Published online by Cambridge University Press: 14 December 2004
The taxonomy and phylogenetic relationships of fish trypanosomes are uncertain. A collection of 22 cloned trypanosome isolates from 14 species of European freshwater fish and 1 species of African freshwater fish were examined by molecular phylogenetic analysis. The small subunit ribosomal RNA (ssu rRNA) genes of 8 clones were sequenced and compared with ssu rRNA gene sequences from a wider selection of vertebrate trypanosome isolates by phylogenetic analysis. All trypanosomes from freshwater fish fell in a single clade, subdivided into 3 groups. This clade sits within a larger, robust clade containing trypanosomes from marine fish and various amphibious vertebrates. All 22 trypanosome clones were analysed by random amplification of polymorphic DNA. The resulting dendrogram shows 3 groups, which are congruent with the groups identified in the ssu rRNA gene phylogeny. Two of the groups contain the majority of trypanosome isolates and within-group variation is slight. These groups do not separate purported trypanosome species distinguished by morphology or host origin, and thus these criteria do not appear to be reliable guides to genetic relationships among fish trypanosomes. However, we suggest that the 2 groups themselves may represent different species of fish trypanosomes. The polymorphic DNA markers we have identified will facilitate future comparisons of the biology of these 2 groups of fish trypanosomes.
Trypanosomes are important pathogens of humans and livestock in tropical regions of the world, but are also widespread parasites of other vertebrates. Both marine and freshwater fish commonly carry trypanosomes, which are transmitted by bloodsucking aquatic leeches (Lom, 1979; Letch, 1980). Although ubiquitous, the taxonomy and phylogenetic relationships of fish trypanosomes are uncertain. In the past, each fish trypanosome isolated from a different species of host fish was named as a new species (Lom, 1979). From the multitude of species thus established, later workers endeavoured to winnow the synonyms by examining morphology and host range. Cross-transmission experiments have proved that particular trypanosome species are not restricted to the host species from which they were originally recovered (Lom, 1973; Khan, 1977; Letch, 1979; Woo & Black, 1984), at variance with the findings of earlier workers (Laveran & Mesnil, 1902; Breindl, 1915). While successful experimental cross-infection may prove that a trypanosome is not strictly host specific, failure of infection may be due to immunity of the recipient fish due to a previous infection, or lack of susceptibility to a particular trypanosome population. As regards morphology, one confusing factor is that fish trypanosomes increase markedly in length during infection and thus individuals of a single species may show large disparities in size (Letch, 1979). European freshwater fish trypanosomes can be grouped into those species that are monomorphic and those which show pleomorphism. The first (e.g. T. carassii) have a more or less similar morphology in the initial, peak and chronic phase of infection, and differ only in size. Those in the second group also vary in size, but in addition show different morphs in a chronic infection. They may have a different shape (e.g. T. elegans), or conspicuous subpellicular bundles of microtubules (myonemes, T. percae), or cytoplasmic granules (e.g. T. granulosum). It is these characteristics that give the impression of distinct species, although it is acknowledged that species differences may also be cryptic. Other biological criteria, such as the morphology of culture or vector stages, or the sequence of stages within the leech vector, have also proved unsatisfactory for the reliable differentiation of fish trypanosome species.
Molecular methods have provided further tools to examine the relationships of fish trypanosomes. By isoenzyme analysis, trypanosome isolates from the minnow (Phoxinus phoxinus) and stone loach (Nemacheilus barbatulus) in UK, which nominally belonged to 2 different trypanosome species, were indistinguishable using 11 enzymes (Letch, 1979). Similarly, Zajicek (1991) found that 16 trypanosome isolates from 10 species of freshwater fish from the Czech Republic were very similar by isoenzymes, with 3 of the 6 enzymes used being invariant. Although 2 other enzymes gave several different banding patterns, there was no correlation between enzyme pattern and host species. Analysis of the kinetoplast DNA of fish trypanosomes showed that the size of minicircles was conserved among freshwater, but not marine, fish trypanosomes (Jirku et al. 1995). Comparison of DNA sequences of the conserved region of the minicircle showed that the freshwater fish trypanosomes formed a compact group among trypanosomatids and 2 groups could be distinguished based on a sequence inversion (Kolesnikov et al. 1995). However, these groups were not evident from comparison of 12S ribosomal RNA (rRNA) gene sequences of the kinetoplast maxicircles, for which pairwise comparison of genetic distances suggested continuous variation, with no indication of discontinuities attributable to barriers to gene flow, and did not correlate with either geographical origin or host species (Figueroa et al. 1999). Paradoxically, phylogenetic analysis suggested the existence of 2 major clades (Figueroa et al. 1999), but because only one isolate was common between this study and that of Kolesnikov et al. (1995), it is not possible to compare the groups directly. Analysis of partial nuclear small subunit (ssu) rRNA gene sequences subdivided trypanosome isolates from 4 species of freshwater fish into 2 closely related groups, Types A and B (Overath et al. 1999). Both types could be isolated from the same individual fish, depending on the culture conditions used. These authors identified the T. cobitis isolate from a stone loach characterized by Letch (1979) and Stevens et al. (1999), and all the trypanosomes studied by Figueroa et al. (1999) as Type B.
Here we have used ssu rRNA sequences and the more discriminatory technique of random amplification of polymorphic DNA (Welsh & McClelland, 1990; Williams et al. 1990) on a larger collection of isolates to elucidate genetic relationships among freshwater fish trypanosomes.
Freshwater fish trypanosome isolates are listed in Table 1. Trypanosomes were grown in L4NHS medium (Baker, Liston & Selden, 1976) or hypo-osmotic biphasic blood-agar medium (T. granulosum, CLAR; J. Lom and H. Peckova, unpublished) or SNB-9 blood-agar medium (MS, MARV; Lom & Dykova, 1992), or in vivo in goldfish (isolate MARV). In the last case, blood obtained by heart puncture was diluted with PBSG and allowed to sediment overnight at 4 °C, followed by low speed centrifugation; trypanosomes in the supernatant were recovered by centrifugation. DNA was prepared by standard methods (Van der Ploeg et al. 1982).

The ssu rRNA gene was amplified by PCR either as a continuous 2 kb fragment or as overlapping fragments using the primers described (Maslov et al. 1996; Stevens et al. 1999). Sequences were aligned using Clustal_X (Thompson et al. 1997), using default settings and with final adjustments made by eye. Positions in which gaps were postulated were excluded from analyses. All analyses were carried out using the program PAUP* version 4.0b10 (Swofford, 2003). The maximum likelihood (ML) model consisted of a generalized reversible rate mutation matrix, a four-category gamma distribution (Γ) and a parameter to accommodate the proportion of invariant sites. ML model parameters were estimated by an automated reiterative heuristic search, using the tree branch reconnection (TBR) method with a reconnection limit of 8. The nucleotide base composition was incorporated empirically into all cases. For ML bootstrapping, ML parameter values were calculated from the tree and were set for the analysis. 100 bootstrap replicates were performed. For maximum parsimony analysis, heuristic searches were performed with 100 random addition replicates and TBR branch swapping. A total of 1000 bootstrap replicates were calculated, using the simple addition algorithm.
Random amplification of polymorphic DNA (RAPD: Welsh & McClelland, 1990; Williams et al. 1990) was performed as described by Kanmogne et al. (1996). The 10-mer arbitrary primers used had previously been selected for RAPD analysis of Trypanosoma brucei subspecies (Tibayrenc et al. 1993): P2, GTCGAGCCGT; P4, GTCGGGCTAA; P5, GTTCTGGGGA; P7, GTGGGCAAAG; P8, GGATGCAGTG; P9, CCGCAATGGG; P10, GACGCTAGTG. The PCR conditions were essentially those used by Williams et al. (1990) using 5–10 ng DNA as template. Products were separated through 1·5% agarose gels in Tris-Acetate-EDTA, pH 8·3 buffer. The results from the 7 primers were compiled into a single data matrix of 70 characters, with each band (character) recorded as present or absent for each isolate. The complete data matrix was then subjected to numerical analysis using the unweighted pair group method using arithmetic averages (UPGMA) in PAUP* version 4.0b10 (Swofford, 2003).
The 8 ssu rRNA gene sequences obtained in this study were aligned with those from a wide diversity of trypanosomes, including 4 species isolated from marine or freshwater fish. In trees derived from this alignment, all the fish trypanosome isolates fell in the aquatic clade. This clade also contains trypanosomes from amphibia, reptiles and the duck-billed platypus, and is associated with transmission by aquatic bloodsucking leeches (Maslov et al. 1996; Stevens et al. 1999, 2001; Jakes, O'Donoghue & Adlard, 2001). To elucidate the relationships within this clade, the ssu rRNA gene sequences of all aquatic clade trypanosomes and representative isolates from 3 other trypanosome clades (T. lewisi, a trypanosome of rats; T. avium, a trypanosome of birds; T. grayi, a trypanosome of the crocodile) were aligned (27 trypanosome taxa in total). Phylogenies constructed from this alignment robustly supported the aquatic clade (Fig. 1A, B). The deepest split within this clade divided it into 2 subclades: one containing trypanosomes from amphibia and a chameleon (T. therezieni), and the other containing trypanosomes mainly from fish, but also including trypanosomes from an aquatic tortoise (T. chelodinae), the duck-billed platypus (T. binneyi) and an unknown species from an aquatic leech (Piscicola geometra) collected from a canal in the UK (T. sp. K&A leech).

Fig. 1. Phylogenetic analysis of ssu rRNA gene sequences from vertebrate trypanosomes. (A) Phylogram constructed by bootstrap (1000 bootstrap replicates) maximum parsimony analysis of 27 trypanosome taxa. The tree is the single most parsimonious tree (length=511, CI=0·736, RI=0·800), based on an alignment of 1301 characters, of which 134 were parsimony informative. Bootstrap values are shown for all major nodes and all branches receiving bootstrap support values [ges ]50%. The tree is rooted on 3 outgroup taxa. Database accession numbers are given for all sequences used in the analysis. (B) Maximum likelihood tree based on the same alignment (−ln L 4686.37081). Bootstrap values are shown for all major nodes and all branches receiving bootstrap support values [ges ]50% (%: 100 replicates).
In the maximum parsimony tree (Fig. 1A), all 10 sequences of freshwater fish trypanosomes fell in a single clade, which was separate from that for trypanosomes of marine fish (T. triglae, T. boissoni). However, in the maximum likelihood tree (Fig. 1B), these trypanosomes appeared paraphyletic at the base of the fish trypanosome clade. Three subgroups of freshwater fish trypanosome isolates were distinguishable; one group contained isolates of diverse origins (MARV from Czech Republic, CLAR from an imported African catfish, and a Portuguese isolate of T. granulosum), while the rest of the European isolates were divided into 2 groups, A and B.
Seven primers (P2, P4, P5, P7, P8, P9, P10; Tibayrenc et al. 1993; Kanmogne et al. 1996) were used to amplify multiple fragments from fish trypanosome DNA. Fig. 2 shows the results obtained using primer P2. Isolates found to have similar banding patterns were re-run in adjacent lanes on subsequent electrophoretic gels to facilitate comparison of band sizes. A total of 25 trypanosome DNA samples representing 22 isolates (Table 1) were scored for presence or absence of a total of 70 bands (characters) from the 7 primers. Not all bands visible on the gel photo were included; weak or inconsistent bands were omitted. The resulting data matrix was analysed by UPGMA (PAUP* version 4.0b10; Swofford, 2003), and the dendrogram is shown in Fig. 3. The 2 samples from Clarias angolensis (CLAR) gave identical results with all 7 primers and grouped separately from the other isolates in the dendrogram (group C). The rest of the isolates formed 2 broad groups, with characteristic patterns for some primers, e.g. primer P2 (Fig. 2) and primers P9 and P10 (Fig. 4). These 2 groups tally with the groups evident from the phylogenetic analysis based on the ssu rRNA gene: Ts-Tt-HOD and R6 are in group A, while Ts-Se-BL and Ts-Ab-Tb are in group B. The only inconsistency is the position of T. granulosum, which is on the periphery of group A in the RAPD dendrogram, but is in group B in the ssu rRNA tree (Fig. 1).

Fig. 2. Ethidium bromide-stained gel showing RAPD results for primer P2 using various fish trypanosome DNAs as template. M, marker. Lanes 1–25: 1, Ts-Se-SP1 clone 1; 2, Ts-Pf-V2 clone 2; 3, Ts-Rr-SP2 clone 1; 4, Ts-Se-BL clone 1; 5, Ts-LC clone 2; 6, Ts-Ab-TB clone 1; 7, Ts-Caa-1 clone 3; 8, Ts-Bb-LS clone 2; 9, Ts-Pf-Mus clone 2; 10, Ts-Tt-VS clone 1; 11, MARV clone 11 (culture); 12, Ts-AA-HO clone 1; 13, Ts-Caa-PO clone 2; 14, LP clone 2; 15, R6 clone1; 16, Ts-Gg-PO clone 1; 17, S clone 2; 18, Ts-Cc-SP clone 1; 19, Ts-Tt-HOD clone 1; 20, CLAR clone 1; 21, CLAR clone 1; 22, E clone 1; 23, R6 clone1; 24, MARV clone 11 (blood); 25, Trypanosoma granulosum clone 1.

Fig. 3. UPGMA dendrogram of RAPD results including 70 characters for 23 fish trypanosome samples. The ssu rRNA gene of asterisked samples was also sequenced.

Fig. 4. Ethidium bromide-stained gels showing RAPD results for primers P9 and P10 using fish trypanosome DNA templates of groups A or B. Primer 9, Lanes 1–11: 1. Ts-Pf-Mus clone 2, 2. Ts- Tt-VS clone 1, 3. E clone 1, 4. Ts-AA-HO clone 1, 5. Ts-Caa-PO clone 2, 6. LP clone 2, 7. R6 clone1, 8. Ts-Gg-PO clone 1, 9. R6 clone1, 10. Ts-Tt-HOD clone 1, 11. S clone 2. Primer 10, Lanes 1–10: 1. Ts-Bb-LS clone 2, 2. Ts- Tt-VS clone 1, 3. Ts-Caa-1 clone 3, 4. Ts-AA-HO clone 1, 5. Ts-Caa-PO clone 2, 6. LP clone 2, 7. Ts-Rr-SP2 clone 1, 8. R6 clone1, 9. Ts-Gg-PO clone 1, 10. S clone 2.
Two samples of isolate MARV were analysed, but gave dissimilar results with some primers, e.g. for primer P2, compare lanes 11 and 24 in Fig. 2. Since one sample was purified from fish blood, while the other was grown in culture, we assume that some of the extra bands may have resulted from contamination with fish DNA from the blood, because RAPD primers are not organism-specific. Only results from the culture-grown sample were included in the UPGMA analysis. MARV showed distinctive RAPD patterns for most primers, reflected in its peripheral position in the dendrogram with CLAR (group C). Although this relationship is strongly supported by the ssu rRNA data (Fig. 1A, B), the grouping of CLAR and MARV in the RAPD dendrogram (Fig. 3) may simply reflect the grouping of 2 very divergent samples.
In phylogenetic analysis based on the ssu rRNA gene sequence, all trypanosome isolates from freshwater fish grouped in a single clade. This clade is nested within the larger aquatic clade, which also contains trypanosomes from amphibia, reptiles and the duck-billed platypus, and is associated with transmission by aquatic bloodsucking leeches ( Maslov et al. 1996; Stevens et al. 1999, 2001; Jakes et al. 2001). There is extreme variation in the rate of evolution of the ssu rRNA gene among taxa within the aquatic clade, with most of the freshwater fish trypanosomes being on very short branches compared to trypanosomes from marine fish and other aquatic vertebrates for which branch lengths are long. Such disparities may have caused long branch attraction, leading to false grouping of the fast-evolving taxa.
The freshwater fish trypanosomes are further subdivided into at least 3 subgroups by both phylogenetic analysis of the ssu rRNA gene and RAPD analysis. Two of the subgroups (A and B) show little within-group variation and yet contain a mixture of fish trypanosome ‘species’ known to be morphologically dissimilar as trypomastigotes in the host bloodstream. For example, T. granulosum, a trypanosome of the eel characterized by densely staining cytoplasmic granules and a pronounced undulating membrane, is grouped with T. cobitis and T. percae in group B. From these results we conclude that fish trypanosome morphology may be a poor guide to the genetic relationships between isolates. Both size and morphology are known to vary markedly during trypanosome growth, and have been shown in some cases to depend on conditions within different host species (Woo, 1994); for example, it is possible that the phenotype characteristic of T. percae is elicited by the particular conditions within the perch host. In addition, it is clear that the trypanosome genotypes investigated here were not strictly specific to the hosts from which they were recovered. We may also speculate that a number of slightly different trypanosome populations infect this range of freshwater fish hosts, showing little, if any, host specificity. These conclusions are in agreement with the findings of previous studies (Letch, 1979; Zajicek, 1991; Figueroa et al. 1999).
It is difficult to compare the subgroups of fish trypanosomes identified here with those identified in previous studies, except where the same isolates have been characterized. Of 16 clones typed isoenzymically by Zajicek (1991), one (Ts-Ab-R6) belongs to group A, and 3 (Ts-Se-BL, Ts-Bb-LS, Ts-Caa-1) to group B; however, the isoenzyme patterns are too variable to make meaningful comparisons. Figueroa et al. (1999) also examined 3 clones examined here (Ts-Aa-HO, Ts-Lc clone 2, S clone 2) and found that Ts-Aa-HO and Ts-Lc clone 2 differed by only 4 nucleotides in 12S rRNA sequences, while S clone 2 differed from both by 10 nucleotides; these differences placed S clone 2 in a separate group from the other 2 clones in phylogenetic analysis. Our study divided the clones in the same way by RAPD analysis, with S clone 2 in group A, and Ts-Aa-HO and Ts-Lc clone 2 in group B. Kolesnikov et al. (1995) also used clone Ts-Ab-TB for analysis of kDNA minicircle sequences, and found that it had the conserved region considered typical of T. carassii. This finding predicts that other group B clones may be characterized by this type of minicircle. Lastly, Overath et al. (1999) identified two sequence variants among partial ssu rRNA gene sequences, which shared 97·3% similarity over 300 bp; clone TsCc-NEM K1 from carp was classified as Type A, together with another carp isolate and several tench isolates, while clone TsCc-NEM 38p13 from carp was classified as Type B, together with T. cobitis from stone loach and the pike isolate El-CP (=group B in this study). Comparison of the Type A sequence of Overath et al. (1999) with the group A sequence represented by clones Ts-Tt-HOD and R6 here, reveals 99·0% identity over 300 bp, and it therefore seems likely that Type A is synonymous with group A; the availability of longer gene sequences from Type A would allow confirmation of this point. In summary, it is possible that the 2 groups A and B identified by Overath et al. (1999) and in this study by ssu rRNA gene polymorphisms and RAPD patterns may also be distinguished by sequence polymorphisms in the 12S rRNA gene and the kDNA minicircle conserved region. We tentatively suggest that the 2 groups may comprise 2 related but divergent species of freshwater fish trypanosomes. Although it is tempting to name the groups as different species, it is unclear which prior species name should be used, as some species belong to more than one group. On the other hand, to propose new names would mean changing all previous species into nomina nuda, claiming that they were described before the defining molecular character could be taken in account. Either option presents taxonomic difficulties and is certain to lead to more confusion rather than the clarification we seek. Thus, at this stage we suggest the pragmatic option of designating the groups by informal names as follows: group A, tincae group and group B, carassii group. The availability of genotypic markers will allow potential biological differences between these groups to be identified in future studies.
The third subgroup was distinct and contained a trypanosome from an African catfish (CLAR), together with 2 more closely related trypanosomes – a Portuguese isolate of T. granulosum, and clone MARV, a trypanosome from carp, also grown experimentally in goldfish in this study. Thus, the 2 European isolates of T. granulosum studied here are genetically different, although this species has a distinctive morphology in host blood. T. granulosum is the trypanosome of the European eel (Anguilla anguilla). Although the life-cycle of A. anguilla involves periods in freshwater and marine environments, T. granulosum is transmitted by the freshwater leech Hemiclepsis marginata (Zintl, Voorheis & Holland, 2000). This agrees with the placement of T. granulosum in the clade of freshwater, rather than marine, fish trypanosomes.
A single isolate (Trypanosoma sp. K&A leech) from a UK specimen of Piscicola geometra also fell within the fish trypanosome clade, suggesting it might be a fish trypanosome, but the vertebrate host is unknown. This isolate is distant from all the fish trypanosomes so far sampled: the ssu rRNA gene sequence differs from those of freshwater fish trypanosomes by 3·5–4·2%, whereas the maximum sequence divergence within this group is 1·6%. This suggests that more extensive sampling might reveal greater diversity within fish trypanosomes than presently suspected.
In conclusion, we may still be far from understanding the taxonomy of freshwater fish trypanosomes, but the availability of polymorphic genetic markers will facilitate future studies of biological variation.
We are grateful to The Royal Society for funding collaborative work between the Czech Republic and UK, and to Iva Dyková for all her support. We thank The Wellcome Trust for financial support to Vanessa Ferris and Patrick Hamilton.

Table 1. Freshwater fish trypanosome isolates used in this study

Fig. 1. Phylogenetic analysis of ssu rRNA gene sequences from vertebrate trypanosomes. (A) Phylogram constructed by bootstrap (1000 bootstrap replicates) maximum parsimony analysis of 27 trypanosome taxa. The tree is the single most parsimonious tree (length=511, CI=0·736, RI=0·800), based on an alignment of 1301 characters, of which 134 were parsimony informative. Bootstrap values are shown for all major nodes and all branches receiving bootstrap support values [ges ]50%. The tree is rooted on 3 outgroup taxa. Database accession numbers are given for all sequences used in the analysis. (B) Maximum likelihood tree based on the same alignment (−ln L 4686.37081). Bootstrap values are shown for all major nodes and all branches receiving bootstrap support values [ges ]50% (%: 100 replicates).
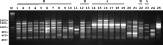
Fig. 2. Ethidium bromide-stained gel showing RAPD results for primer P2 using various fish trypanosome DNAs as template. M, marker. Lanes 1–25: 1, Ts-Se-SP1 clone 1; 2, Ts-Pf-V2 clone 2; 3, Ts-Rr-SP2 clone 1; 4, Ts-Se-BL clone 1; 5, Ts-LC clone 2; 6, Ts-Ab-TB clone 1; 7, Ts-Caa-1 clone 3; 8, Ts-Bb-LS clone 2; 9, Ts-Pf-Mus clone 2; 10, Ts-Tt-VS clone 1; 11, MARV clone 11 (culture); 12, Ts-AA-HO clone 1; 13, Ts-Caa-PO clone 2; 14, LP clone 2; 15, R6 clone1; 16, Ts-Gg-PO clone 1; 17, S clone 2; 18, Ts-Cc-SP clone 1; 19, Ts-Tt-HOD clone 1; 20, CLAR clone 1; 21, CLAR clone 1; 22, E clone 1; 23, R6 clone1; 24, MARV clone 11 (blood); 25, Trypanosoma granulosum clone 1.
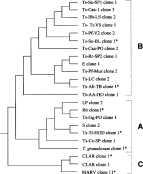
Fig. 3. UPGMA dendrogram of RAPD results including 70 characters for 23 fish trypanosome samples. The ssu rRNA gene of asterisked samples was also sequenced.
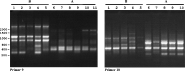
Fig. 4. Ethidium bromide-stained gels showing RAPD results for primers P9 and P10 using fish trypanosome DNA templates of groups A or B. Primer 9, Lanes 1–11: 1. Ts-Pf-Mus clone 2, 2. Ts- Tt-VS clone 1, 3. E clone 1, 4. Ts-AA-HO clone 1, 5. Ts-Caa-PO clone 2, 6. LP clone 2, 7. R6 clone1, 8. Ts-Gg-PO clone 1, 9. R6 clone1, 10. Ts-Tt-HOD clone 1, 11. S clone 2. Primer 10, Lanes 1–10: 1. Ts-Bb-LS clone 2, 2. Ts- Tt-VS clone 1, 3. Ts-Caa-1 clone 3, 4. Ts-AA-HO clone 1, 5. Ts-Caa-PO clone 2, 6. LP clone 2, 7. Ts-Rr-SP2 clone 1, 8. R6 clone1, 9. Ts-Gg-PO clone 1, 10. S clone 2.