Introduction
Delirium, also called acute confusional state, is an unspecific manifestation of acute illness occurring in post cardio-surgical patients (Rudiger et al., Reference Rudiger, Begdeda and Babic2016) and characterized by disturbances in consciousness or attention and cognition, an abrupt onset and fluctuating course caused by underlying etiologies (DSM 5) (American Psychiatric Association, 2013). Delirium is common in the intensive care units (ICUs) reaching an incidence of 80% and causes significant distress for patients, families, and caregivers. The management of delirium is controversial. Whereas the outdated American Psychiatric Associations Guidelines (Practice guideline for the treatment of patients with delirium, American Psychiatric Association, 1999) recommended the use of antipsychotics, in particular haloperidol as the gold standard, the newer National Institute for Clinical Excellence (NICE) guidelines (Royal College of Physicians (UK), 2010) advocate the cautious use of antipsychotics only for patients in distress caused by delirium. Further, previous reviews supported the use of antipsychotics, whereas a later review disapproved of their use (Barbateskovic et al., Reference Barbateskovic, Larsen and Oxenboll-Collet2016; Kishi et al., Reference Kishi, Hirota and Matsunaga2016; Neufeld et al., Reference Neufeld, Yue and Robinson2016; Burry et al., Reference Burry, Mehta and Perreault2018; Nikooie et al., Reference Nikooie, Neufeld and Oh2019; Riviere et al., Reference Riviere, van der Mast and Vandenberghe2019).
The management schedule implemented in this study compromised between these approaches, administering haloperidol for delirious patients in distress and experiencing hallucinations.
Haloperidol is a typical antipsychotic with primarily inverse agonism on the dopamine (D)-2 to -4 receptors, silent agonism on the alpha-1a and negligible effects on the histaminergic and muscarinergic receptors. Like all enteral antipsychotics, haloperidol can prolong the QTc in a dose-dependent manner likely caused by hERG inhibition (Crumb et al., Reference Crumb, Ekins and Sarazan2006) causes QTc prolongation, torsades de pointes, and sudden cardiac death. In particular, the parenteral formulation has been associated with QTc prolongation and torsades de pointes (Metzger and Friedman, Reference Metzger and Friedman1993). In the 1980s, sudden cardiac death may have been caused by very high doses of haloperidol (Henderson et al., Reference Henderson, Lane and Henry1991). As shown in deaths caused by methadone, another aspect of parenteral formulations is the use of additives: Chlorobutanol, a preservative, caused substantial QTc prolongation by inhibition of the human ether-a-go-go related gene responsible for cardiac potassium channels and reduced potassium elimination causing a prolongation of the repolarization (Kornick et al., Reference Kornick, Kilborn and Santiago-Palma2003). For parenteral haloperidol, some generic formulations contain chlorobutanol, whereas, e.g., the brand formulation does not. Additionally, recent evidence suggests that QTc prolongation is clinically less important than previously assumed (Tables 1 and 2). Further, QRS morphology might be more relevant to QTc prolongation, torsades de pointes, and sudden cardiac death (Attin and Davidson, Reference Attin and Davidson2011).
Table 1. Studies with significant QTc prolongation
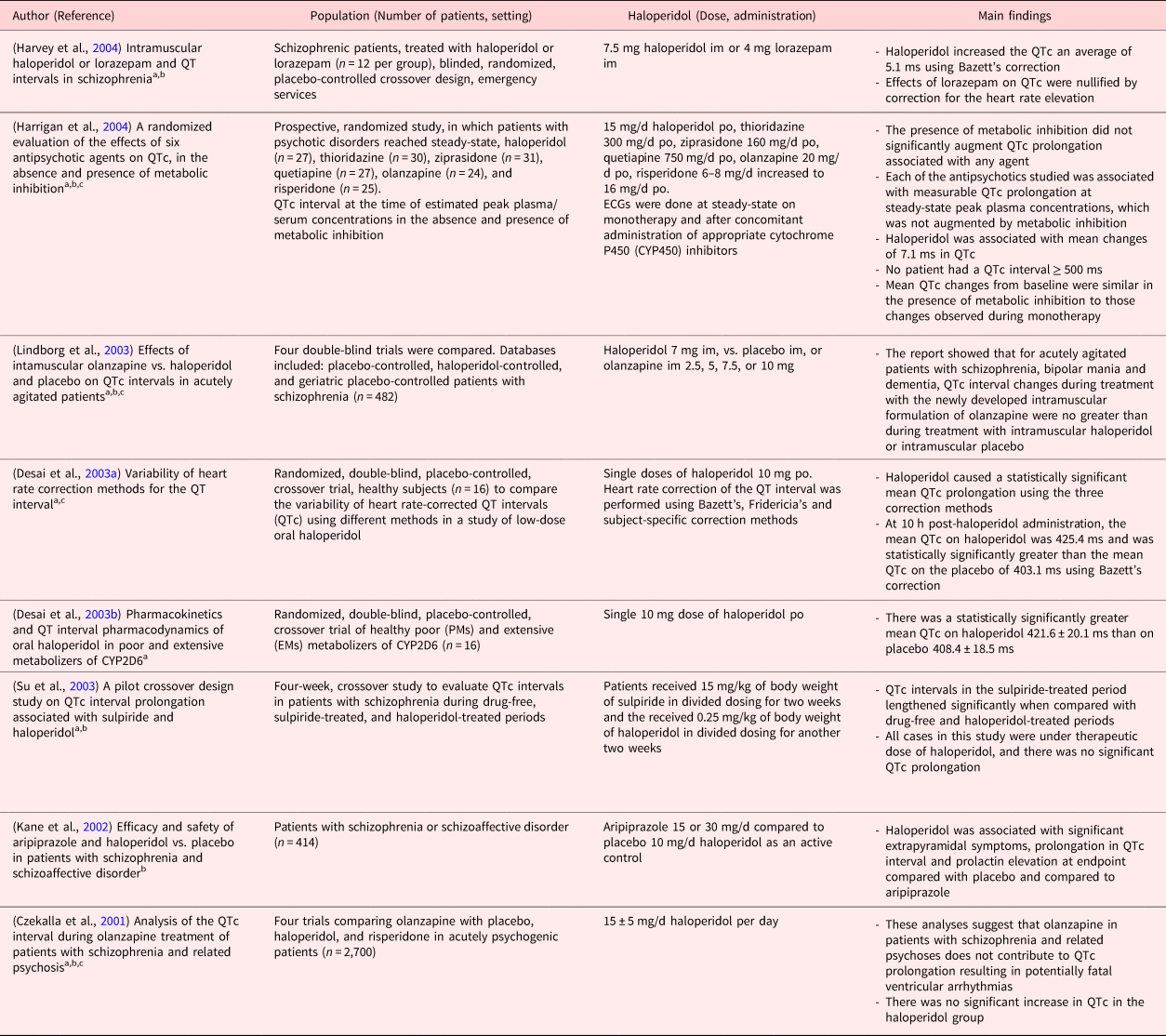
Clinical studies in PubMed were searched using the following terms.
a Haloperidol and QT prolongation (16 studies).
b Haloperidol and long QT (13 studies).
c Haloperidol and QTc prolongation (19 studies).
Table 2. Studies without significant QTc prolongation
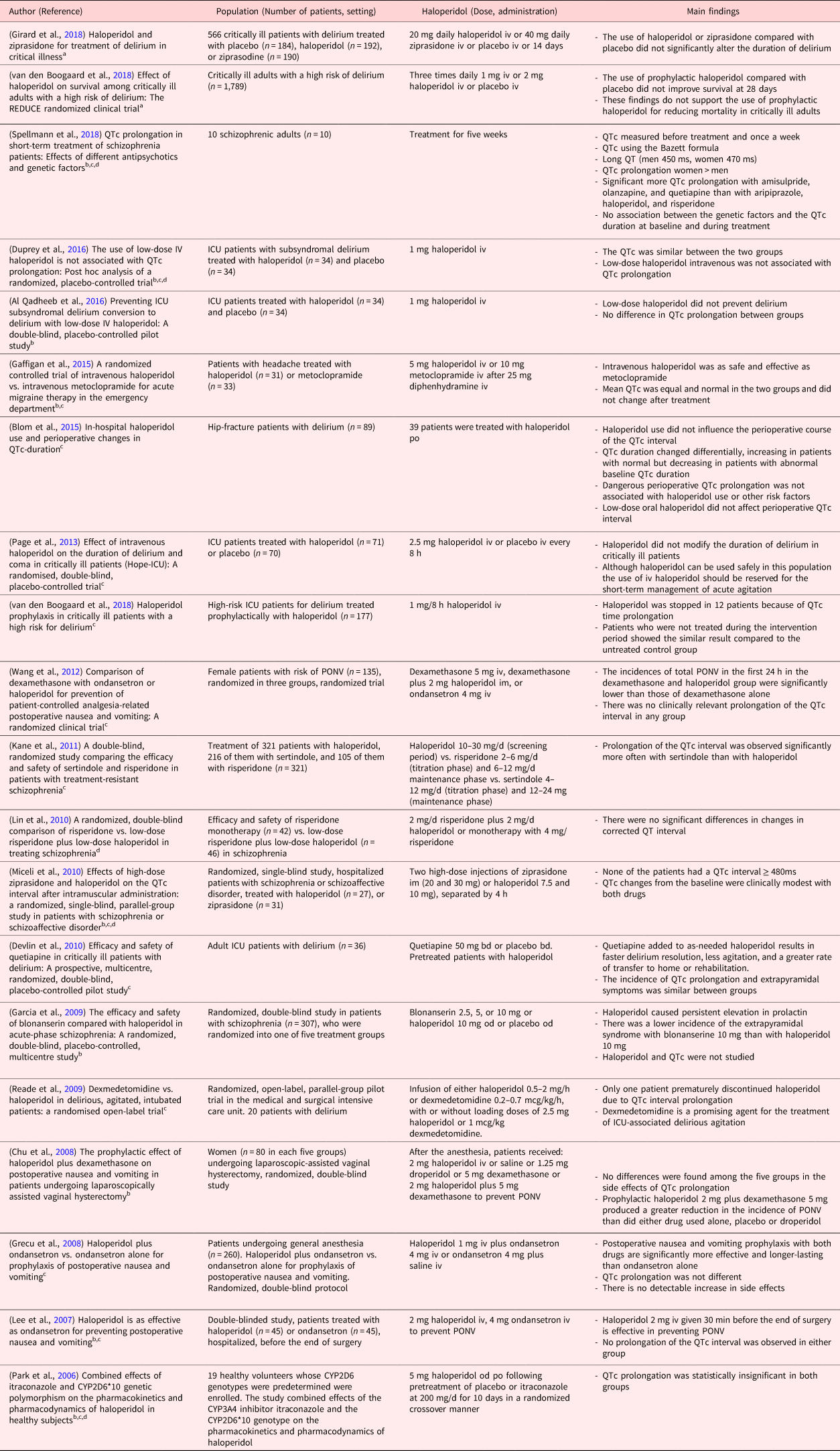
Clinical studies in PubMed were searched using the following terms.
a Haloperidol and delirium and critical illness (10 studies).
b Haloperidol and QT prolongation (16 studies).
c Haloperidol and QTc prolongation (19 studies).
d Haloperidol and long QT (13 studies).
Hence, it remains unclear, whether haloperidol administration prolongs the QTc interval in a vulnerable population after cardiac surgery. We hypothesize that the current formulation of haloperidol has no clinically relevant adverse effect on the QTc time. The aim of this study is to assess the risk of QTc prolongation after haloperidol administration in ICU patients after cardiac surgery and to compare our results with reports from the literature.
Methods
Patients and procedures
This retrospective cohort study was reported according to STROBE guidelines (von Elm et al., Reference von Elm, Altman and Egger2014) and conducted on the cardio-surgical ICU at the University Hospital Zurich, Switzerland. From January 1 to December 31, 2014, 1,181 patients were managed on this 12-bed unit, mostly post cardiovascular surgery. The ICU is led by certified specialists in intensive care medicine. The treatment goals of our cardio-surgical ICU patients have been summarized (Hauffe et al., Reference Hauffe, Kruger and Bettex2015, Reference Hauffe, Kruger and Bettex2016). Neurologists and psychiatrists are available for expert consultation at all hours. The daily records of all patients admitted to the ICU were screened in order to identify patients with delirium managed with enteral and/or parenteral haloperidol. Inclusion criteria were age >18 years, management with haloperidol intravenously, orally or either, and an electrocardiogram (ECG) prior and after haloperidol administration. Exclusion criteria were the absence of ECG in the given time frame within 24 h after haloperidol administration and a pacemaker ECG. Patients were only once included in the analysis, repeated occurrences were omitted.
This study was approved by the ethics committee of the Canton of Zurich (KEK-ZH-Nr. 2012-0263).
Variables
The severity of disease at the ICU was calculated with the Simplified Acute Physiology Score (SAPS) II (Le Gall et al., Reference Le Gall, Lemeshow and Saulnier1993). The ECG after haloperidol administration was performed within 24 h and compared with the ECG recorded at ICU admission. The QT time was measured by the electrocardiograph Cardiovit AT 10 (Schiller, Baar, Switzerland) and by manual analysis. QT times were corrected with the Bazett formula: ![]() ${\rm QTc} = {\rm QT}/\surd {\rm heart}\,{\rm rate}$. Long QT was defined according to the American Heart Association as QTc >450 ms for men and QTc >460 ms for women (Rautaharju et al., Reference Rautaharju, Surawicz and Gettes2009).
${\rm QTc} = {\rm QT}/\surd {\rm heart}\,{\rm rate}$. Long QT was defined according to the American Heart Association as QTc >450 ms for men and QTc >460 ms for women (Rautaharju et al., Reference Rautaharju, Surawicz and Gettes2009).
Delirium management schedule
The delirium management schedule (DelirPath) (Schubert et al., Reference Schubert, Schurch and Boettger2018) consists of a screening and a management algorithm. All patients on the ICU are regularly screened thrice daily with the Intensive Care Delirium Screening Checklist (ICDSC) and on suspicion of incident delirium, the Confusion Assessment Method for the ICU (CAM-ICU) was performed.
The ICDSC (Devlin et al., Reference Devlin, Fong and Schumaker2007) is a screening instrument including eight items based on the DSM-IV TR criteria specifically designed for the intensive care setting with two points: absent or present. This scale was designed for patients with limited communication abilities such as intubated patients. The maximum score is eight; scores of more than three indicate the presence of delirium. Each item is rated on the patient's behavior over the previous 24 h, and the inter-rater reliability between intensive care staff was considered adequate (Bergeron et al., Reference Bergeron, Dubois and Dumont2001).
The CAM-ICU (Ely et al., Reference Ely, Margolin and Francis2001) is based on the CAM (Inouye et al., Reference Inouye, van Dyck and Alessi1990) reflecting the DSM-III-R criteria (American Psychiatric Association, 1987) and designed for patients with limited communication abilities. This scale contains four features with two levels: absent and present. The nonverbal items achieve a lower sensitivity than the verbal items. The inter-rater reliability ranges from 0.79 to 0.95 (McNicoll et al., Reference McNicoll, Pisani and Ely2005).
Once delirium was determined with the ICDSC and CAM-ICU, the management algorithm (Schubert et al., Reference Schubert, Schurch and Boettger2018) was initiated. In patients with hyperactive delirium, pipamperone was administered orally. In delirious patients with hallucinations or aggressive behavior, enteral and/or parenteral administration of haloperidol was added. Oral haloperidol (Haldol® 2 mg/ml, Janssen-Cilag AG, Zug, Switzerland) containing the preservative E 218 was administered as drops in doses of 0.5 or 1 mg. Intravenous haloperidol (Haldol® 5 mg/ml, Janssen-Cilag AG, Zug, Switzerland) containing acidum lacticum and aqua ad iniectabilia q.s. ad solute was injected in steps of 0.5–1 mg iv. The cumulative dose was adjusted by the physician according to the clinical condition. Neither enteral nor intravenous haloperidol contained the preservative chlorobutanol.
Vegetative symptoms were blunted with the α-2 agonists clonidine (as short or continuous infusions) or dexmedetomidine (continuous infusions only). In individuals with nocturnal agitation, insomnia or a risk of a nonconvulsive epilepsy, intravenous midazolam was continuously infused at doses of 0.05–0.1 mg/kg/h and interrupted daily at 6 am.
Data sources
Physiological variables were collected from the hand-written ICU charts and transferred into an electronic database. Results from patient history and clinical examination, the surgical reports, the ECG, as well as the results from laboratory analyses were extracted from the electronic patient documentation system. Physiological and laboratory variable were collected on the day haloperidol was started. No additional study-specific interventions were performed.
Statistical analysis
All analyses were performed with the use of IBM SPSS Statistics (IBM Corp, Armonk, NY). Baseline characteristics were determined for all included patients and described as medians and interquartile ranges based on their parametric properties, in addition to percentages for categorical variables. Subsequent group comparisons were made between patients who developed a new long QT after haloperidol administration and patients who did not. Values are given as median (interquartile range) or numbers (percentages), as appropriate. Groups were compared using the Mann–Whitney U test or the Fisher's exact test, as appropriate. Pearson or Spearman correlations were chosen to determine the dose-dependent effect of haloperidol on QTc. The null hypothesis was rejected with a two-sided p-value < 0.05.
Literature review
A limited literature search was conducted on key resources, including PubMed, as well as focused internet search. PubMed was searched with the filter for clinical trials using the terms “haloperidol AND QT prolongation,” “haloperidol AND QTc prolongation,” “haloperidol AND long QT,” and “haloperidol AND delirium AND critical illness.” The search was also limited to English language documents published between March 2001 and February 2019.
Results
Study population
During the 24-month study period, 2,216 patients were admitted to the cardio-surgical ICU. The patients were rather sick, as evidenced by a median SAPS and ICU mortality of 31 and 4.3%, respectively. On hospital admission, 129 patients had objected to the scientific use of their medical data and, therefore, were not screened. Overall, 169 patients had an incomplete documentation and were, therefore, not screened, too. Of the 1,918 patients screened, 146 received haloperidol. Of these, on 72, no ECG was performed and another six had a pacemaker rhythm and were consequently excluded (Figure 1). In the end, 68 patients were included in this analysis. The median age of patients was 71 years, and 77% were male. All patients underwent cardiac surgery and both median SAPS and ICU LOS were 41 and 6 days, respectively, and mortality did not occur. The interval between ICU admission and haloperidol administration was three days, and the cumulative haloperidol dose was 4 mg. Haloperidol was administered enterally in 7.4%, intravenously in 84%, and both enterally and intravenously in 8.8%.

Fig. 1. Exclusion process.
QTc measurements
There were no differences between machine and manual measurements of the QTc time (data not shown). The median QTc time difference between follow-up and baseline was 3 (−23 to 32) ms. In total, 53% experienced QTc prolongation after haloperidol administration with 31 (12–47) ms.
At baseline, 31% (21/68 patients) had a long QT, and following, after haloperidol administration, 28% (19/68 patients). Only 17% with QTc within normal limits at baseline had a newly onset long QT after haloperidol administration. Patients with and without a newly onset long QT were compared, and the results are displayed in Tables 3 and 4. Inter-group analyses did not reveal significant risk factors. Further, haloperidol was not associated with a dose-dependent QTc prolongation as displayed in Figure 2.

Fig. 2. Correlation between haloperidol dose and QTc intervals. Pearson correlation coefficient: new long QT (squares, n = 8): r = 272, p = 0.515; Controls (circles, n = 60): r = 0.082, p = 0.536.
Table 3. Reviews and meta-analysis
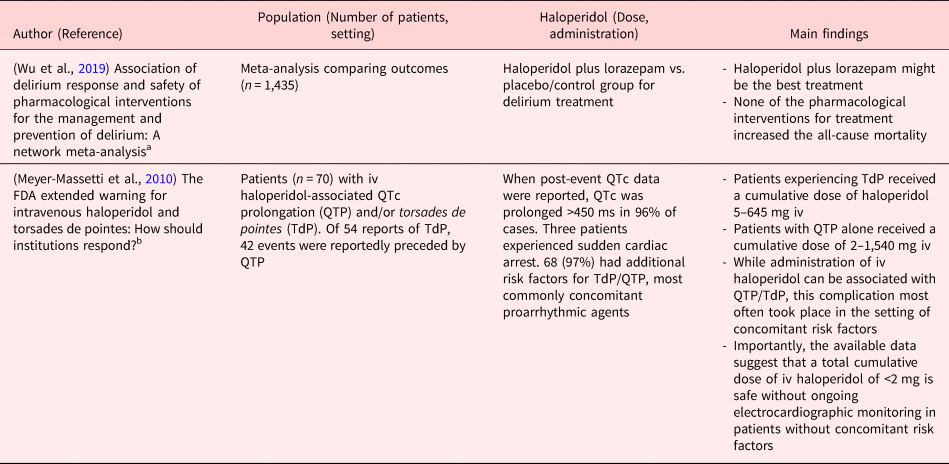
ICU, Intensive Care Unit; iv, Intravenously; po, per os; im, Intramuscular; od, once daily; bid, bis in die (twice daily); PONV, Postoperative Nausea and Vomitus.
Clinical studies in PubMed were searched using the following terms.
a Haloperidol and delirium and critical illness (10 studies).
b FDA haloperidol QTc.
Table 4. Baseline and medical characteristics of patients with new long QT and controls

Results are represented as median (interquartile range). Controls are patients preexisting long QT at baseline (n = 21) and normal QTc time after haloperidol administration (n = 39).
Discussion
Summary of main findings
In this clinical observation study performed in a cardio-surgical ICU, we investigated the relationship between haloperidol administration for delirium and QTc prolongation. First, one-third of patients had a long QT prior to haloperidol administration, and this number did not increase with haloperidol. Second, there was no correlation between haloperidol dose and QTc time, suggesting that haloperidol by itself does not cause relevant QTc prolongations. Third, a detailed analysis between patients with and without a newly onset long QT revealed no significant risk factors. Of note, the time interval between the first dose of haloperidol and the ECG was not different between the two groups. In addition, inter-group differences between the patient with and without a new long QT, the route of haloperidol administration and cumulative haloperidol doses did not exist.
Review of the literature and comparison to the existing literature
The search strategy in PubMed for clinical trials and with the terms “haloperidol and QT prolongation” yielded 16, “haloperidol and QTc prolongation” 19, and “haloperidol and long QT” 13 publications. Further, using the search terms, “haloperidol AND delirium AND critical illness” returned 10 clinical publications. A summary of the 32 retrieved publications is given in Tables 1 and 2. In the more recent studies published between April 2006 and June 2018 (Table 2), the QTc prolongation after haloperidol was considered not significant and across studies, doses were low (between 1 and 5 mg/d). The findings in these studies (Table 2) are comparable with our results. One study (Wang et al., Reference Wang, Tsay and Huang2012) investigated intramuscular haloperidol at 7.5 and 10 mg separated by 4 h: the changes in QTc from baseline were modest and eventually, no patient displayed a QTc interval ≥480 ms. Another study (van den Boogaard et al., Reference van den Boogaard, Schoonhoven and van Achterberg2013) used haloperidol at 10–30 mg/d; however, the route of administration is unknown, and the effect on QTc was not compared vs. placebo.
Conversely, throughout the publications between March 2001 and October 2004 (Table 1), significant QTc prolongations were reported after haloperidol administration. However, the administration of lower doses of haloperidol (up to 0.25 mg/kg) in split doses was not associated with significant QTc prolongations (Desai et al., Reference Desai, Li and Desta2003a). Similarly, another study was not able to replicate significant QTc prolongation after 15 ± 5 mg/d haloperidol vs. other antipsychotics (Kane et al., Reference Kane, Carson and Saha2002). In addition, a cumulative dose of haloperidol <2 mg intravenously was safely administrated in patients without concomitant risk factors without ongoing electrocardiographic monitoring (Lin et al., Reference Lin, Kuo and Chou2010).
This might support the notion as shown in mortalities on methadone that an additive in addition to high dosing might have been responsible for the past reports on QTc prolongation, torsades de pointes, and sudden cardiac death (Kornick et al., Reference Kornick, Kilborn and Santiago-Palma2003). Commonly overseen, haloperidol, like most first-generation antipsychotics, has multiple neurotoxic effects vs. second-generation antipsychotics (Nasrallah and Chen, Reference Nasrallah and Chen2017) and the in vivo extent of this neurotoxicity remains understudied.
Strengths and limitations of the study
This study had few strengths and limitations: over 2 years, 2,216 patients were screened and systematically assessed. Eventually, few patients could be included, preventing the meaningful use of multivariate testing. Apparently, haloperidol was not the first-line treatment of delirium in this ICU — vs. the α-2 agonists clonidine and dexmedetomidine — but reserved for delirious patients with predominant hallucinations and/or aggressive behavior. Further, it was not possible to collect all relevant parameters relevant to QTc interval prolongation; however, this effect might be negligible. Of note, one-third of patients had a long QT before starting haloperidol. Further with a randomized controlled design are required to confirm these findings.
Interpretation
Low-dose intravenous haloperidol is not clinically relevant as risk factor for the development of a newly onset long QT syndrome: For (1) the number of patients with long QT did not increase with haloperidol administration; (2) there was no dose-dependent effect of haloperidol on QTc intervals; and (3) no inter-group differences between the oral and intravenous formulation existed. These results are supported by recent publications inside and outside the ICU setting, and hence, the requirement of ECG monitoring when administering intravenous haloperidol might be unnecessary.
Generalizability
One strength of this study was its setting, the daily clinical practice. However, only ICU patients post major cardiovascular surgery, a vulnerable population for the development of arrhythmia, was included. Underlying cardiac diseases, as well as the cardiac surgery by themselves, represent risk factors for the development of long QT. Hence, these findings may overestimate the frequency of long QT syndromes vs. other critically ill patients. Further studies may benefit from the inclusion of other clinical settings.
Conclusions
In this clinical observation study performed in a cardio-surgical ICU, we investigated the relationship between haloperidol administration for delirium and QTc prolongation. These results indicate the safety of low-dose intravenous haloperidol in the management of delirium; thus, haloperidol did not represent a clinically relevant risk factor for the development of a new long QT syndrome and further support recent publications investigating the safety of low-dose haloperidol inside and outside the ICU setting. Hence, the requirement of cardiac monitoring when administering intravenous haloperidol is questionable for delirious patients suffering from hallucinations and aggressive behavior.
Funding
This work was supported by departmental funds of the Institute of Anaesthesiology, University Hospital Zurich, Zurich.
Conflict of interest
There are no conflicts of interest.









