitzgerald, Laird and Maller(33).
It remains unclear how the impairment of executive functions contributes to the altered emotional processing in MDD. Therefore, we aimed to identify brain regions specifically related to impaired executive functions while performing an emotional task. To that end, we compared 20 medication-free depressed patients with an acute MDD episode to 29 matched healthy controls. Both groups performed an emotional task during functional magnetic resonance imaging (fMRI) applying visual emotional stimuli from the International Affective Picture System (IAPS) Reference Lang, Bradley and Cuthbert(34).
Furthermore, they completed the intra–extra dimensional set shift (IED) test Reference Robbins, James and Owen(35). This test contains different stages; at each stage a different set criterion of learning had to be satisfied. By means of the test results, we identified the stage that specifically distinguished the performance of MDD patients and healthy controls. Then we correlated the neuropsychological results of that stage to the changes in fMRI BOLD signal in MDD patients and healthy subjects. As executive functions have been related predominately to the prefrontal cortex, and since the IED test is known to be sensitive for impairments of the same region our analysis focusses on this part of the cortex.
Materials and methods
Twenty medication-free depressed patients (11 women, 9 men; age: 40.00 ± 9.89; mean ± SD) with an acute MDD episode (DSM-IV, APA, 1994) were recruited from the In-patient Department of Psychiatry at the University of Zurich. Inclusion criterion was a score of at least 24 on the 21-item Hamilton rating scale for depression (HDRS) Reference Hamilton(36) (33.12 ± 7.13). Exclusion criteria were any neurological or medical disorder and any psychiatric disorder other than MDD. Patients had been free of psychotropic medication for a minimum of 1 week at scanning (9.12 ± 7.98). One patient had to be excluded from the sample because of the structural abnormalities in the 3D T1-weighted anatomical scan. Patients showed the following characteristics: number of episodes: 1.8 ± 2.2, duration of current episode: 15.83 ± 16.24 weeks, duration of illness: 6.6 ± 8.1 years. All patients completed the Beck Depression Inventory (BDI; 29.94 ± 4.93) Reference Beck(37).
We also investigated 29 healthy subjects (21 women, 8 men; average age: 35.32 ± 7.26) without any psychiatric, neurologic or medical illness. The groups did not differ with respect to age, IQ, education and handedness. Intelligence was tested using a word recognition test (MWT-B) Reference Lehrl, Triebig and Fischer(38), which is functionally equivalent to the widely used NART test Reference Nelson and O’Connell(39). IQ scores in controls (IQ 113 ± 13) and patients (IQ 109 ± 13) did not differ significantly. All subjects were right- handed as assessed by the Edinburgh Inventory for Handedness Reference Oldfield(40). After a detailed explanation of the study design and any potential risks, all subjects gave their written informed consent. The study was approved by the institutional review board of the University of Zurich/Switzerland. The same sample has also been used by Grimm et al. Reference Grimm, Boesiger and Beck(3,Reference Grimm, Beck and Schuepbach19).
Neuropsychological testing
A test of attentional set formation and shifting (IED) from the Cambridge Neuropsychological Test Automated Battery (CANTAB) Reference Robbins, James and Owen(35), previously shown to be sensitive to frontal lobe dysfunction, was applied. The IED examines a subject's ability to attend to the specific attributes of compound stimuli, and to shift attention when required. Two artificial dimensions are used: colour-filled shapes and white lines. Simple stimuli are made up of one of these dimensions, whereas compound stimuli are made up of both. Subjects progress through the test by satisfying a set criterion of learning at each stage. If the subject failed to reach this criterion after 50 trials, the test ended. To begin with, participants were given a simple simultaneous discrimination (SD) in which the stimuli varied along one of the two dimensions for deriving the stimuli. For the second stage (SDR), the discriminanda remained the same, but the previously incorrect choice became the correct one and vice versa. At the third stage (CD), the second dimension was introduced with one exemplar of each dimension paired together to form a compound stimulus in two of the response boxes. To succeed, a participant had to continue to respond to the correct exemplar of the previous stage. The stimuli for the fourth stage (CD) were also compounds, but the two exemplars from the different dimensions were superimposed. The contingencies were again unchanged from those for the previous two stages. A reversal then occurred at the fifth stage (CDR). New exemplars for both dimensions were introduced at the sixth stage, the intradimensional shift (IDS), but the relevant dimension was unchanged from Stage 1. This was followed by a further reversal at the seventh stage (IDR). For the penultimate stage, the extradimensional shift (EDS), new exemplars were again introduced, but success at this point depended on the participant shifting response set to the exemplars of the previously irrelevant dimension. Finally, contingencies were reversed to the previously incorrect exemplar of the new dimension (EDR). Measures of performance on this task were the number of trials needed to reach the criterion as well as the number of errors made summed over the stages and at the EDS stage. For participants failing the test at earlier stages, 25 errors were substituted for their score.
fMRI paradigm
The fMRI paradigm has been described elsewhere in full detail Reference Grimm, Schmidt and Bermpohl(41). Subjects were asked to view photographs taken from the IAPS with positive (IAPS norm ratings: 7.32 ± 2.06) and negative (IAPS norm ratings: 2.24 ± 2.67) valence. The picture sets were counterbalanced across all subjects as well as within each subject according to the two categories of valence as well as according to dominance, intensity, human faces and human figures. All pictures were centred on a black background. All IAPS stimuli were only presented once per subject. The IAPS pictures were presented for 4 s. In half of the presented pictures, subjects had to judge the pictures as to whether they were positive or negative in content [picture judgement (PJ)]; this was indicated by the letters ‘P/N’ in one corner of the picture. Reaction times and ratings (positive/negative) were recorded. In the other half of the presented pictures, subjects had to passively view the picture [picture viewing (PV)]. The subjects were instructed to arbitrarily press a button without making any judgement.
After the presentation of each picture, a fixation cross was presented for 6–8 s (6, 6.5, 7, 7.5 and 8 s). This allowed the subjects to recover from emotional stimulation and served as baseline condition Reference Stark and Squire(42). The baseline duration was randomly varied accounting for variable stimulus onset asynchrony. A total of 80 trials were presented for PV and 79 trials for PJ. The different task conditions were pseudorandomised within and across the runs. The subjects were familiarised with the paradigm by completing a test run of 10 trials.
Data analysis
Neuropsychological and behavioural data were analysed using the Statistical Package for the Social Sciences (SPSS) Windows version 11.0. Performance on the IED set-shifting task was compared between groups using independent group t-test. To investigate the effect of task difficulty (stage) on the number of trials and errors a repeated measures analysis of variance (ANOVA) was conducted, with stage as the within-subjects factor and group as the between-subjects factor. We followed the CANTAB method of adjusting the number of errors such that if subjects failed to complete stages at a particular level of task difficulty, more difficult test levels were not administered, and performance at those levels was assumed to be random (50% correct). Performance on the IED was also examined according to the percentage of subjects succeeding or failing to reach the criterion at each stage of the task using chi-squared tests. Exploratory correlational analyses were conducted to assess associations between test performance and clinical symptom ratings.
Reaction times and judgements (positive/negative rating) of the emotional task were analysed in a multivariate ANOVA with the factors group (healthy subjects/MDD patients), valence (positive/negative pictures) and task (PJ/PV).
fMRI data acquisition and analysis
Measurements were performed on a Philips Intera 3T whole-body MR unit equipped with an eight-channel Philips SENSE head coil. Functional time series were acquired with a sensitivity encoded Reference Pruessmann, Weiger and Scheidegger(43) single-shot echo-planar sequence (SENSE-sshEPI). The following acquisition parameters were used in the fMRI protocol: TE = 35 ms, FOV = 22 cm, acquisition matrix = 80 × 80, interpolated to 128 × 128, voxel size = 2.75×2.75 × 4 mm3, SENSE accelera- tion factor R = 2.0. Using a mid-saggital scout image, 32 contiguous axial slices were placed along the anterior–posterior commissure plane covering the entire brain with a TR = 3000 ms (θ = 82°). The first three acquisitions were discarded because of T1 saturation effects. A 3D T1-weighted anatomical scan was obtained for structural reference.
Image analyses were carried out using MATLAB 6.5.1 (The Mathworks, Inc., Natick, MA, USA) and SPM2 (http://www.fil.ion.ucl.ac.uk) Reference Friston, Frith and Turner(44). One-thousand-and-twenty volume images were corrected for differences in slice acquisition time, re-aligned to the first volume, corrected for motion artefacts, mean adjusted by proportional scaling, re-sliced and normalised into standard stereotactic space and smoothed using a 8-mm full-width-at-half-maximum Gaussian kernel. The time series were high-pass filtered (filter width 128 s) and adjusted for systematic differences across trials. The conditions were analysed using a linear convolution model with an assumed haemodynamic response function Reference Friston, Holmes and Poline(45). For each subject we defined a design matrix modelling emotional judgement, emotional perception and the baseline as separate events. Additionally, the parameters obtained in the re-alignment procedure were included as regressors in the design matrix. Specific effects were tested by applying linear contrasts to the parameter estimates for each condition, resulting in a t-statistic for each voxel.
The individual IED scores (EDS errors) were included as regressors in the design matrix for the healthy subjects and the MDD patient group separately Reference Grimm, Schmidt and Bermpohl(41,Reference Anderson, Christoff and Stappen46). This yielded correlation maps for the relationship between t-values and subjects' scores. The threshold of significance was set to p < 0.005 uncorrected (k > 10).
As the IED test is known to be sensitive for frontal lobe impairments we focussed the further analysis on those regions. The effect sizes (% signal change) for significantly correlating regions were extracted and then correlated with the individual IED scores using Pearson correlation analysis. Region of interests (ROIs) were functionally defined by the peak voxels of activation during PJ > PV. For the extraction of effect sizes, we used Marsbar (http://www.sourceforge.net/projects/marsbar), which allows showing the effect size for each ROI for all conditions. Signal changes are shown relative to the mean signal across the whole experiment.
Finally, using regression maps as described above, we also correlated signal changes during PJ > PV with HDRS scores in MDD subjects. This yielded correlation maps for the relationship between signal intensity and depression severity. The threshold for significant correlation was set to p < 0.005, uncorrected, k > 10. An ROI in the perigenual anterior cingulated cortex (PACC) (Reference Bermpohl, Pascual-Leone and Amedi8,Reference Oldfield40,Reference Northoff, Grimm and Boeker10) was functionally defined by the peak voxel of activation during PJ > PV. The effect sizes were extracted using Marsbar and then correlated with the individual HDRS scores using Pearson correlation analysis.
Results
Neuropsychological data
There were significant group differences in the overall number of stages completed (t = 2.56, df = 21.49, p = 0.02), the number of trials (t = −2.23, df = 21.91, p = 0.04) and the number of errors (t = −2.37, df = 21.72, p = 0.03). Analysis of the effect of task difficulty (stage) revealed a significant stage effect (F = 5.05, df = 8, p = 0.00), but no group effect and interaction effect, on the number of trials. Analysis of group differences in the number of errors revealed a significant effect of both group (F = 7.72, df = 1, p = 0.001) and stage (F = 19.57, df = 2, p = 0.00) as well as a significant group by stage interaction (F = 3.27, df = 2, p = 0.00). The interaction was caused by a significantly divergent performance at the EDR stage with the MDD group committing significantly more errors than the control group (t = −2.49, df = 28.90, p = 0.02). There were no differences at the other stages (Fig. 1).

Fig. 1 Bar diagrams (representing means and SD) show results in IED for healthy controls and MDD patients. Bars are demonstrated for (a) stages completed, (b) number of trials and errors during all stages and EDS stage and (c) percentage of subjects succeeding or failing to reach the criterion at IDR and EDS stage of the task.
Performance on the ID/ED set shift was also examined according to the percentage of subjects succeeding or failing to reach the criterion at each stage of the task. Eighty-six per cent of control subjects were able to complete all stages successfully compared to 53% of MDD subjects (χ 2 = 6.55, df = 1, p = 0.02). Groups did also differ at the EDS stage with 90% of controls compared to 63% of MDD subjects being able to reach criterion (χ 2 = 4.88, df = 1, p = 0.04) (Fig. 1). There was no association between test performance and clinical symptom ratings (BDI/HDRS score).
Behavioural data
Reaction times were longer in emotional judgement compared to PV (F = 13.99, df = 1, p = 0.00). In addition, MDD patients showed significantly longer reaction times (F = 157.58, df = 1, p = 0.00). This effect concerned both PV (healthy: 1.58 ± 0.60; MDD: 1.80 ± 0.78; t = −7.94, df = 1886.37, p = 0.00; df scores reflect the number of pictures) and emotional judgement (healthy: 1.63 ± 0.63; MDD: 1.89 ± 0.81; t = −8.75, df = 1844.5537, p = 0.00). The results are indicative of a consistent psychomotor impairment in patients with MDD in comparison to healthy subjects.
During the fMRI experiment, MDD patients judged the pictures significantly more negative than healthy subjects (F = 74.66, df = 1, p = 0.00). This difference particularly concerned the positive picture (healthy: 7.24 ± 2.17; MDD: 6.90 ± 2.54; F = 32.90, df = 1, p = 0.00).
fMRI results
The number of errors made at the EDS stage, correlated with contrast estimates of PJ > PV, showed various correlation in healthy volunteers (Table 1) and patients (Table 2). According to our focus on prefrontal regions the right ventrolateral prefrontal cortex (VLPFC) and right orbitofrontal cortex (OFC) in healthy volunteers as well as the right DLPFC and the left DMPFC (dorsomedial prefrontal cortex) in MDD patients were subjected for further analysis.
Table 1 Regions showing significant positive or significant negative correlation between the fMRI BOLD signal (contrast PJ > PV) and the EDS errors for healthy subjects
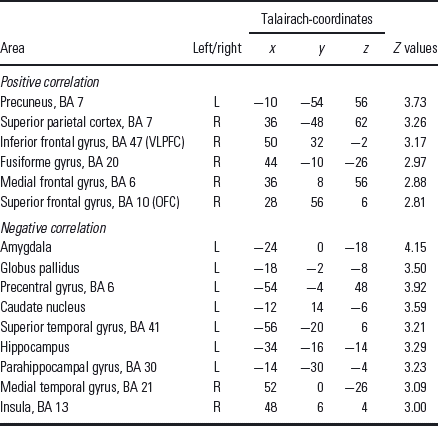
EDS, extradimensional shift; OFC, orbitofrontal cortex; VLPFC, ventrolateral prefrontal cortex.
The threshold of significance was set to p < 0.005 uncorrected (k > 10).
Table 2 Regions showing significant positive or significant negative correlation between the fMRI BOLD signal (contrast PJ > PV) and the EDS errors for MDD patients
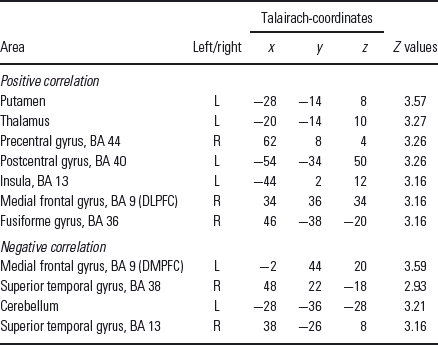
EDS, extradimensional shift; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex.
The threshold of significance was set to p < 0.005 uncorrected (k > 10).
Healthy subjects showed a significant positive correlation of EDS errors with signal changes in the right VLPFC (talairach-coordinates x = 50, y = 32, z = −2, r = 0.43, p < 0.05; Fig. 2) and the right OFC (talairach-coordinates x = 28, y = 56, z = 6, r = 0.47, p < 0.05; Fig. 2). In order to test for a possible influence of the outlier (Fig. 2) we tentatively excluded them and repeated the correlation analysis. This modified correlation analysis equally showed a significant positive correlation in the right VLPFC (r = 0.45, p = 0.019) as well as in the right OFC (r = 0.46, p = 0.016).

Fig. 2 Prefrontal regions with a significant correlation between the per cent signal change of the fMRI BOLD signal (contrast emotional judgement is greater than emotional perception) and the EDS (extradimensional shift) errors for healthy subjects. The threshold of significance was set to p < 0.005 uncorrected (k > 10). The right ventrolateral prefrontal cortex (VLPFC, Talairach-coordinates x = 50, y = 32, z = −2) and the right orbitofrontal cortex (OFC Talairach-coordinates x = 28, y = 56, z = 6) showing significant positive correlation. * indicates a significant correlation (p < 0.05 using Pearson correlation analysis).
MDD subjects in contrast showed a significant positive correlation in right DLPFC (talairach-coordinates x = 34, y = 36, z = 34, r = 0.5, p < 0.05; Fig. 3) and a significant negative correlation in the left DMPFC (talairach-coordinates x = −2, y = 44, z = 20, r = −0.78, p < 0.01; Fig. 3).
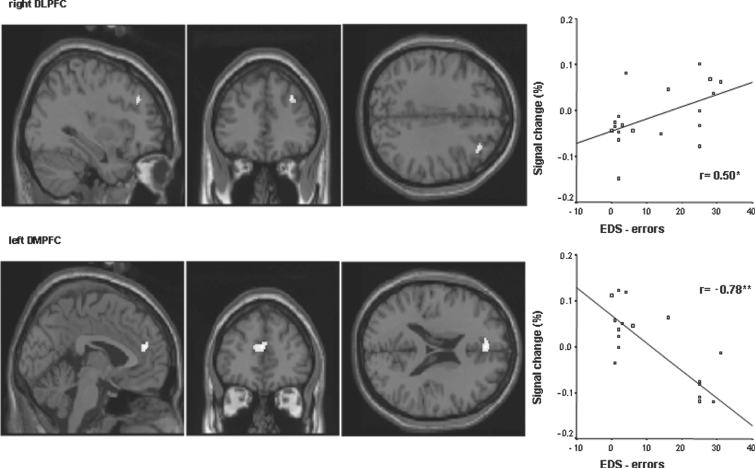
Fig. 3 Prefrontal regions with a significant correlation between the per cent signal change of the fMRI BOLD signal (contrast emotional judgement is greater than emotional perception) and the EDS (extradimensional shift) errors for MDD patients. The threshold of significance was set to p < 0.005 uncorrected (k > 10). The right dorsolateral prefrontal cortex (DLPFC, Talairach-coordinates x = 34, y = 36, z = 34) shows a positive significant correlation, whereas the left dorsomedial prefrontal cortex (DMPFC Talairach-coordinates x = −2, y = 44, z = 20) shows negative significant correlation. * indicates a significant correlation (p < 0.05 using Pearson correlation analysis). ** indicates a significant correlation with p < 0.01.
In order to show the psychopathological relevance of altered neural activity in MDD, contrast estimates of PJ > PV were correlated with patients HDRS scores. Ratings of depression differed significantly between healthy and MDD subjects (healthy: 3.69 ± 1.56; MDD: 33.12 ± 7.13; t = −16.78; p = 0.00). MDD patients' subjective ratings of depression correlated significantly with signal changes in the PACC (talairach-coordinates x = 8, y = 40, z = 10, r = 0.63, p < 0.01). The higher signal intensities in this region, the higher patients' depressive symptoms were rated in the HDRS (Fig. 4).
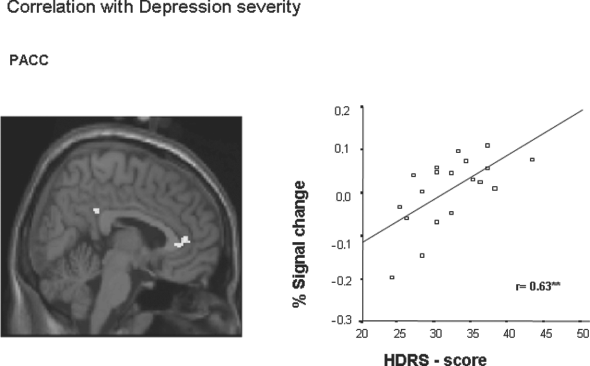
Fig. 4 Prefrontal regions with a significant correlation between the per cent signal change of the fMRI BOLD signal (contrast PJ > PV) and the HDRS scores in MDD subjects. This yielded correlation maps for the relationship between signal intensity and depression severity. The threshold of significance was set to p < 0.005 uncorrected (k > 10). MDD patients' subjective ratings of depression correlated significantly with signal changes in the perigenual anterior cingulated cortex (Talairach-coordinates x = 8, y = 40, z = 10). ** indicates a significant correlation (p < 0.01 using Pearson correlation analysis).
Discussion
The present study examined the relationship of executive functions and prefrontal signal changes during emotion processing in medication-free patients suffering from MDD compared to healthy controls. Results show impaired set shifting, psychomotor slowing and abnormal modulation in MDD.
The depression group was impaired on the task of attentional set shifting, completing fewer stages, requiring more trials to criterion and making more errors than controls. Significantly less subjects of the depressed group were able to learn the extradimensional set shift, which indicates that patients experienced difficulty in shifting attention to a previously irrelevant stimulus dimension in order to discriminate between stimuli. Consistent with the results of Purcell et al. Reference Purcell, Maruff and Kyrios(29), we showed that half of the depression group failed to complete all nine stages of the set-shifting task. Several authors suggested that impaired cognitive flexibility might be the most specific neuropsychological deficit in MDD (Reference Veiel22,Reference Austin, Ross and Murray23,Reference Stordal, Lundervold and Mykletun47,Reference Beblo and Herrmann48). Using the IED set-shifting task Beats et al. Reference Beats, Sahakian and Levy(27) found that elderly patients were more likely to fail at both the IDS and the EDS stages, suggesting difficulties with set maintenance and shifting in older patients with depression. Our results regarding the EDS stage suggest that deficits in medication-free middle-aged patients with severe MDD concern mainly set shifting but not set maintenance (IDS) and reversal learning (IDR).
During emotional stimulation, patients with depression were slower than controls to judge the pictures as to whether they were positive or negative in content. This finding is consistent with observations of psychomotor slowing and increased reaction times in depressed patients (Reference Hammar49–Reference Christensen, Griffiths and Mackinnon52).
The number of errors made at the EDS stage of the set-shifting task were correlated with contrast estimates of PJ > PV to investigate the relationship between executive functions and neuronal activity in the prefrontal cortex. Our results in healthy subjects showed a significant positive correlation of EDS errors in the right VLPFC and the right OFC. The more errors at the EDS stage the more activation in the right VLPFC and the right OFC. MDD patients showed a different correlation pattern with a significant positive correlation in the right DLPFC as well a significant negative correlation in the DMPFC. Thus, an unimpaired attentional shift of stimulus–response associations in healthy subjects may correspond to an increased activation in ventromedial and ventrolateral regions. MDD patients showed a different pattern with decreased dorsomedial activation and increased dorsolateral prefrontal activation.
Animal studies have shown that lateral prefrontal cortex is crucial in shifting an attentional set between perceptual dimensions (EDS) Reference Dias, Robbins and Roberts(53,Reference Dias, Robbins and Roberts54). This is in accordance with our observation of a positive correlation of EDS errors in the right VLPFC in healthy subjects. However, in contrast to their results, we found an additional correlation of EDS errors in the right OFC.
Moreover, our results did not show a relationship between behavioural shifts and DLPFC activity in healthy subjects, but a correlation between increased EDS errors and DLPFC activity in MDD patients. Previous fMRI studies could at least to some extent replicate the finding of animal lesion studies which showed, that DLPFC lesions impair EDS by causing failure to re-direct attention away from those perceptual aspects of the stimuli on which the previous behaviour was based Reference Rogers, Andrews and Grasby(55). Deficits in EDS are also explained with the inability to maintain an integrated representation of the information necessary for identifying and then shifting to a newly relevant rule. A working memory deficit might therefore prevent the identification of changed stimulus-reward contingencies Reference Goldman-Rakic, Plum and Mountcastle(56).
Regarding the association of distinct prefrontal regions with the different IED components, Shafritz et al. Reference Shafritz, Kartheiser and Belger(57) discussed a dorsal neural circuit comprised of the DLPFC, ACC and intraparietal sulcus and a ventral neural circuit comprised of the VLPFC, ACC and striatum. Shifts in cognitive set are specifically mediated by the ventral system. In this system the VLPFC may serve to inhibit the previous stimulus–response pattern, necessary for the successful implementation of the new cognitive set Reference Konishi, Nakajima and Uchida(58,Reference Smith, Taylor and Brammer59). Our findings in healthy subjects are in accordance with the assumption of a ventral system processing shifts in cognitive set.
Our observation of altered neural activity in right DLPFC and left DMPFC in MDD fits in with other studies that suggested a relation of cognitive symptoms to dorsal frontal regions (Reference Dolan, Bench and Liddle30–Reference Okada, Okamoto and Morinobu32).
Moreover, these regions have also been observed in studies applying emotional tasks. The DMPFC has frequently been associated with emotional processing Reference Phan, Wager and Taylor(60). It is predominantly modulated when emotions are evaluated (Reference Northoff, Heinzel and Bermpohl9,Reference Gusnard, Akbudak and Shulman61,Reference Lane, Fink and Chau62). It may be speculated that this evaluative function is related to, or influenced by, the central executive functions that are impaired in MDD.
Grimm et al. Reference Grimm, Beck and Schuepbach(19) showed hypoactivity in the left DLPFC and hyperactivity in the right DLPFC in MDD. Additionally, they observed that the reduced activity in the left DLPFC was associated with the emotional task, whereas increased activity in right DLPFC was related to attention to this task. Our results further specify the role of the right DLPFC in MDD by showing an involvement in impaired executive functions.
Our findings in healthy subjects indicated that the shifts in cognitive set tested by the IED are processed by a ventral system. In contrast, in MDD patients dorsal regions of the prefrontal cortex were activated during this task. It might be hypothesised that this difference in neural processing reflects the use of different strategies in their performance of cognitive tasks relative to healthy controls Reference Rogers, Bellgrove and Chiu(63). As according to Damasio Reference Damasio(5) cognitive and emotional processing are strongly dependant on each other, one may speculate that any change in the strategy for cognitive processing influences emotional tasks and vice versa. Thus, although our task, applied during the fMRI measurement, did not explicitly demand the use of executive functions they implicitly contribute to the emotional task. By this means impaired executive functions, as reflected by altered processing in right DLPFC and left DMPFC, may influence emotional processing in patients suffering from MDD. This is in line with Ochsner and Gross Reference Ochsner and Gross(12) emphasising the importance of cognitive control of emotion for human adaption.
As the differences in neural activation between healthy controls and MDD patients are corresponding to a difference in the performance, we mainly interpreted the neural differences as a reflexion of the dysfunctional neural processing in MDD patients. Alternatively, they may, at least in part, reflect a compensatory strategy that is used to improve the dysfunctional neural circuitry in MDD patients. Such compensatory strategies have been identified in other patients. For example, Staffen et al. Reference Staffen, Mair and Zauner(64) found evidence that patients suffering from multiple sclerosis show altered neural activation pattern as an expression of a compensatory mechanism. However, in contrast to our results, the patients in their study showed an intact performance in the applied behavioural task supporting the idea of a compensatory mechanism. Yet, a similar mechanism cannot be principally ruled out in MDD patients.
The influence of symptom severity represents a possible confound in the study. Therefore, we separately correlated the BOLD signal with symptom severity measured by the HDRS. We found a positive correlation of symptom severity and BOLD signal during emotional judgement in PACC, but not in the dorsal prefrontal cortex. Therefore, we suggest that the altered neural processing in right DLPFC and left DMPFC is not directly related to symptom severity assessed by HDRS scores. However, it has to be noted that the symptom severity may also be evaluated by other tests such as the BDI. As we only tested for a correlation with the HDRS scores, a possible influence of symptom severity as assessed by other tests cannot be excluded.
There are some methodological issues that have to be considered. Firstly, it may be questioned if the neural activity observed necessarily reflects the executive functions. In principle, it cannot be excluded that other types of processing that are equally correlated with the scores of the IED may confound our results. However, the experimental setup was chosen to minimise such possible influences as much as possible. To focus on the cognitive component involving the executive functions of the emotional task we subtracted emotional viewing from emotional judgement. In order to further dissect the resulting network with regard to the involvement of the executive functions we correlated the results of the emotional task with those of the IED. Thus, the observed prefrontal activity consists only of those regions that show significant activation contrasting PJ with PV and correlate with the scores of the IED.
Secondly, the IED results were obtained after the scanning procedure. It has to be acknowledged that the link between the fMRI BOLD signal and the results of the IED might have been more direct if both had been recorded during the fMRI measurement. However, we purposely chose not to do so, since we focussed on impaired executive functions during emotional processing. Emotional processing and specific cognitive testing (e.g. performance of IED) cannot both be performed during the scanning procedure. Therefore, we used an emotional task containing a cognitive component (i.e. judgement of emotional pictures) during the scanning procedure and the IED was performed immediately afterwards. This experimental setup allowed us to probe for an influence of impairments of the executive functions on emotional processing. In contrast, the performance of the IED during the scanning procedure would not have similarly permitted to address this question.
Thirdly, because of our a priori hypothesis-driven approach based on an anatomically specified hypothesis on prefrontal activation, we set the level of significance for the covariate maps at p < 0.005, uncorrected. This allowed us to achieve a high level of sensitivity for detection. It is in accordance with other studies in the field of affective neuroscience aiming to avoid false-negative results (Reference Herwig, Baumgartner and Kaffenberger65–Reference Phelps, O’Connor and Gatenby67). However, it has to be noted that the high level of sensitivity implies an increased risk of false-positive results. Therefore, our results should be considered as initial findings regarding the influence of impaired executive functions on emotional processing in patients suffering from MDD.
Finally, it has to be noted that our results show a pure correlation between the impaired executive functions and the altered neural processing. Thus, one has to be careful to draw conclusions concerning the causation. The establishment of causal relationships is a principal problem in functional imaging studies on human subjects because of the restrictions by our ethical standards. Therefore, Panksepp Reference Panksepp(68) suggested the use of animal studies in combination with human studies to generate causal data.








