Introduction
During the past few decades, a distinct change in the Arctic environment may have been seen (Chapman and Walsh Reference Chapman and Walsh1993; Tynan and DeMaster Reference Tynan and DeMaster1997; Serreze and others Reference Serreze, Walsh, Chapin, Osterkamp, Dyergerov, Romanovsky, Oechel, Morison, Zhang and Barry2000). Since the late 19th century, the annual average air temperature in the region has risen by 1.5°C (Overpeck and others Reference Overpeck, Hughen, Hardy, Bradley, Case, Douglas, Finney, Gajewski, Jacoby, Jennigs, Lamoureux, Lasca, MacDonald, Moore, Retelle, Smith, Wolfe and Zieliński1997). Coverage of the Arctic Ocean sea ice decreases systematically (Serreze Reference Serreze, Maslanik, Scambos, Fetterer, Stroeve, Knowles, Fowler, Drobot, Barry and Haran2003; Lindsay and Zhang Reference Lindsay and Zhang2005) with increasing amounts of precipitation (Maxwell Reference Maxwell, Oechel, Callaghan, Gilmanov, Holten, Maxwell, Molau and Sveinbjörnsson1997). The consequence of these processes is a transformation in the functioning of terrestrial ecosystems: coverage of permafrost (Anisimov and Nelson Reference Anisimov and Nelson1996) and the depth of the active layer (Anisimov and others Reference Anisimov, Shiklomanov and Nelson1997), an increase in plant biomass (Epstein and others Reference Epstein, Walker, Chapin and Starfield2000), and changes in stocks of biogenic elements in soils (McGuire and others Reference McGuire, Melillo, Kicklighter and Joyce2005). It is estimated that the Boreal and the Arctic have between 20% and 60% of global soil carbon (Schlesinger Reference Schlesinger1977; Post and others Reference Post, Emanuel, Zinke and Stangerberger1982; Gorham Reference Gorham1991). The temperature rise, as a result of global warming, will probably increase the rate of soil biological activity of the polar regions, and, as a result, an additional amount of CO2 can be put into the atmosphere (Luo and Zhou Reference Luo and Zhou2006).
The biological activity of the soil is determined by direct and indirect methods. The total number of microorganisms is determined by the direct method (Holding Reference Holding, Bliss, Heal and Moore1981; Simankova and others Reference Simankova, Kotsyurbenko, Steckebrandt, Kostirikina, Lysenko, Osipow and Nozhevnikova2000), while indirect methods are based on the products of their activity, such as released CO2 or the activity of certain enzymes. One of the methods of assessment of the biological activity of soil is also the determination of the total cellulolytic activity (Russel and others Reference Russel, Górska and Wyczółkowski2005). Gravimetric methods, based on the weight loss of cellulosic material placed in an analysed environment or medium, are often used for the determination of cellulolytic activity. The disadvantage of these methods is getting results that are inflated in relation to the real rate of organic matter decomposition (Niewinna Reference Niewinna2009). However, they are widely used due to their low costs and easy implementation (Bieńkowski Reference Bieńkowski1990a; Niewinna Reference Niewinna2009). Gravimetric methods are connected with the determination of mass loss of linen fabrics, filter paper or other cellulosic materials buried for a limited time. These methods were applied in determining the influence of acidification of the Silesia, Poland, soils region on their degree of biological activity (Bieńkowski Reference Bieńkowski1990a). Drewnik (Reference Drewnik1996, Reference Drewnik2006) used the method of mass loss of cellulose filter papers for the description of the influence of environmental factors on the differentiated rate of organic matter decomposition in soils of the Carpathian mountains. A gravimetric method was also used for the determination of cellulolytic activity of tundra soils of the Yamal Peninsula and the Ural mountains (Andreyashkina and Peshkova Reference Andreyashkina and Peshkova2001). Fisher and others (Reference Fisher, Niewinna and Yasulbutaeva2006), on the basis of results obtained by the same method, carried out a comparative study of the biological activity of soils of different ecosystems (desert, steppe, mountain) in Dagestan. Previous studies on cellulolytic activity showed an extremely low rate of organic matter decomposition in arctic soils (Rosswall Reference Rosswall and Holdings1974; Bieńkowski Reference Bieńkowski1990b). Because of the large diversity of soils of polar ecosystems, the current level of knowledge about their functioning is insufficient. This is particularly important in the context of determining the paths of transformation of the soil environment as a result of still changing climate conditions.
The aim of this research is to determine the cellulolytic activity of soils of coastal lowlands of west Spitsbergen. An important aspect of the study was to determine the influence of the basic properties of soils on the rate of cellulose decomposition. This objective was based on a comparative analysis of cellulolytic activity in two locations representing different soil conditions. These studies will be, one hopes, important as a ‘reference point’ for future teams preparing to study the dynamics of the soil environment of the west Spitsbergen tundra under a changing climate.
Subject and methods
West Spitsbergen is a mountainous region covered extensively (40–60%) by glaciers. The presence of glacial and fluvioglacial relief forms is connected with glaciation: frontal and lateral moraines, outwash plains, fluvioglacial cones and more specific forms are observed.
Along the coast there are coastal plains made up of a system of marine terraces raised as a result of Pleistocene and Holocene glacial isostatic movements (Lindner and others Reference Lindner, Marks, Roszczynko and Emil1991; Landvik and others Reference Landvik, Bondevik, Elverhoi, Fjeldskaar, Mangerud, Salvigsen, Siegert, Svendsen and Vorren1998). One of these plains is covered by the area studied named Calypsostranda lying in the northwest part of Wedel Jarlsberg Land. This plain is located in the southern part of Bellsund and on the western coast of Recherche Fiord. The width of Calypsostranda reaches up to 2 km, and its length is about 5 km. Seven terraces with a height of 2 to 85 m above sea level are distinguished within Calypsostranda (Zagórski Reference Zagórski2002). The highest and oldest terraces (VII and VI) are abrasive platforms, created as a result of destructive activity of sea waves, uplifted probably in previstulian period, modified by glacial exaration. The lower, younger terraces (V to I) are accumulative and are covered by Quaternary glacial, fluvioglacial and marine sediments (Pękala and Repelewska-Pękalowa Reference Pękala, Repelewska–Pękalowa, Pękala and Repelewska–Pękalowa1990; Salvigsen and others Reference Salvigsen, Elgersma, Landvik, Pękala and Repelewska–Pękalowa1991).
The vegetation of Calypsostranda, where research station Calypsobyen is situated is less developed in comparison with the communities described in the proximity of other research stations located on the west coast of Spitsbergen (Hornsund and Kaffiøyra). This is due to lower precipitation and lower average temperatures in the summer which relate to Calypsostranda being sheltered from direct exposure to moist air masses from the Atlantic (Borysiak and Ratyńska Reference Borysiak, Ratyńska, Kostrzewski, Pulina and Zwoliński2004). Święs (Reference Święs, Pękala and Repelewska–Pękalowa1988) has identified eight major complex plant communities. Dry lichen-moss tundra is the most common and is typical for the broad, flat marine terraces. It is divided into initial and deflationary tundra, with Dryas octopetala and grey lichen tundra with Cladonia sp. In addition, in Calypsostranda there are also plant communities of arctic deserts, dry moss associations on rock denudation, foothill peat mosses, bog moss, grass and moss community edges of lakes and waterways. The most numerous species present are, among others: Salix polaris, Salix reticulata, Oxyria digyna, Dryas octopetala, Polygonum viviparum, Cerastium arcticum, Silene acaulis, Saxifraga oppositifolia and Saxifraga caespitosa (Rzętkowska Reference Rzętkowska1987).
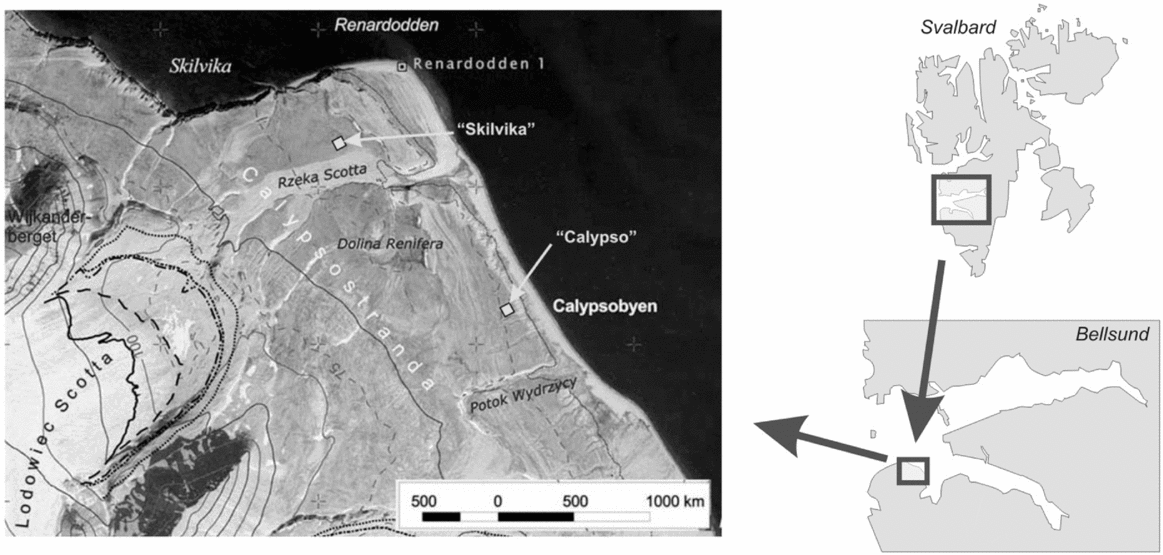
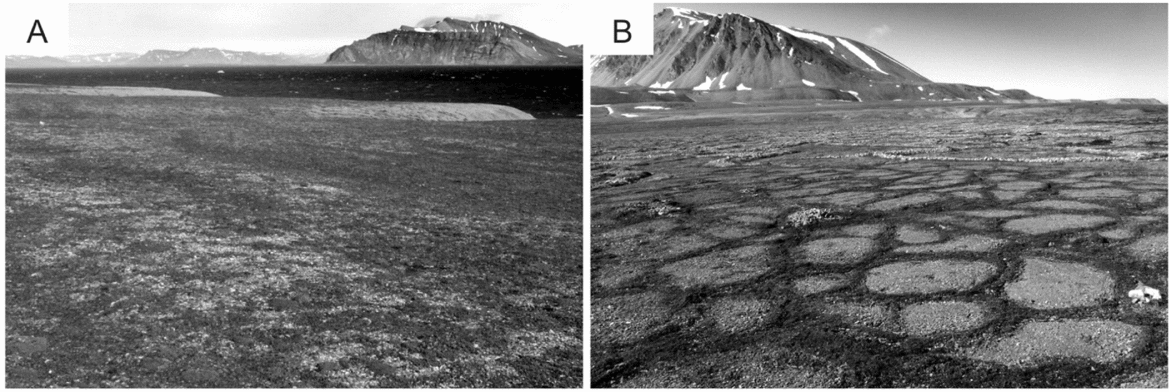
Two locations were selected for the study of soil cellulolytic activity, representing the most common soil types of Calypsostranda. The distance between the positions in a straight line is about 2 km (Fig. 1). The first position (Calypso) is situated at a height of 19 m.a.s.l., approximately 200 m from the Bellsund coast near the research station Calypsobyen. Within the position, slightly convex polygons occur which are called tundra ‘Jahn’ polygons (Fig. 2), resulting from the impact of cryogenic processes of marine sediments, sandy gravels or gravelly sands. These polygons are of considerable size, from a few metres to several metres in diameter, and their occurrence is particularly common in the lower marine terraces of Calypsostranda (Klimowicz and others Reference Klimowicz, Melke, Uziak and Chodorowski2008). The polygons are connected with Arctic tundra brown soils. According to the WRB classification (IUSS-FAO 2007), these soils are classified as Turbic Cryosols (Skeletic) and Turbic Cambic Cryosols (Skeletic). The typical morphology of the arctic brown soil profile (A-Bw-C (r)) is characterised by the presence of both a well-developed surface horizon of humus accumulation (about 2% Corg.) and the horizon of enrichment in the compounds of iron, passing smoothly at the depth of several tens of centimetres into the parent rock, showing gleying properties often associated with the influence of a permafrost ceiling. This position is characterised by a significant degree of coverage (70–100%) by dry tundra vegetation dominated by Salix polaris, Polygonum viviparum, Cerastium arcticum, Silene acaulis, Saxifraga oppositifolia.

Fig. 1. Location of research positions. Fragment of orthophotomap northwest part of Wedel Jarlsberg Land (Zagórski Reference Zagórski2005).
The second position (Skilvika) is located north of the Scott River outwash plain at a height of 16 m.a.s.l. In terms of geomorphology, it represents remnant depression of the former sea gulf within the terrace III (Szczęsny and others Reference Szczęsny, Dzierżek, Harasimiuk, Nitychoruk, Pękala and Repelewska–Pękalowa1989). Skilvika represents the Arctic gleyic soils associated with the occurrence of gleyed polygonal (cell) grounds created in a strong influence of cryogenic processes (Fig. 2). The central part of the cell is a strongly gleyed mineral material with a texture of sandy clay with a significant amount of silt, showing no morphological differentiation into horizons. Particular cells are limited by cracks in the form of polygons with a diameter of 0.5–1.0 m which accumulate very coarse (stony) rock material along with organic matter in varying degrees of decomposition. Crack depth ranges from several centimetres to tens of centimetres. The vegetation coverage rate is about 50%, and vegetation – mosses, Salix polaris, Polygonum viviparum, Saxifraga oppositifolia or Saxifraga caespitosa – develops mainly within the cracks.

Fig. 2. Research positions: A - Calypso, dry deflation tundra; B – Skilvika – gleyed polygonal soils.
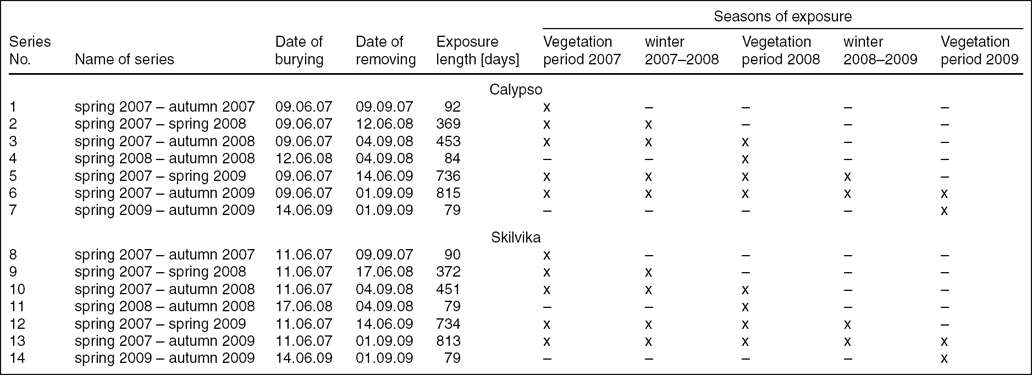
Field studies were conducted during the growing seasons (June–September) in 2007–2009. Cellulolytic activity of the soils was determined by a gravimetric method (Russel and others Reference Russel, Górska and Wyczółkowski2005) based on weight loss of cellulosic material buried in the surface layers of soil. Discs of filter paper (previously weighed and numbered) were buried at a depth of 0–10 cm within each test area in the centre of the tundra Jahn polygons (Calypso) and cell polygons (Skilvika). These discs had a diameter of 90 mm and were attached to glass plates (area of 100 cm2). The whole set was packed in nylon net, facilitating the removal of partially digested filter paper discs from the soil and protecting them from the sticking of soil peds to the cellulosic substrate. For each of Calypso and Skilvika seven series of determinations were performed (20 repetitions in each series) with different lengths of time and dates of exposure of filter papers (Table 1). The rate of cellulose decomposition was expressed as annual (g · g−1 · year−1) and daily (mg · g−1 · 24h−1) fractional weight losses and recalculated to k coefficient using the equation for organic matter decomposition with no production (Olson Reference Olson1963; Carnevale and Levis Reference Carnevale and Lewis2001):
k = − (ln(W/Wi))/t; where W – weight of partially decayed cellulosic discs, Wi – initial weight of cellulosic discs, t – time (year). Furthermore, the time for 95% (3/k) weight loss was calculated (Olson Reference Olson1963).
During the course of the study, basic elements of weather were recorded. In order to determine the basic soil properties, soil material was collected from a depth of 0–5 and 10–15 cm, as composite samples (Petersen and Calvin Reference Petersen, Calvin, Sparks, Page, Helmke and Loeppert1996) to provide reliable description of sites. In fine earthy particles (with a diameter <2 mm), the following determinations were made:
Texture by areometric-sieve method, (Casagrande, modified by Prószyński);
Organic carbon content (Corg.) by Tiurin method (Lityński and others Reference Lityński, Jurkowska and Gorlach1976);
Total nitrogen content (Forster Reference Forster1996);
Total phosphorus content (Grimshaw Reference Grimshaw and Rowland1987);
Carbonate content by Scheibler volumetric apparatus (Lityński and others Reference Lityński, Jurkowska and Gorlach1976);
Soil pH electrometrically in H2O and 1 M KCl (Thomas Reference Thomas, Sparks, Page, Helmke and Loeppert1996);
Exchangeable cations content by Mehlich method (Lityński and others Reference Lityński, Jurkowska and Gorlach1976).
Table 1. Length and period of discs exposure.

x – period of discs decomposition in soil
Results
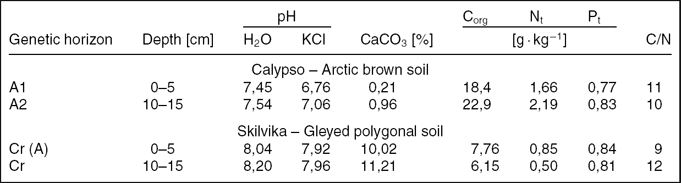
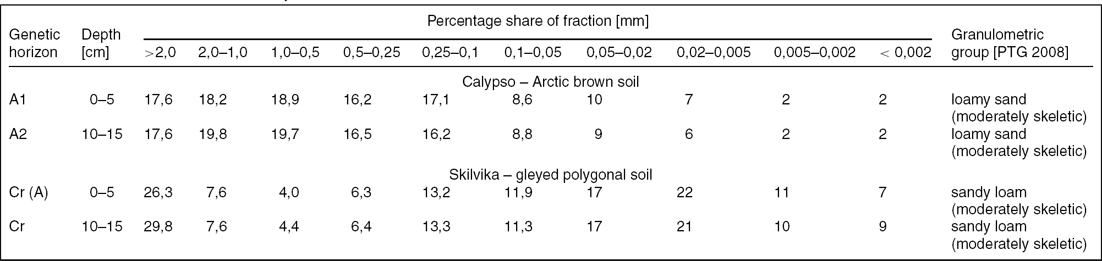
Surface humus horizons of arctic brown soils occurring within the Calypso position were characterised by a significant content of sand fractions (approximately 80%) and extremely low content of clay fractions (Table 2). Coarse parts in the form of gravel and stones (> 2 mm) constituted over 17%. Gleyed polygonal soils of Skilvika contained far greater amounts of silt fractions (approximately 50%) and clay (7–9%) at a much lower content of sand fractions. The share of coarse parts in these soils was considerable and ranged from 26–30%.
Table 2. Grain size distribution of soils analysed.
The content of organic carbon and total nitrogen was much higher in the surface horizons of Arctic brown soils in comparison with similar horizons of gleyed polygonal soils. Both test plots contained similar amounts of phosphorus, regardless of the depth of sampling. The humus horizon of arctic brown soils was neutral, while the soils of Skilvika exhibited an alkaline reaction that was connected to the presence of carbonates, which was much higher in the gleyed polygonal soils (Table 3).
Table 3. Selected chemical and physicochemical properties of soils analysed.

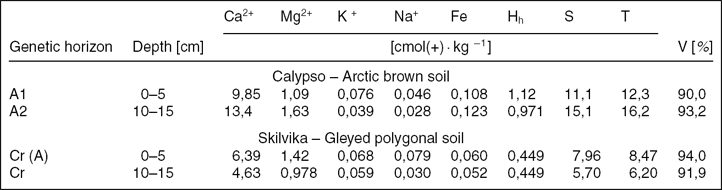
Calypso surface soils were characterised by a higher cation exchange capacity when compared to Skilvika soils (Table 4). Regarding the composition of the exchangeable cations of both types of soil dominated by Ca2+, a significant share of the cations Mg2+ and H+ was observed. The lowest content was noted for K+, Na+, and Fe. Due to the high content of calcium cations, the degree of saturation of the sorptive complex by base cations was significant and equalled or exceeded 90% in all cases.
Table 4. Properties of sorptive complex of soils.

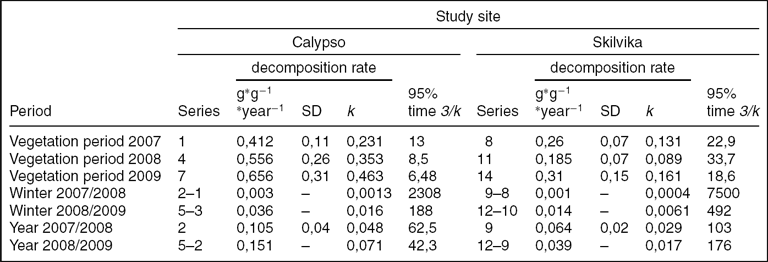
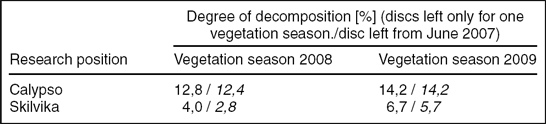
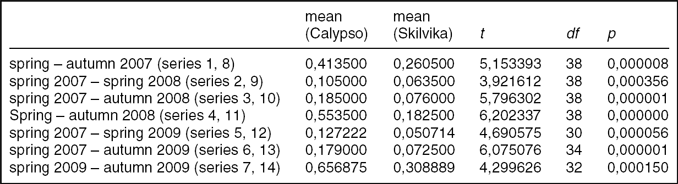
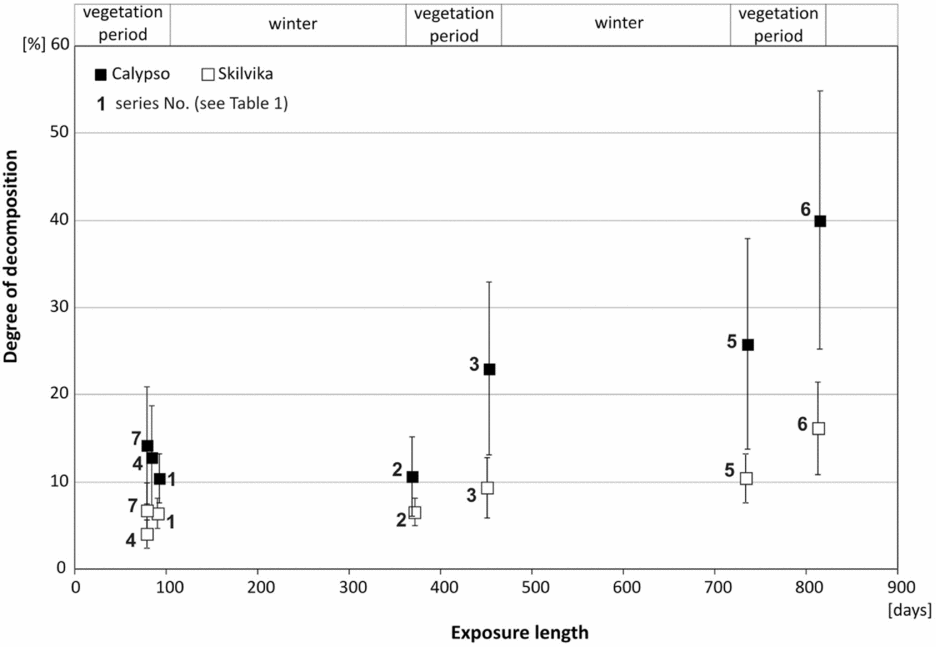
Despite the very long exposure of the cellulose discs, the degree of decomposition was relatively low (Fig. 3). In addition, there were significant differences in cellulolytic activity between the two research locations. Mean weight losses of cellulose discs for growing the seasons (vegetation periods) 2007, 2008 and 2009 (series of spring–autumn) in the Arctic brown soils were 10.4% (± 2,8 SD), 12.8% (± 5,9 SD) and 14.2% (± 6,7 SD), respectively. In the gleyed Skilvika polygonal soils, weight losses were even two or three times lower: 6.4% (± 1,75 SD), 4.0% (± 1,6 SD) and 6.7% (± 3,1 SD), respectively. The annual fractional weight losses during vegetation periods ranged between 0,412–0,656 in Calypso and 0,089–0,161 (g · g−1 · year−1) in Skilvika (Table 5). Similar results of the decomposition rate of cellulose discs in the growing seasons of 2008 and 2009 were also obtained from a comparison of their degree of decomposition in the series left for a longer period (Table 6). For this purpose, a series of discs buried in the spring of 2007 and dug out at the beginning and end of growing seasons in successive years (2008 and 2009) were used. The data obtained were used for calculation of the average rate of cellulose decomposition in the resting periods 2007/08 and 2008/09 (snow cover presence besides the growing period). The degree of decomposition of buried filter papers in that period was extremely low and amounted to 0.2 and 2.8% (0,003–0,036 (g · g−1 · year−1)) in arctic brown soils and 0.1 and 1.1% (0,001–0,014 (g · g−1 · year−1)) in the gleyed polygonal soils. For purposes of comparison with results obtained by other authors, the degrees of decomposition in the growing seasons 2007, 2008 and 2009 have been converted to a rate of decomposition of cellulose, expressed in mg · g−1 · 24h−1. These values were, respectively: 1.13, 1.52 and 1.80 for Calypso and 0.71, 0.51 and 0.85 for Skilvika. A comparison of these values shows that the average rate (for all three growing seasons) of cellulose decomposition was higher in the area of Calypso by 2.23 times. Differences in decomposition rate between both sites were tested statistically, for all research periods (Table 7).
Table 5. Cellulose decomposition rate and parameters.

Table 6. Influence of exposure length on their degree of decomposition.

Table 7. Statistics of cellulose decomposition rate (g*g*year−1) for Calypso and Skilvika, for particular research periods.


Fig. 3. Comparison of average (± SD) rate of decomposition of cellulose discs.
Discussion
The soils under study are influenced by similar climatic conditions. Significant differences in cellulolytic activity must therefore be associated with different soil properties in both locations of the research. The main factor determining the biological activity of soil is organic matter content. Surface horizons of Arctic brown soils contained 2.3 times more organic carbon than gleyed polygonal soils, which corresponds to more than double the cellulolytic activity of these soils. It should also be noted that gleyed polygonal soils have a much lower ratio of humic to fulvic acids in humus compounds which is strongly associated with the mineral part of soil (Klimowicz and others Reference Klimowicz, Melke, Uziak and Chodorowski1999). In gleyed soils, the value of this ratio is only 0.17, while in the Arctic brown soils, it increases to 0.59 (Klimowicz and others Reference Klimowicz, Melke, Uziak and Chodorowski2008). The relatively high content of organic matter in soils of Calypso is the effect of considerable coverage by deflationary tundra vegetation; this, in turn, is a result of the stability of the land and low intensity of cryogenic separation processes (Klimowicz and others Reference Klimowicz, Melke, Uziak and Chodorowski2008). The increased content of organic matter in Arctic brown soils contributed to an increased cation exchange capacity, especially the content of calcium and hydrogen ions which have an impact on soil microbial activity. Another factor that influences the activity of soil microorganisms in arctic brown soils is their better air-water conditions connected to a coarser texture. Gleyed Skilvika polygonal soil contains much higher silt and clay fractions. For this reason, during the period of snow melting and thawing of ceiling parts of the active layer of permafrost, soils are characterised by a moisture content close to the maximum water capacity, hindering the development of higher plants in the central part of the polygon. Previous studies on redox potential have confirmed the dominance of reducing conditions. There is even a strong anaerobiosis in the water-saturated gleyed soils of Calypsostranda (Melke and Uziak Reference Melke and Uziak1989).
The results showed that it would take 42,3–62,5 years (Calypso) and 103–176 years (Skilvika) to decompose 95% of organic matter in the studied soils. The results are consistent with the data provided by other authors. In tundra soils it is around 100 years. The time required for 95% of organic matter to decompose in temperate deciduous forests is four times longer whereas in savannah it takes only 1 year (Montagnini and Jordan Reference Montagnini and Jordan2005).
Comparison of the degree of decomposition of cellulose discs in subsequent growing seasons showed significant differences in particular years. These differences may be due to variable climatic conditions. In subsequent seasons of research (6 June 2007–9 September 2007, 16 June 2008–7 September 2008, 11 June 2009–9 September 2009), the average soil temperatures at a depth of 5 cm were, respectively: 5.8, 5.5 and 5.9°C, while precipitation varied as follows: 52.1, 58.8 and 32.7 mm. In gleyed polygonal soils of Skilvika, the annual trend of changes in decomposition of cellulose discs is similar to the changes in average soil temperatures in each season of research. In the Arctic brown soils, a steadily increasing degree of decomposition of cellulose discs was observed (Fig. 3, Table 5). This increase does not reflect changes in average precipitation and soil temperatures calculated for the entire growing seasons. Finding the relationship between changing climatic conditions and cellulolytic activity of these soils therefore requires a more detailed analysis. It is possible that the biological activity of these soils, due to the large amount of organic matter and favourable air-water conditions, is not so sensitive to small changes in weather conditions, as it is in gleyed polygonal soils.
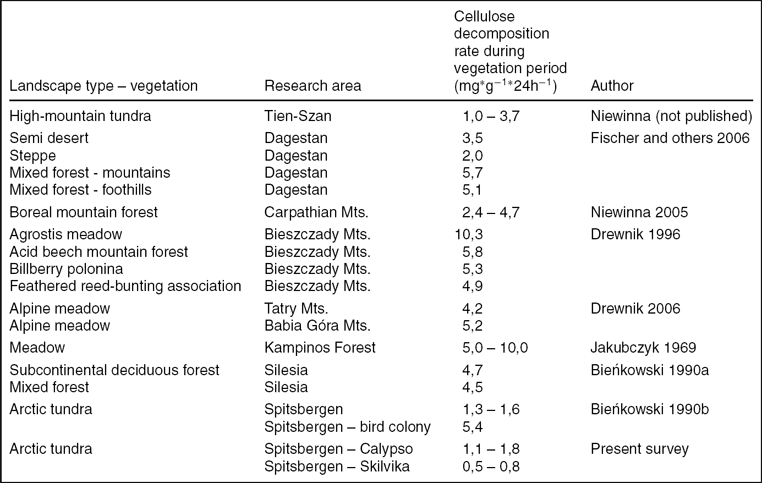
In comparison with the soil ecosystems of lower latitudes, the soils analysed are characterised by low cellulolytic activity resulting from the harsh climatic conditions (Table 8). In the forest and meadow soils in mountain areas of the Carpathians and the Caucasus, the rate of decomposition of cellulose is several times higher (Drewnik Reference Drewnik1996, Reference Drewnik2006; Niewinna 2005; Fisher and others Reference Fisher, Niewinna and Yasulbutaeva2006). A similar or slightly higher rate of degradation was observed in alpine tundra soils of Tien-Szan (Fisher and others Reference Fisher, Niewinna and Yasulbutaeva2006 after Niewinna unpublished). In comparison with the results for other areas of Spitsbergen, the rate of decomposition of cellulose in the Arctic brown soils on the area of Calypso was almost identical to the rate of this process in the tundra soils surrounding Horsund (Bieńkowski Reference Bieńkowski1990b). Among the soils of Spitsbergen, the largest cellulolytic activity was found in organic soils under the influence of nutrients flowing from the little auk (Alle alle) colony (Bieńkowski Reference Bieńkowski1990b). The lowest rate of cellulose decomposition in the growing season, less than 1 mg · g−1.24h−1, was definitely found in gleyed polygonal Skilvika soils.
Table 8. Comparison of cellulose decomposition rate in various ecosystems (Fisher and others Reference Fisher, Niewinna and Yasulbutaeva2006, modified).

An additional methodological aspect of the interpretation of results is the possibility to compare the degree of decomposition of filter papers to evaluate the impact of the length of exposure time of filter papers on the rate of their decomposition. In all the analysed cases, the length of time of remaining filter papers in the soil did not significantly change the rate of decomposition. The average degree of decomposition of filter papers buried only for the vegetation periods of 2008 and 2009 was almost identical to the average degree of decomposition calculated from the differences between the respective series of filter papers buried in June 2007 (Table 6).
Conclusions
1. The soils of the study area are characterised by very low cellulolytic activity, as shown by the low degree of decomposition of cellulose discs on both positions of the research.
2. Higher cellulolytic activity was observed in Arctic brown soils. The lower rate of cellulose decomposition in gleyed polygonal soils is probably due to a significantly lower content of organic matter resulting from the coverage of the central part of polygons by vegetation.
3. The indirect factor influencing cellulolytic activity of the soils is the grain size distribution. In the soils of Skilvika, the higher content of silt and clay fractions causes unfavourable water-air conditions, thereby reducing the development of higher plants in the central part of the polygon, and affects only a small accumulation of organic matter in surface horizons.
4. Excessive soil moisture of gleyed polygonal soils during snow melting and thawing of ceiling parts of the active layer of permafrost may impede the cellulolytic activity of these soils in relation to the better-aerated Arctic brown soils.
5. The length of exposure of cellulose discs did not affect significant changes in the rate of decomposition.
Acknowledgements
The study was conducted under a grant of the Ministry of Science and Higher Education N N305 118 31/3955.