Introduction
The determination of the possible ecology or behavior of extinct animals has been an enjoyable challenge for scientists and the popular media alike. Studies of comparative anatomy from Richard Owen to the present day have compared the anatomy of extinct animals with extant relatives (or analogues) and drawn conclusions about the behavior of the extinct forms in a qualitative fashion. During the past few decades, researchers have devoted considerable effort to the development of quantitative modes of analysis for inferring the behavior and ecology of extinct species. Such studies fall under the general rubric of “ecomorphology,” whereby the morphology of an animal is functionally correlated with its ecology or behavior (see Wainwright Reference Wainwright1991). Ecomorphological studies of extinct species include multivariate morphometrics on various skeletal elements to statistically assess the association between the morphology of a skeletal structure (dependent variables) in the extant forms with their ecology or behavior (independent variable). Morphological traits associated with known behaviors may be used as indicators of ecology and behavior in extinct taxa (e.g., Slater and Van Valkenburgh Reference Slater and Van Valkenburgh2008; Meachen-Samuels and Van Valkenburgh Reference Meachen-Samuels and Van Valkenburgh2009; Figueirido and Janis Reference Figueirido and Janis2011; Samuels et al. Reference Samuels, Meachen and Sakai2013; Janis and Figueirido Reference Janis and Figueirido2014).
Biomechanical modeling can also be used to reconstruct the paleoecology of extinct species, as it uses the morphology of skeletal structures and the physical properties of biomaterials to evaluate functional performance of anatomical arrangements (e.g., Alexander Reference Alexander1985). A recent example of such an approach is finite element analysis, in which the virtual performance of skeletal elements of extinct animals under simulated stress has been compared with that of their living relatives to infer performance and function (e.g., McHenry et al. Reference McHenry, Wroe, Clausen, Moreno and Cunningham2007; Wroe Reference Wroe2008; Rayfield Reference Rayfield2007; Tseng and Wang Reference Tseng and Wang2010; Tseng et al. Reference Tseng, Antón and Salesa2011; Gill et al. Reference Gill, Purnell, Crumpton, Brown, Gostling, Stampanoni and Rayfield2014).
Paleoecological inferences are likely to be more reliable if there are living relatives and if a clear analogy can be established with performance in an extant animal. However, we know of various types of morphologies in extinct mammals in which there are no extant analogues: examples include saber-toothed carnivores (e.g., Emerson and Radinsky Reference Emerson and Radinsky1980) and large, clawed herbivores (e.g., Coombs Reference Coombs1983). If living relatives are absent and/or there are no analogous forms, paleoecological inferences are likely to be more ambiguous, especially in the absence of an extant phylogenetic bracket (Witmer Reference Witmer1995). The temptation to shoehorn an extinct animal into the ecomorphological role of an extant one may lead to premature conclusions. For example, the extinct short-legged barrel-bodied rhino Teleoceras is often portrayed as having a hippo-like mode of life, but careful examination of the evidence (in this instance, isotopic evidence of a preference for a terrestrial habitat) shows that to be incorrect (MacFadden Reference MacFadden1998). Additionally, an extinct animal may behave in a way seen in no extant taxon, such as the proposed unique mode of swimming in the archaeocete Ambulocetus (Thewissen and Fish Reference Thewissen and Fish1997) or the proposed bipedal walking locomotion in sthenurine kangaroos (Janis et al. Reference Janis, Buttrill and Figueirido2014). In such cases, the selection of highly functional—or “taxon-free”—traits to derive ecomorphological conclusions is crucial, because analyses will then show its function, whether or not the anatomy of an extinct animal falls within the boundaries of the range of morphologies of extant ones. If it does not fall within these boundaries, it may have had a type of ecomorphology not seen today.
There are also a number of other approaches to the ecology of extinct taxa that capture aspects of behavior during the animal’s lifetime: these could be termed “eco-physiological,” as they reflect lifetime habits rather than adaptive morphology revealed by ecomorphological or biomechanical traits. Such approaches can be invaluable for studying extinct animals without living analogues. These include wear on the teeth to determine diet, whether macrowear (e.g., Janis Reference Janis1979), mesowear (e.g., Fortelius and Solounias Reference Fortelius and Solounias2000), or microwear (e.g., Solounias et al. Reference Solounias, Rivals and Semprebon2010). Another approach involves geochemistry: the study of the isotopic signatures in dental and bone tissues incorporated during food and water consumption (e.g., Cerling and Harris Reference Cerling and Harris1999; Palmqvist et al. Reference Palmqvist, Perez-Carlos, Janis, Figueirido, Torregrosa and Gröcke2008). However, while both of these methodologies are extremely useful in the derivation of feeding behavior or habitat preference, they cannot be employed to determine other behavioral aspects of extinct species such as hunting style or locomotor strategy.
In this paper we demonstrate the potential of ecomorphological methodologies to infer aspects of behavior in extinct species without living relatives by using a functional and quantifiable morphological trait. Here we use the shape of the anterior surface of the humeral distal epiphysis (from here on referred to as the elbow joint) as a morphological indicator to infer the predatory behavior of the emblematic Australian marsupial lion (Thylacoleo carnifex). We propose that, despite the fact that Thylacoleo is usually portrayed as a catlike predator because of its rather feline-like appearance, it may have had a predatory behavior unlike that seen in any extant carnivore.
Thylacoleo carnifex, known as the pouched (or marsupial) lion, was a carnivorous marsupial of the Australian Pleistocene. It was originally described in 1859 by the renowned paleontologist Sir Richard Owen: the genus name Thylacoleo (from the Greek thylakos and leo) means “pouched lion,” and the species name carnifex (from the Latin carnifex) means “executioner.” In the same study, Owen (Reference Owen1859) identified Thylacoleo as “one of the fellest and most destructive of predatory beasts.” The marsupial lion is the youngest species of the genus Thylacoleo (T. crassidentatus and T. hilli being known from the Pliocene), the largest member of all thylacoleonids (~100–160 kg; Wroe et al. Reference Wroe, Myers, Wells and Gillespie1999, Reference Wroe, Myers, Seebacher, Kear, Gillespie, Crowther and Salisbury2003), and the most geographically dispersed of the three.
The family Thylacoleonidae belongs to the order Diprotodontia (Mammalia; Metatheria), which today comprises only omnivorous or herbivorous forms such as possums, koalas, wombats, and kangaroos (e.g., Finch Reference Finch1982; Wells et al. Reference Wells, Horton and Rogers1982, Reference Wells, Murray and Bourne2009; Case Reference Case1985; Wroe et al. Reference Wroe, Ebach, Ahyong, de Muizon and Muirhead2000; Wroe Reference Wroe2003). There has been much debate and controversy about the probable diet of the marsupial lion. Owen (Reference Owen1859) initially characterized Thylacoleo as a carnivore based on its large and sectorial third lower and upper premolars (from now on referred to as carnassials), its canine-like incisors, and the extreme reduction of the other cheek teeth. In contrast, other contemporaneous researchers raised doubts about Owen’s hypothesis (e.g., Krefft Reference Krefft1866; Flower Reference Flower1868; Cope Reference Cope1882; De Vis Reference De Vis1883; Lydekker Reference Lydekker1894), in particular because the angle, orientation, and morphology of its caniniform incisors raised doubts about their use for killing prey (e.g., Anderson Reference Anderson1929; Gill Reference Gill1954). However, all of the evidence obtained during the last few decades, from both adaptive (Finch Reference Finch1982; Wells et al. Reference Wells, Horton and Rogers1982; Wroe et al. Reference Wroe, McHenry and Thomason2005, Reference Wroe, Lowry and Anton2008) and ecophysiological (Nedin Reference Nedin1991; Gröcke Reference Gröcke1997; Wells et al. Reference Wells, Horton and Rogers1982) approaches, indicates that Thylacoleo was indeed a hypercarnivorous animal. Thylacoleo thus represents an extinct hypercarnivorous species without living analogues (Wroe Reference Wroe2000), as all living diprotodontids are omnivores or herbivores (e.g., Finch Reference Finch1982; Wells et al. Reference Wells, Horton and Rogers1982, Reference Wells, Murray and Bourne2009; Case Reference Case1985; Wroe et al. Reference Wroe, Ebach, Ahyong, de Muizon and Muirhead2000; Wroe Reference Wroe2003).
Despite the unequivocal evidence of carnivory, the specific way in which the marsupial lion killed its prey (i.e., its predatory behavior) remains more uncertain. This is probably because the morphology of its postcranial skeleton has received considerably less attention than that of the skull and dentition. A detailed study of the postcranial morphology of Thylacoleo was lacking until Wells and Nichol (Reference Wells and Nichol1977) presented a description of its manus and pes, concluding that Thylacoleo had a digitigrade posture in the manus and a plantigrade stance in the pes. Wells and Nichol (Reference Wells and Nichol1977) also noted an efficient and powerful grasping mechanism of the forelimbs together with the possession of a short, very robust, and pseudo-opposable thumb with a large hooded claw. Both Wells and Nichol (Reference Wells and Nichol1977) and Wells et al. (Reference Wells, Murray and Bourne2009) interpreted the morphology of the manus and pes of Thylacoleo as ideally adapted to a climbing grasp, inferring a scansorial habit (i.e., both terrestrial and capable of climbing). However, Finch (Reference Finch1982), in a study of limb proportions, concluded that Thylacoleo was not particularly climbing adapted. The difference of opinion between these authors may relate to the fact that the hands of generalized terrestrial carnivores and arboreal ones have some traits in common: a hand well adapted to a climbing grasp could equally be well adapted for holding prey (Wells and Nichol Reference Wells and Nichol1977), and both activities require a high degree of forearm maneuverability. A more extensive study on the potential abilities of Thylacoleo to either climb trees or manipulate prey would offer additional evidence on the predatory behavior deployed by the marsupial lion and to further understanding of its paleobiology.
In this paper we investigate the forearm anatomy of Thylacoleo to determine its probable predatory behavior. We use the shape of the elbow joint, a highly functional morphological trait considered an indicator of forearm maneuverability (Andersson and Werdelin Reference Andersson and Werdelin2003; Andersson Reference Andersson2004, Reference Andersson2005; Figueirido and Janis Reference Figueirido and Janis2011; Figueirido et al. Reference Figueirido, Martín-Serra, Tseng and Janis2015), and we employ landmark-based methods of geometric morphometrics to compare the elbow of Thylacoleo with the elbow of extant mammals.
Material and Methods
The sample of Thylacoleo carnifex (Metatheria; Diprotodontia) includes three specimens collected from James Quarry cave, Naracoorte, South Australia (collectively SAM-P12384) and housed at the South Australian Museum in Adelaide (South Australia) (Fig. 1A). The photographs of isolated humeri were taken by C.M.J.

Figure 1 Anterior surface of the humeral distal epiphysis (proxy for elbow-joint shape). A, Elbow joints of the three specimens of Thylacoleo carnifex sampled. From left to right: SAM-P12384a (reversed), SAM-P12384b, and SAM-P12384c (reversed). Scale bar, 3cm. B, Elbow joints of different species of placental and marsupials used for comparison. First row (from left to right): koala (Phascolarctos cinereus), wombat (Vombatus ursinus), and Tasmanian devil (Sarcophillus harrisi). Second row (from left to right): Tasmanian tiger (Thylacinus cynocephalus), Hoffmann’s two-toed sloth (Choloepus hoffmanni), and placental tiger (Panthera tigris). The area of the trochlea is represented in light gray (in blue in the online version) and the area of the capitulum is represented in dark gray (in pink in the online version). Scale bar, 1cm.
We also collected data on the humeri of 190 specimens belonging to 78 extant species from the placental orders Carnivora, Primates, and Pilosa and the marsupial orders Diprotodontia, Dasyuromorphia, and Peramelemorphia (Table 1). Although it may appear that our sample size is unbalanced in favor of placentals, we note that marsupials only represent 4% of the species diversity of mammals (Nowak Reference Nowak1999).
Table 1 Sample size used in this study. The ecological categories used in CVA and LDA are also shown. Bibliographic sources: 1, Samuels et al. (Reference Samuels, Meachen and Sakai2013); 2, McDonald (Reference MacDonald1984); 3, Nowak (Reference Nowak1999); 4, Gompper & and Decker (Reference Gompper and Decker1998); 5, Meachen-Samuels and Van Valkenburgh (Reference Meachen-Samuels and Van Valkenburgh2009); 6, Hayssen (Reference Hayssen2010); 7, Quinn and Wilson (Reference Quinn and Wilson2002); 8, Jones et al. (Reference Jones, Jones, Jones and Wilson1996); 9, Groves (Reference Groves1971); 10, Jones et al. (Reference Jones, Rose and Burnett2001); 11, Jones (Reference Jones2003); 12, Jones and Stoddart (Reference Jones and Stoddart1998); 13, Procter-Gray and Ganslosser (Reference Procter-Gray and Ganslosser1986); 14, Lindenmayer et al. (Reference Lindenmayer, Cunningham, Pope and Donnelly1999); 15, Johnson and Johnson (Reference Johnson and Johnson1983); 16, Iwaniuk et al. (Reference Iwaniuk, Pellis and Whishaw2000); 17, Wilson and Mittermeier (Reference Wilson and Mittermeier2009); 18, Poglayen-Neuwall and Toweill (Reference Poglayen-Neuwall and Toweill1988); 19, Andersson and Werdelin (Reference Andersson and Werdelin2003); 20, Sillero-Zubiri and Marino (Reference Sillero-Zubiri and Marino2004); 21, Powell (Reference Powell1981); 22, Taylor (Reference Taylor1974); 23, Gebo and Rose (Reference Gebo and Rose1993); 24, Ford and Hoffmann (Reference Ford and Hoffmann1988); 25, Mendel (Reference Mendel1985); 26, Mendel (Reference Mendel1981); 27, White (Reference White1993); 28, Taylor (Reference Taylor1978); 29, Youlatos (Reference Youlatos1996); 30, O’Connor and Rarey (Reference O’Connor and Rarey1979); 31, Figueirido and Janis (Reference Figueirido and Janis2011); 32, Warburton et al. (2011); 33, Grand and Barboza (Reference Grand and Barboza2001). For the museum numbers see Supplementary Table S1. †Extinct taxon.

We included a wide sample of placentals with various degrees of forearm maneuverability. Taxa with a high degree of forearm maneuverability (i.e., with a great ability for forearm pronation and supination), including predominantly arboreal or scansorial forms, either for climbing trees, holding onto branches (e.g., the American black bear, Ursus americanus; the orangutan, Pongo pygmaeus; the brown-throated three-toed sloth, Bradypus variegatus), or manipulating food (e.g., the kinkajou, Potos flavus). Taxa with a moderate degree of forelimb maneuverability include generalized terrestrial forms (e.g., the tiger, Panthera tigris, which can use its forelimbs to grapple with its prey). Taxa with the most restricted capacity to pronate and supinate the forearm include cursorially adapted forms (e.g., the African hunting dog, Lycaon pictus).
For the marsupials, we collected data from arboreal and terrestrial Diprotodontia: these included Vombatiformes (e.g., the koala, Phascolarctos cinereus; and the wombat, Vombatus ursinus) and Phalangeriformes (possums; and Macropodiformes such as tree kangaroos Dendrolagus spp.; and the swamp wallaby, Wallabia bicolor). Other terrestrial forms included Peramelemorphia (the greater bilby, Macrotis lagotis) and Dasyuromorphia (carnivorous marsupials, including the Tasmanian devil, Sarcophillus harrisi; the recently extinct thylacine Thylacinus cynocephalus; and the spotted quoll, Dasyurus maculatus). Some examples are shown in Figure 1B. These data were obtained from the American Museum of Natural History (New York) and the Museum of Comparative Zoology of Harvard University (Cambridge, Mass.).
We used the approach of Andersson and Werdelin (Reference Andersson and Werdelin2003) and Andersson (Reference Andersson2004) to capture the shape of the elbow joint (Fig. 2A), collecting digital pictures on the anterior surface of the humeral distal epiphysis with a scale bar and at an appropriate distance. We digitized six homologous landmarks in two dimensions (Fig. 2B) with TPSdigv.2 (Rohlf Reference Rohlf2008), and we used the Measure tool of this software to incorporate a measure of size.

Figure 2 Morphometric data collected for analysis. A, Elbow-joint shape of the marsupial lion showing anatomical features. B, Six landmarks digitized on the high-resolution digital images to recover the shape of the elbow joint. The area of the trochlea is represented in light gray (in blue in the online version) and the area of the capitulum is represented in dark gray (in pink in the online version). Scale bar, 3cm. Abbreviations: ca, capitulum; ef, entepicondylar foramen; le, lateral epicondyle; me, medial epicondyle; rf, radial fossa; tr, trochlea; trc, trochlear crest (or groove).
All the specimens were aligned using Procrustes superimposition (Dryden and Mardia Reference Dryden and Mardia1998) to remove the effects of rotation, translation, and scaling. We used centroid size (Cs; the square root of the sum of the Euclidean distances between each of the landmarks and the centroid; Bookstein Reference Bookstein1991) and Procrustes coordinates (i.e., aligned x,y landmark coordinates) as proxies for size and shape, respectively. The Procrustes superimposition method was performed with MorphoJ (Klingenberg Reference Klingenberg2011).
We assembled a phylogeny following various published sources (see Fig. 3) with Mesquite (Maddison and Maddison Reference Maddison and Maddison2011). We then quantified the phylogenetic signal in elbow shape and size using a permutation test developed by Laurin (Reference Laurin2004) for univariate traits and extended for multivariate analyses by Klingenberg and Gidaszewski (Reference Klingenberg and Gidaszewski2010) to simulate the null hypothesis of complete independence (e.g., Gidaszewski et al. Reference Gidaszewski, Baylac and Klingenberg2009; Figueirido et al. Reference Figueirido, Serrano-Alarcón, Slater and Palmqvist2010, Reference Figueirido, Tseng and Martín-Serra2013; Klingenberg and Marugán-Lobón Reference Klingenberg and Marugán-Lobón2013; Martín-Serra et al. Reference Martín-Serra, Figueirido and Palmqvist2014a,Reference Martín-Serra, Figueirido and Palmqvistb, Reference Martín-Serra, Figueirido, Pérez-Claros and Palmqvist2015) using MorphoJ (Klingenberg Reference Klingenberg2011).

Figure 3 Phylogeny used in this study. The main tree topology is based on Bininda-Emonds et al. (Reference Bininda-Emonds, Cardillo, Jones, MacPhee, Beck, Grenyer, Price, Vos, Gittleman and Purvis, A2007). The phylogenetic relationships for the Carnivora are based on Nyakatura and Bininda-Emonds (Reference Nyakatura and Bininda-Emonds2012) and for the Primates on the updated consensus 10 kTree website (Version 3; Arnold et al. Reference Arnold, Matthews and Nunn2010). The phylogenetic relationships for marsupials were taken from Johnson (Reference Johnson2014). Node numbers refer to the ancestral shapes reconstructed in Fig. 4.
Additionally, a multivariate regression analysis (Monteiro Reference Monteiro1999) of shape on size was performed to test the influence of allometry. The statistical significance was tested with a permutation test against the null hypothesis of complete independence of shape on size (Drake and Klingenberg Reference Drake and Klingenberg2008). However, as species are not independent data points, we also applied independent contrasts analysis (IC; Felsenstein Reference Felsenstein1985) to take phylogenetic effects into account. The statistical significance was again tested with a permutation test against the null hypothesis of complete independence of shape on size (Drake and Klingenberg Reference Drake and Klingenberg2008). The independent contrast analyses and the permutation tests were performed with MorphoJ (Klingenberg Reference Klingenberg2011).
To control for the possibility that larger species might require a less flexible elbow simply to brace their body weight, or that small terrestrial species might encounter more situations that require them to climb more often simply because of their small size, and so require a more flexible elbow, we eliminated the predicted component of shape due to size differences by computing the residuals from the evolutionary regression analyses following Klingenberg and Marugán-Lobón (Reference Klingenberg and Marugán-Lobón2013) and Martín-Serra et al. (Reference Martín-Serra, Figueirido and Palmqvist2014a,Reference Martín-Serra, Figueirido and Palmqvistb, Reference Martín-Serra, Figueirido, Pérez-Claros and Palmqvist2015). These residuals were used in all subsequent multivariate analyses as data free of allometry. Here we show the results including allometric effects (i.e., those results obtained from Procrustes coordinates), but the results without allometric effects (i.e., residuals) are shown in the supplementary material. However, these results without allometric effect should be interpreted with caution, because body size is a variable that influences the degree of substrate use in living taxa, and it is a strong limiting factor for arboreal species (e.g., Taylor Reference Taylor1974; Van Valkenburgh Reference Van Valkenburgh1987). If size-related shape changes (allometric effects) are removed, it is possible that the “substrate signal” in elbow shape would be also erased.
We reconstructed the hypothetical morphology of the ancestral nodes in the phylogenetic tree shown in Figure 3 (e.g., McArdle and Rodrigo Reference McArdle and Rodrigo1994; Martins and Hansen Reference Martins and Hansen1997; Garland et al. Reference Garland, Midford and Ives1999; Polly Reference Polly2001; Rohlf Reference Rohlf2001; Finarelli and Flynn Reference Finarelli and Flynn2006; Astúa Reference Astúa2009; Figueirido et al. Reference Figueirido, Serrano-Alarcón, Slater and Palmqvist2010, Reference Figueirido, Tseng and Martín-Serra2013; Almécija et al. Reference Almécija, Tallman, Alba, Pina, Moyà-Solà and Jungers2013; Martín-Serra et al. Reference Martín-Serra, Figueirido and Palmqvist2014a,Reference Martín-Serra, Figueirido and Palmqvistb) using the square-changed parsimony method of Maddison (Reference Maddison1991) with MorphoJ (Klingenberg Reference Klingenberg2011). We used this approach to specifically compare the elbow of Thylacoleo with the ancestral states of other mammalian groups and with other tips of the phylogeny.
To investigate the ordination of the taxa in the phenotypic space, we performed a principal components analysis (PCA) from the covariance matrix of the Procrustes coordinates. The hypothetical ancestral shapes were then plotted onto the phenotypic space, and the branches were later connected to create elbow phylomorphospaces (see, e.g., Klingenberg and Ekau Reference Klingenberg and Ekau1996; Rohlf Reference Rohlf2002; Gidaszweski et al. 2009; Figueirido et al. Reference Figueirido, Serrano-Alarcón, Slater and Palmqvist2010, Reference Figueirido, Tseng and Martín-Serra2013; Klingenberg and Gidaszewski Reference Klingenberg and Gidaszewski2010; Klingenberg et al. Reference Klingenberg, Dutke, Whelan and Kim2012; Martín-Serra Reference Martín-Serra, Figueirido and Palmqvist2014a,Reference Martín-Serra, Figueirido and Palmqvistb; Sherratt et al. Reference Sherratt, Gower, Klingenberg and Wilkinson2014) using MorphoJ (Klingenberg Reference Klingenberg2011). To explore the influence of phylogeny on the first two PCs, we mapped the scores of the species on these eigenvectors onto the phylogeny shown in Figure 3 using squared-changed parsimony, assuming a Brownian motion model of evolution, with the PDAPtree module of Mesquite (Midford et al. Reference Midford, Garland and Maddison2002).
We also conducted a canonical variates analysis (CVA) to determine the features distinguishing among taxa with different degrees of forearm mobility (Table 1). The extant taxa were classified according to degree of object manipulation with their forelimbs (low, medium, and high). Species with high forelimb mobility are those that are capable of putting food into their mouths using their forearms or that have a wide angle of forearm rotation measured in vivo (mainly in primates). Species with low forearm mobility are those with limited capacity for forearm supination due to cursorial adaptations. Those species with moderate forearm mobility still are able to perform some forearm movements for grappling with prey or for grasping food, but they usually do not use their forearms to put food in their mouths, because they do not have the same freedom of movement.
These criteria to quantify “elbow mobility” were appropriate for all the taxa except the more terrestrial primates such as the rhesus macaque (Macaca mulatta): like all primates, this taxon can put food into its mouth but has a mean range of radio-ulnar pronation and supination (79°) much lower than the orangutan (Pongo, 150°) and gibbons (Hylobates, 163°), as seen in experimental studies (O’Connor and Rarey Reference O’Connor and Rarey1979). Furthermore, this macaque is clearly more terrestrial than any other primate included in our sample (Table 1), and for this reason we classified it has having moderate elbow mobility.
The statistical significance of the pairwise differences in mean shapes among the three groups was assessed with a permutation test using both the Mahalanobis distances (MDs) and the Procrustes distances (PDs) between groups using MorphoJ (Klingenberg Reference Klingenberg2011). The test operates by randomly reassigning the specimens into the groups compared 10,000 times. The means of all random groups are then calculated, and the pairwise distances among them are computed. The test provides a p-value, which is the proportion of permutations that result in a pairwise distance between groups equal to or less than the observed one. Therefore, if our groups are significantly different according to the morphology of the elbow, the pairwise distances obtained in each permutation should be lower than the one obtained with the original data.
To assess how much of the variation is due to phylogenetic relationships of the species under study, we performed phylogenetic MANOVAs from the species scores on both canonical axes. To do this, we used the aov.phylo function included in the Geiger package (Harmon et al. Reference Harmon, Weir, Brock, Glor and Challenger2008) for R. We used Brownian motion as a model for evolutionary change, and ran 1000 simulations to create an empirical null distribution of F-values to compare with our sample.
Both of the functions obtained from the sample of living taxa in CVA were later applied to the Procrustes coordinates of all thylacoleonid elbow shapes. The percentage of probability of living species to belong to any of the groups was assessed using the direct method of leave-one-out cross-validation procedure (e.g., Timm Reference Timm2002) with SPSS, Version 19. The Thylacoleo specimens were classified according to their proximity to group centroids.
As shown in the results section, we determined that the morphology of the elbow joint of Thylacoleo was indicative of a highly mobile forelimb. Because there is little anatomical difference between the forelimbs of terrestrial carnivores that usually manipulate prey and those of arboreal mammals, since both require highly mobile forelimbs (Wells and Nichol Reference Wells and Nichol1977; Fabre et al. Reference Fabre, Cornette, Slater, Argot, Peigné, Goswami and Pouydebat2013), we also performed a linear discriminant analysis (LDA) to separate the living arboreal and terrestrial taxa (Table 1). Again, the statistical significance of pairwise differences in mean shapes between the two groups was assessed with a permutation test using both the MDs and the PDs between groups after 10,000 permutations. The reliability of the discrimination was assessed by the leave-one-out cross-validation method (e.g., Timm Reference Timm2002), and the classification of thylacoleonids into one of the two groups was determined according to their proximity to group centroids.
Results
Allometry and Phylogeny in Elbow-Joint Shape
The permutation test indicated a strong phylogenetic signal in both distal humeral size and shape (LogCs: tree length=7.642, p<0.0001; Procrustes coordinates: tree length=0.555, p<0.0001). The presence of a phylogenetic signal does not invalidate this structure as a functional trait, as it mainly reflects forearm motion and body weight support. Rather, this simply means that this structure has an evolutionary history, as few cases exhibit a homoplastic degree high enough to mask the presence of phylogenetic signal.
Although the multivariate regression of Procrustes coordinates on LogCs (species averages) was not statistically significant (n=81; p=0.0967), the multivariate regression of both contrasted variables yielded a clear significant association (n=79; p=0.0030) (see Fig. 4). This result indicates that placentals and marsupials follow different allometric trends, and this is the reason why the interspecific regression was not significant. However, when phylogeny was taken into account, the association between the contrast of size and the contrast of shape was significant, which means that changes in elbow size between nodes are accompanied by changes in elbow shape. In any event, our results indicate that evolutionary allometry is a significant source of elbow shape variation.

Figure 4 Multivariate regression of elbow shape on size. A, Interspecific allometry regression analysis of the 81 species analyzed in this study. The 95% confidence ellipses for placentals and marsupials are also shown. B, Evolutionary allometry regression analysis of the contrast of elbow shape on the contrast of size. In both cases the thin-plate spline diagrams represent size-related shape changes accounted for each regression vector shown as deviations from the average shape (gray dots) to the predicted shape change over one unit of centroid size (black dots); the lollipops indicate the corresponding landmark shift.
Functional Anatomy of the Elbow Joint
The reconstructed shapes at the internal nodes and some of the tips of the phylogeny are depicted in Figure 5. Relative to other marsupials such as the wombat, other diprotodonts or dasyuromorphians, or even other thylacoleonids such as the Miocene Priscileo (personal observation of Priscileo pitikantensis SAM-P37720), both the trochlea and the capitulum of Thylacoleo are large (Fig. 5). The articular surface of the trochlea is less proximodistally extended than in the wombat (Fig. 5B), the ancestral state for dasyuromorphians (Fig. 5D), all carnivorans (Fig. 5G), and pantherine felids (i.e., the large felids) (Fig. 5H). In contrast, the trochlea of Thylacoleo is more proximodistally extended than in the koala (Fig. 5A) and the ancestral shape for xenarthrans (Fig. 5E). The extension of the capitulum of Thylacoleo is similar to the capitulum of dasyuromorphians (Fig. 5D), xenarthrans (Fig. 5E), and pantherine felids (Fig. 5H). The capitulum is less lateroproximally extended than in the koala (Fig. 5A) or the ancestral state for diprotodontids (Fig. 5C), but more so than in the wombat (Fig. 5B) and primates (Fig. 5F).

Figure 5 Morphometric comparison of the elbow-joint anatomy of Thylacoleo with other taxa and hypothetical shapes. The diagrams represent deformation grids showing the morphological change obtained from the elbow of Thylacoleo (black outline) to the elbow of: A, the koala (Phascolarctos cinereus); B, the wombat (Vombatus ursinus); C, the ancestral state for extant diprotodontians (Node 70, Fig. 3); D, the ancestral state for dasyuromorphians (Node 82, Fig. 3); E, the ancestral state for xenarthrans (Node 63, Fig. 3); F, the ancestral state for primates (Node 50, Fig. 3); G, the ancestral state for all carnivorans (Node 3, Fig. 3); and H, the ancestral state for pantherine felids (Node 34, Fig. 3). The ancestral states (shapes of the internal nodes) were inferred using squared-change parsimony (see methods section). Note that as the branch lengths were not included, the reconstructed ancestral shapes could be considered as an average or a consensus shape for the group.
The articular surface of the Thylacoleo humeral distal epiphysis is not as shallow as in the koala (Fig. 5A), xenarthrans (Fig. 5E), or primates (Fig. 5F), due to the trochlea being extended medially, resulting in a large distal trochlear crest. In contrast, the articular surface of Thylacoleo is more shallow than that of the wombat (Fig. 5B), the ancestral state for dasyuromorphians (Fig. 5D), all carnivorans (Fig. 5G), and pantherine felids (Fig. 5H). Although the elbow shape of Thylacoleo is similar to that of the ancestor for all diprotodontids (Fig. 5C), the trochlea is more mediodistally extended and the capitulum is less proximally extended, rendering it less shallow.
A large trochlea and a rounded condyle-like capitulum allow for a high ability to supinate the forearm (Taylor Reference Taylor1974; Argot Reference Argot2001), and thus we can deduce that the forearm of Thylacoleo was well able to perform this movement. The articular surface of the Thylacoleo elbow is also characterized by a large trochlear crest, which increases stabilization of the forearm (Jenkins Reference Jenkins1973; Figueirido and Janis Reference Figueirido and Janis2011; Janis and Figueirido Reference Janis and Figueirido2014; Figueirido et al. Reference Figueirido, Martín-Serra, Tseng and Janis2015). Our results thus indicate that Thylacoleo was able to stabilize the forearm to a greater degree than the koala but less so than other more terrestrial taxa (excluding the wombat, see discussion section). This anatomy is probably indicative of the ability to stabilize the forearm on the ground, the typical condition of terrestrial species. Thus the elbow-joint anatomy of Thylacoleo indicates that while it was able to lock the arm into a prone position, as in living terrestrial species, it also retained the ability to supinate the forearm, as seen in living arboreal species.
The Phenotypic Space of the Mammalian Elbow Joint
As the first two PCs explained ~75% of the original variance, we show here only these components, because they provide a reasonable approximation for the total shape variation. The third component explained less than 10% of the original shape variance, and the inspection of this eigenvector did not reveal any relevant morphological pattern. The morphospace depicted from the scores of the taxa on the first two PCs is shown in Figure 6A, with their associated shape changes in Figure 6B, and the respective phylomorphospace in Figure 6C. The PCA performed from the data corrected for allometry yielded very similar results (Supplementary Fig. S1), indicating that the main source of shape variation does not reflect allometry effects.
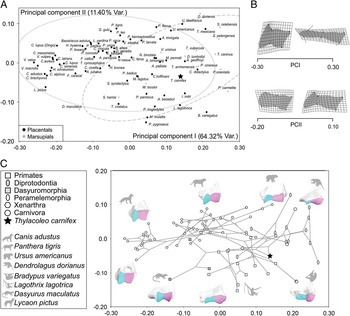
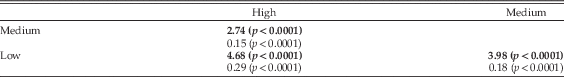
Figure 6 PCA performed on the shape of the elbow of living mammals and Thylacoleo. A, Morphospace depicted from the scores of the species on the first two eigenvectors. The 95% confidence ellipses for placentals and marsupials are also shown. B, The thin-plate spline diagrams representing the shape changes accounted for each PC are shown as deviations from the average or consensus shape (0.0) in each PC (gray straight lines) to the target shapes (black dots). The warping outline of each reconstructed elbow shape is shown for clarity. C, Projection of the phylogenetic tree topology of Fig. 3 onto the phenotypic space depicted from the first two PCs of elbow shape.
The first PC explains 64.32% of the original variance and ordinates the taxa according to forearm mobility: taxa with positive scores have mobile forearms, with a high capacity for supination, while those with negative scores have forearms with more restricted mobility, more locked into a prone position (Fig. 6A,C). Taxa with positive scores on PC1 have distal humeri of a rectangular shape, with the trochlea and capitulum of subequal size, while taxa with negative scores have distal humeri that are more square and box-shaped, with a capitulum that is larger than the trochlea (see Fig. 6B, top).
While it appears that PC1 separates placentals (with more negative scores) from marsupials (with more positive scores), this is because canids occupy the negative portion of this axis. Although there are very few marsupials that resemble carnivoran placentals (i.e., quadrupedal and at least somewhat more terrestrial), note that both the thylacine (Thylacinus cynocephalus) and the quoll (Dasyurus maculatus) fall close to the “placental space” along PC1, as does, to a lesser extent, the Tasmanian devil (Sarcophillus harrisi). The other terrestrial quadrupedal marsupials in this plot, the bilby (Macrotis lagotis) and the wombat (Vombatus ursinus), also plot with less negative scores than most other marsupials, falling in a portion of the morphospace similar to small carnivores with relatively mobile forelimbs (e.g., the African palm civet, Nandinia binotata; the fisher, Martes pennant; and the binturong, Arctictis binturong) (Fig. 6A). More arboreal carnivorans (e.g., the kinkajou, Potos flavus; and the small-toothed palm civet, Arctogalidia trivirgata) have more negative scores on PC1, as do the American and Asiatic black bears (Ursus americanus and Ursus tibethanus, respectively).
The second PC explains 11.4% of the original variance and appears, at least in part, to separate arboreal marsupials (positive scores) from arboreal placentals (negative scores) along the positive side of the first axis (although along the negative side of the first axis marsupials tend to have lower scores than placentals; see, e.g., the placement of the quoll, Dasyurus maculatus) (see Fig. 6A,C). Taxa with positive scores on PC2 have a distal humerus characterized by a pronounced trochlear groove (or a large trochlear crest), while those with negative scores have a less pronounced groove (see Fig. 6B, bottom). Why arboreal marsupials and placentals should be characterized by this difference is not clear, but we note that some of the xenarthrans (the silky anteater, Cyclopes didactylus; the two toed-sloth, Choloepus hoffmanni; and the Northern tamandua, Tamandua mexicana) cluster with the arboreal marsupials, as do some of the South American primates (e.g., the spider monkey, Ateles geoffroyi; and the mantled howler, Allouata palliata).
Mapping the PC scores on the phylogeny shown in Figure 3 using squared-changed parsimony corroborates these results, as neither placentals nor marsupials exhibit a specific range of scores on both eigenvectors (Supplementary Fig. S2). Note, however, that marsupials differ from placentals in their ontogeny (Kelly and Sears Reference Kelly and Sears2011): marsupials are born in a highly altricial state and require well-developed forelimbs to climb to the mother’s teat (Sears Reference Sears2004). This difference in developmental timing between the fore- and hind limbs in marsupials in comparison with placentals (Weisbecker et al. Reference Weisbecker, Goswami, Wroe and Sánchez-Villagra2008; Sears Reference Sears2009; Geiger et al. Reference Geiger, Forasiepi, Koyabu and Sánchez-Villagra2014) might explain why no marsupial has evolved the more restrictive type of elbow joint seen in canids. The supposedly canid-like thylacine does not have a forelimb anatomy in general indicative of canid-like cursorial locomotion (Janis and Figueirido Reference Janis and Figueirido2014).
The elbow shape of Thylacoleo clusters in an intermediate position between that of highly arboreal placentals (i.e., primates and pilosans) and marsupials (i.e., phalangeroids and tree kangaroos). Note, however, that Thylacoleo does not cluster with the arboreal marsupials: its scores on PC1 are similar to the wombat (Vombatus ursinus), the only terrestrial quadrupedal diprotodontid marsupial, and its scores on PC2 are more negative than any marsupial except the mountain cuscus (Phalanger carmelitae). In addition, Thylacoleo is the only hypercarnivorous taxon with this type of elbow morphology: pantherine felids, other hypercarnivorous carnivorans (e.g., the African wild dog, Lycaon pictus; and the gray wolf, Canis lupus), and dasyuromorphians score more positively on both eigenvectors (Fig. 6A,C).
Thylacoleo: A Terrestrial Hypercarnivore with Extreme Forearm Maneuverability.—The CVA performed from the Procrustes coordinates to distinguish among the three groups compared (Table 1) yielded two canonical functions: (CF I: λ=3.954, variance=77.60%; CF II: λ=1.142, variance (%)=22.40). As indicated by the permutation test, both functions allowed a significant separation (p<0.0001) between the three pairs of groups using both MD and PD among groups (Table 2). Furthermore, 87.7% of all the specimens were correctly assigned to their own groups by using the leave-one-out cross-validation procedure. The result of the phylogenetic MANOVA of the specimens scores on both canonical axes was significant for forearm mobility (Wilks=0.17582, F=51.932, p phyl<0.001), indicating that the difference between the three mobility groups is significant even after accounting for phylogenetic relationships.
Table 2 Results of the canonical variates analyses (CVA) performed from the Procrustes coordinates describing elbow shape to separate among high, medium, and low elbow elbow-mobility groups. Numbers in bold type indicate MDs among pairs of groups (and the associated p-values) and normal-type numbers indicate PDs (and the associated p-values).
The pairwise plot depicted from the scores of the specimens on both canonical axes is shown in Figure 7A. The first function mainly separates those taxa with high forearm mobility (scoring positively) from the other two groups (Fig. 7A). Taxa with positive scores have distal humeri that are narrow and rectangular-shaped, with trochlea and capitulum of similar length (Fig. 7B, top left corner). In contrast, taxa with negative scores have a squarer and more box-shaped trochlea (Fig. 7B, top right corner). All thylacoleonids plot within the range of the scores of taxa possessing a highly mobile forearm on the first function, although they do not cluster with them on this plot due to their different scores on the second axis. One specimen of T. carnifex (SAM-P12384c) has scores within the range of taxa with moderate forearm mobility (Fig. 7A), falling close to one of the wombat individuals; but note that a few extant forms with highly mobile elbows, such as bears, also have similar scores on this axis.

Figure 7 CVA performed to determine elbow shape features that best distinguish mammals with high mobility of the forearm from those with medium and low mobility. A, Pairwise plot depicted from the scores on both canonical axes. B, The thin-plate spline diagrams for each canonical function are shown as deviations from the average or consensus shape (0.0) in each discriminant function (gray straight lines) to the target shapes (black dots). The warping outline of each reconstructed elbow shape is also shown for clarity.
The second canonical axis separates those taxa with highly restricted mobility with positive scores and moderately restricted mobility with negative scores (Fig. 7A). Taxa with moderate forearm mobility have a larger trochlea with marked grooves and also a longer capitulum (Fig. 7B, bottom left corner) than those taxa with low forearm mobility (Fig. 7B, lower right corner). Note, however, that the shape variance accounted for by this second axis is influenced by the position of the wombat specimens, which fall outside the range of the defined groups with extremely negative scores on the second axis (Fig. 7A).
This unexpected position of the wombat specimens on the second canonical axis reflects the fact that wombats have a relatively long and rectangular-shaped distal humeral articulatory surface but an extremely well-developed trochlear crest. The combination of these traits reflects moderate forearm mobility combined with the ability to stabilize the forelimbs on the ground. In contrast, other terrestrially adapted marsupials (i.e., dasyuromorphians such as the thylacine, T. cynocephalus) with moderate forearm mobility plot close to pantherine felids (Felidae), and they do not behave as outliers. This is because they have not developed a wombat-like deep trochlear crest: dasyuromorphians resemble placental carnivores in having a trochlea that is relatively shorter than their capitulum and a large and square capitulum, a condition that confers forearm stabilization in a different fashion.
The usual elbow-joint morphology of terrestrial mammals for stabilizing the forelimb on the ground is for a large capitulum, transferring the weight of the animal through the radius to the carpus. However, the retention of the rounded capitulum typical of arboreal taxa in the wombat (allowing the rotation of the radius around the ulna) necessitates an alternative means of stabilizing the forelimb for terrestrial activity, achieved in the wombat via the ulna by means of a large trochlear crest. This, along with other aspects of the forelimb anatomy, results in a secondary restriction of the ability to supinate (Grand and Barboza Reference Grand and Barboza2001). Although the retention of a rounded capitulum reflects phylogenetic inheritance from the ancestral arboreal condition in both the wombat and Thylacoleo, the marsupial lion has not secondarily restricted its forearm mobility in this fashion: the greater degree of forelimb stabilization in the wombat may relate to its digging adaptations.
All of the thylacoleonids fall within the range of taxa with moderate forearm mobility on this axis, closest to the wombats among extant taxa, although both of these marsupials plot outside of the 95% of confidence ellipses, occupying an empty part of the morphospace. The thylacoleonid individuals occupy an intermediate position between the wombats and those taxa with high forearm mobility such as primates, pilosans, and koalas. This result is also supported when the CVA is performed with data corrected for allometry (Supplementary Fig. S3), which indicates that the main source of shape variation is not strongly influenced by size differences. The main difference between both analyses is that in the CVA morphospace free of allometric effects, the specimens of Thylacoleo score less negatively on the second axis. As in the PCA, Thylacoleo does not cluster with other hypercarnivorous species such as pantherine felids.
As previously discussed, the forelimb development of marsupials is different from that of placentals (Weisbecker et al Reference Weisbecker, Goswami, Wroe and Sánchez-Villagra2008; Sears Reference Sears2009; Geiger et al. Reference Geiger, Forasiepi, Koyabu and Sánchez-Villagra2014), and so we made a more direct comparison of Thylacoleo with marsupials alone. We performed a linear discriminant analysis (LDA) to determine whether Thylacoleo clustered with extant arboreal or terrestrial forms. The LDA yielded a function (λ=4.564), which allowed the discrimination of the 85.7% of the taxa (MD=4.248 [p<0.001]; PD=0.257 [p<0.001]).
In this first analysis, we included the wombats as unknowns along with the Thylacoleo specimens, because wombats were also outliers in CVA, and we were curious to see how they would be classified. All of the specimens of wombats and Thylacoleo cluster with terrestrial taxa (Fig. 8A). Repeating the analysis, but now including the wombats as known terrestrial forms, provided similar results (λ=4.564; 90.1% of the taxa correctly classified; MD=3.9179 [p<0.001]; PD=0.207 [p<0.001]). The shape of the distal humerus of arboreal marsupials has a long and very shallow trochlea and a “condyle-like” capitulum (Fig. 8C, right). In contrast, the distal humerus of terrestrial marsupials is characterized by a trochlea and capitulum of similar length and a trochlea with a large crest (Fig. 8C, left). Although the specimens of Thylacoleo cluster in an intermediate region between arboreal and terrestrial species in the latter analysis (Fig. 8B), all individuals were classified as terrestrial. Therefore, we can deduce that Thylacoleo was mainly a terrestrial animal but probably with some abilities for climbing, as revealed by the intermediate position between both ecological groups along the discriminant function. Our results are in accord with those of Wells et al. (Reference Wells, Murray and Bourne2009), who suggested a scansorial habit for the marsupial lion, despite its large body size.

Figure 8 LDA performed from the shape of the elbow joint of marsupials to investigate the most probable substrate use of T. carnifex. A, Discriminant function obtained from the LDA performed from the elbow shape of marsupials, excluding the wombats from the function and including them in the analysis as unknowns, to discriminate between arboreal and terrestrial forms. B, Discriminant function obtained from the LDA performed from the elbow shape of marsupials, now including the wombats as known terrestrial species into the function, to discriminate between arboreal and terrestrial forms. C, Thin-plate spline diagrams for arboreal and terrestrial forms shown as deviations from the consensus shape (gray dots) to the target shapes (black dots) obtained from the analysis of B. The warping outline of each reconstructed elbow shape is also shown for clarity.
The LDAs performed from data corrected for allometry yielded similar results for the living species (Supplementary Fig. S4), which indicates that the discriminant function is not strongly influenced by allometry. However, the specimens of Thylacoleo and some of the wombat individuals now plot with the arboreal taxa (Supplementary Fig. S4). Removing the effects of allometry may also erase important ecomorphological information, as body size is a strong limiting factor for arboreal behavior (e.g., Taylor Reference Taylor1974; Van Valkenburgh Reference Van Valkenburgh1987). In fact, although Thylacoleo is not outside the body size boundaries of arboreal mammals, because it was smaller than large extant apes and many extant bears, it is nevertheless a rather large animal in this respect. Furthermore, the fact that some of the wombat individuals also plot as arboreal forms, if entered as unknowns, underscores the fact that terrestrially adapted mammals may be assigned as arboreal ones if the allometric effects of body size on shape are not taken into account.
We consider that the “arboreal signal” in the elbows of both Thylacoleo and wombats reflects their evolutionary history rather than their actual behavior. Note also that neither taxon plots close to the related, definitively arboreal, koala on either the PCA or the CVA.
Discussion
The results of the comparison of the elbow joint of Thylacoleo with other mammals led to an interesting paleobiological question: If this animal was mostly terrestrial, then why did it retain the degree of forearm maneuverability characteristic of more arboreal forms? Wells and Nichol (Reference Wells and Nichol1977) concluded that the manus of Thylacoleo had an efficient and powerful grasping mechanism, with the pseudo-opposability of digit I against the pisiform combined with a slight capacity for divergence of digit V. Case (Reference Case1985) also suggested the huge, clawed, and possibly opposable pollex of Thylacoleo was related to the ability to manipulate food (Fig. 9).

Figure 9 The manus of Thylacoleo, showing hypothesized movements of the pollex. Right manus with digits II to V flexed and digit I showing the flexion–extension movement in medial (A) and dorsal (B) views. Redrawn and modified from Wells and Nichol (Reference Wells and Nichol1977). Medial (C) and dorsal (D) views of the right manus. Specimen SAM-P16679 from Victoria Fossil cave.
We propose here that Thylacoleo used its high forearm maneuverability for dispatching prey, with a predatory behavior opposite to the placental carnivores with which it is usually compared. That is, while large felids use their forelimbs to grapple with prey, and use their canines to hold and kill it, we propose that Thylacoleo used its large and retractable claw on the semi-opposable thumb to kill its prey, and may have used its supposedly caniniform incisors to subdue it.
Pantherine felids usually kill their prey by suffocation or neural distress, using their canines to exert a prolonged and efficient bite onto the prey’s throat, snout, or neck (Ewer Reference Ewer1973; Biknevicius and Van Valkenburgh Reference Biknevicius and Van Valkenburgh1996). However, there are a number of reasons to doubt that Thylacoleo used its “caniniform” incisors in a similar fashion.
First, in felids, as in all mammals, the lower canines meet the uppers by occluding along the entire length of the anterior margin, exerting a powerful piercing bite. But such piercing would be difficult with the incisors of Thylacoleo, as they meet tip to tip and at an entirely different angle (see Fig. 10A). In addition, the upper incisors of Thylacoleo become blunt with wear, at least in old individuals (Anderson Reference Anderson1929; Wells et al. Reference Wells, Horton and Rogers1982: see Fig. 10C).
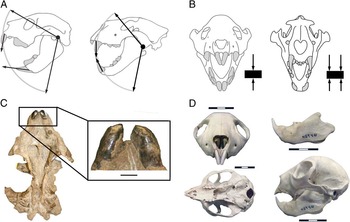
Figure 10 Aspects of the craniodental anatomy of Thylacoleo. A, Schematic drawing showing the hypothesis of Anderson (Reference Anderson1929): the nonparallel, convergent nature of the incisors of Thylacoleo (left) would preclude them meeting as in a placental carnivore such as a lion (right). B, Schematic drawing showing the hypothesis of Gill (Reference Gill1954): the angle and position of the incisors of Thylacoleo may preclude their use for holding prey. The arrows indicate points for holding a hypothetical prey item (rectangle). C, Ventral view of the skull of Thylacoleo (specimen AMNH 19251 from Queensland) illustrating the heavy wear in the upper incisors. Scale bar, 1cm. D, Skull anatomy of Daubentonia (MCZ 45946). Scale bar, 3 cm.
Second, recent biomechanical studies of the skull of Thylacoleo demonstrate a weaker rostrum than in the lion (Panthera leo) under simulated intrinsic forces (Wroe Reference Wroe2008). This was interpreted as reflecting a different style of predatory behavior in Thylacoleo than the “clamp and hold” technique deployed by pantherine felids, although Thylacoleo would be able to employ its massive carnassial-like premolars to scissor through hide and flesh (Wroe Reference Wroe2008). However, this evidence could also support our hypothesis that the incisors were not used to administer a killing bite.
Third, extant large felids possess transversely oriented incisor rows that form protruding arcades, isolating them from the rest of the dentition to enable them to tear the flesh of their prey (Biknevicius and Van Valkenburgh Reference Biknevicius and Van Valkenburgh1996). It is obvious that one of these incisor functions is lacking in the marsupial lion: If the incisors were “canine-like,” then how was Thylacoleo able to tear the flesh and the skin of its prey? And if the incisors were not as “canine-like” as previously thought, then how did Thylacoleo kill its prey?
A qualitative comparison between the skulls of Thylacoleo and the aye-aye (Daubentonia madagascarensis; Fig. 10D) may provide some insights to these questions. Sir Richard Owen (Reference Owen1871) compared the skulls of both taxa, as their external morphological resemblance is exceptional, despite the obvious difference in size. Both mammals share, among other traits, an extreme development of the median incisors meeting at a similar occlusal angle (Owen Reference Owen1871). In the aye-aye these incisors are used for gnawing holes in wood at specific points along the tree bark when the grubs that they feed on are detected (Erickson Reference Erickson1991), although the aye-aye has ever-growing incisors and lacks the dental specialization toward flesh eating seen in Thylacoleo. The incisors of Thylacoleo may have been used to tear skin and flesh, rather than tree bark, a behavior leading to craniodental similarity with the aye-aye, at least in some respects.
The blunting of the incisors of Thylacoleo might have resulted from the skin or the flesh of its preferred prey being very hard and/or very abrasive. Fossil remains of the marsupial lion have been found associated with remains of large macropodids of the genera Macropus and Sthenurus (Horton and Wright Reference Horton and Wright1981), and the exceptionally tough skin of kangaroos may have posed particular problems for their predators. Note that the leather of the large macropodine kangaroos (Macropus giganteus and Macropus rufus) is unique, because it offers high strength while remaining lightweight and flexible. For this reason kangaroo leather is used for high-performance sporting products (Looney et al. Reference Looney, Kyratzis, Truong and Wassenberg2002). Thus a carnivore specialized for killing kangaroos might face different challenges in removing the skin compared with a carnivore specialized for killing ungulates.
All of these evidences indicate that the “caniniform” incisors of Thylacoleo were not adapted to administering a killing bite, although they may have been used to hold and/or subdue the prey and to bite through hide and flesh. However, the angle and position of these incisors have been interpreted as precluding their use for holding (Gill Reference Gill1954: see Fig. 10B). If the incisors could not be used to subdue the prey, the flexibility of the forearm may have also been important in use for grappling, as well as administering a killing slash with the claw on the pollux.
Conclusions
The determination of the behavior of an extinct animal is always a challenge for paleobiologists, especially when living relatives and/or ecological analogues are absent. This is the case for the Australian “marsupial lion,” Thylacoleo carnifex, whose behavior and ecology has been a matter of debate since Richard Owen’s Reference Owen1859 description, and it is often assumed to have had felid-like behavior. The skull of Thylacoleo is superficially catlike, and large “carnassial” teeth indicate a carnivorous diet: but the marsupial lion lacks large canines, and the proposed “caniniform” incisors seem to be not well equipped to administer a killing bite. Thylacoleo also possessed a greatly enlarged claw on the pollux (Wells and Nichol Reference Wells and Nichol1977; Finch and Freedman Reference Finch and Freedman1988) and wrist anatomy (Weisbecker and Archer Reference Weisbecker and Archer2008) on a highly mobile forelimb. How, then, did Thylacoleo kill its prey?
The osteological design of Thylacoleo is unique among mammals, and predatory behavior is an ecological aspect that cannot be inferred from other ecophysiological methods. Our ecomorphological analysis of the elbow-joint morphology of Thylacoleo sheds light on how it may have dispatched its prey. We show here that Thylacoleo could perform a much greater degree of supination of the manus than seen in extant carnivorous mammals, permitted by a distal humeral articulatory surface with a large and very shallow trochlea and a “condyle-like” rounded capitulum. This exceptional maneuverability of the Thylacoleo forearm, in combination with the possession of an extremely large hooded and retractable claw on the semi-opposable thumb (Wells and Nichol Reference Wells and Nichol1977), is suggestive of a “prey-killing arsenal” (Meachen-Samuels Reference Meachen-Samuels2012) very different to any other known hypercarnivore, extant or extinct. We propose that the robust, powerfully built, and “claw-equipped” forelimb of Thylacoleo (Wroe et al. Reference Wroe, Lowry and Anton2008) played a more active role for dispatching large prey than in living predators (Cox and Jefferson Reference Cox and Jefferson1988; Londei Reference Londei2000; Weisbecker and Archer Reference Weisbecker and Archer2008). The extensive use of the forelimb most probably evolved because its canine-like incisors were not as efficient for prey killing as the true canines of pantherine felids (e.g., Anderson Reference Anderson1929; Gregory Reference Gregory1951; Gill Reference Gill1954; Wells et al. Reference Wells, Horton and Rogers1982). Perhaps with the possession of this claw, the inherited mobile forelimb from a vombatiform arboreal ancestor (Weisbecker and Archer Reference Weisbecker and Archer2008), and the presence of prominent carnassial teeth (Wroe Reference Wroe2008), it was not necessary to develop large canines.
While the predatory behavior of Thylacoleo will never be known for certain, the use of morphometric techniques allows us to infer the probable ecomorphology of this enigmatic predator. Our main conclusion is that Thylacoleo did not have the predatory behavior of an extant large felid as has been traditionally suggested (Owen Reference Owen1859, and many subsequent authors). Our results demonstrate that the forelimb mobility of this animal was unlike that of any known terrestrial mammal: the documented ability for supinating the hand in combination with the enormous sheathed claw on a semi-opposable pollux raises the distinct possibility that this claw was deployed as the mode of killing prey. In addition, despite the evidence for a powerful bite, the mode of occlusion and the wear on the “caniniform” incisors make it unlikely that the incisors were deployed for a killing bite, although they may well have been involved in subduing the prey and/or tearing into the carcass. Thus it is apparent that Thylacoleo, despite being called the marsupial “lion,” probably represents a unique type of predator ecomorph.
Acknowledgments
We are grateful to Judy Chupasko and Mark Omura (Museum of Comparative Zoology, Harvard University), Eileen Westwig, Judy Galkin, and Ruth O’Leary (American Museum of Natural History), and Mary-Anne Binnie (South Australian Museum) for kindly providing access to the specimens under their care. We are especially grateful to Mike Gemmel, Tim Gilchrist, and Marie-Anne Binnie for kindly providing photographs of Thylacoleo manus. The comments of the editor (Bruce MacFadden) and two anonymous reviewers are greatly appreciated. This work was supported by a grant from the Spanish Ministry of Science and Competitiveness to B.F. (CGL2012-37866).
Supplementary Material
Supplemental materials deposited at Dryad: doi:10.5061/dryad.04gj1