Introduction
Plants have evolved a variety of defenses to prevent insect herbivory, which can influence herbivore behavior, growth/development, and fecundity (Schoonhoven et al., Reference Schoonhoven, Van Loon and Dicke2005; Walters, Reference Walters2011). However, these direct plant chemical defenses may indirectly reduce the performance of parasitoids also attacking these herbivore pests (Campbell & Duffy, Reference Campbell and Duffy1979; Barbosa et al., Reference Barbosa, Saunders, Waldvogel, Visser and Minks1982; Turlings & Benrey, Reference Turlings and Benrey1998; Singer & Stireman, Reference Singer and Stireman2003; Hunter, Reference Hunter2003; Ode, Reference Ode2006; Gols et al., Reference Gols, Bukovinszky, van Dam, Dicke, Bullock and Harvey2008). Among the parasitoid types most affected, koinobionts may suffer more than idiobionts because they allow insect herbivores to continue to feed and develop after parasitization, permitting insect hosts to further ingest toxic plant tissues (Harvey et al., Reference Harvey, Harvey and Thompson1995; Strand, Reference Strand, Hochberg and Ives2000). Therefore, the quality of plants consumed by herbivore hosts after parasitization may affect koinobionts more than idionbionts, which arrest host development and prevent further feeding.
Thus, understanding genetic variation in plant defenses is important for resolving how artificial (e.g., plant domestication) and natural selection influence plant phenotypes (Underwood et al., Reference Underwood, Morris, Gross and Lockwood2000). Variation in constitutive defense among artificially selected cultivars derived from the same parent species may indicate the potential extent to which natural selection can shape herbivore resistance in natural populations. So, genetic variation in plant resistance has been widely used to artificially select for pest-resistant crops (Kennedy & Barbour, Reference Kennedy, Barbour, Fritz and Simms1992). During this artificial selection process, however, the potential impacts of crop plants on parasitoids are infrequently considered. Studies into the influence of host plant genotype on the performance of parasitoids are scarce and predominantly conducted on gall-forming herbivores and their natural enemies (Johnson, Reference Johnson2008; Schädler et al., Reference Schädler, Brandl and Kempel2010). In studies examining the impact of pest-resistant crops and wild relatives on parasitoids, plant genotype is most often analyzed as a fixed effect variable. Such studies are limited in their scope of inference because these findings cannot be extrapolated to new taxonomic groups (Bolker et al., Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2009). Therefore, to fully understand the effects of plant resistance to herbivorous hosts on parasitoids, it is of necessity to quantify the variability in parasitoid life history traits among levels of the plant resistance to hosts.
In this study, we randomly selected seven soybean cultivars from a stock of soybean genotypes varied in resistance to herbivorous Spodoptera litura (Lepidoptera: Noctuidae) larvae, to examine how these soybean cultivars influence variability in developmental and reproductive parameters of the higher trophic level parasitoid Meteorus pulchricornis (Hymenoptera: Braconidae).
Materials and methods
Plants and insects
We randomly chose seven soybean cultivars (‘PI227686’, ‘Larma’, ‘Kefeng No. 1’, ‘NN1138-2’, ‘NNCD5’, ‘JLNMH’, and ‘FPHD’) from the National Soybean Germplasm Collection in Nanjing Agricultural University, which had 20 soybean cultivars. This line of soybean genotypes shows varying levels of resistance to the tobacco cutworm S. litura (Zhan & Gai, Reference Zhan and Gai2000; Wu et al., Reference Wu, Wu, Wu, Wang, Gai and Yu2006). The soybean cultivars were sown in plastic pots (20 cm in diameter and 18 cm in depth) covered with gauze net in an open field. The plants were used in experiments when they reached the 6–7th trifoliate leaf stage.
Laboratory colonies of S. litura and M. pulchricornis were established in 2010 from soybean fields in the suburb of Nanjing (32.0°N and 118.7°E). S. litura is a polyphagous herbivore capable of feeding on 150 plant species and one of the most economically important insect pests in Asia with the larvae inflicting severe damage to soybean plants in eastern China (Rao et al., Reference Rao, Wightman and Rao1993; Zhan & Gai, Reference Zhan and Gai2000). S. litura was maintained in colony on artificial diet (Shen & Wu, Reference Shen and Wu1995) in incubators (25°C ± 1, 60 ± 10% RH, and 14:10 h light/dark photoperiod). Emerged adults were transferred in groups to a cage (23 cm long × 22.5 cm wide × 32 cm high) with 40-mesh nylon organza over wooden frames to oviposit, where supplemental food of 10% honey liquid was mediated in cotton and paper strips provided as the substrate for eggs.
Meteorus pulchricornis is a thelytokous solitary endoparasitoid of numerous free-living lepidopteran larvae exposed on plant foliage (Maeto, Reference Maeto1989). It is one of the most important natural enemies of lepidopteran pests in East Asia, including Helicoverpa armigera and Spodoptera species (Takashino et al., Reference Takashino, Kobayashi and Okada1998; Liu & Li, Reference Liu and Li2006, Reference Liu and Li2008). In New Zealand, a uniparental strain was first detected in 1996 and attacks a wide range of indigenous species of Lepidoptera (Berry & Walker, Reference Berry and Walker2004). Its biparental strain was introduced from Europe into the USA for biological control of the gypsy moth, Lymantria dispar (Fuester et al., Reference Fuester, Taylor, Peng and Swan1993). M. pulchricornis attacks medium-aged larvae (Liu & Li, Reference Liu and Li2006, Reference Liu and Li2008), but prefers younger larvae and survives better in the 2nd than in the 4th instar S. litura larvae (Chen et al., Reference Chen, Li and Meng2011). A laboratory colony was maintained using S. litura 3rd instar larvae as hosts. Parasitoids used in the experiments were all 6–8 days old without experience of parasitism.
Developmental and reproductive performances of parasitoids
Spodoptera litura 2nd and 4th instar larvae were used as hosts. To obtain host larvae for parasitism, we transferred S. litura egg masses to potted soybean seedlings of respective testing cultivars. When the larvae reached the 2nd or 4th instar, they were individually weighed (AL204-IC, Mettler Toledo Microbalance) and then exposed to a naïve 6–8 days old wasp for parasitism in a glass tube (2 cm in diameter and 8 cm in depth). The parasitoids without stinging hosts within 10 min were replaced. Once the larvae were attacked, they were individually transferred to Petri dishes (5 cm in diameter and 1.3 cm in depth) where fresh leaves excised from the same soybean cultivars as before parasitism were provided as food and replaced daily. Feeding stopped when parasitoids larvae egressed from hosts or host larvae pupated. Parasitoid pupae were individually collected in glass tubes (2 cm in diameter and 8 cm in depth), which were covered with gauze, maintained in an incubator and examined daily for adult emergence. Emerging wasps were individually introduced into containers (14 cm in diameter and 6 cm in depth) without supplemental food, where 10 S. litura 2nd instar larvae were provided as hosts for parasitism. The host larvae were replaced daily until the parasitoid died. Those replaced larvae were subsequently dissected to confirm parasitism under a stereo-microscope (Olympus, SZ-CTV) 36–48 h later (the time for eggs swelled to be easily recognized). The dead wasps were then measured in the hind tibia length under a microscope (Motic Digital Microscope, DM-143, PAL system). We recorded parasitoid's developmental time (egg-to-larval egression from hosts), survival success to adult, adult body size (measured as hind tibia length), and realized lifetime fecundity (measured as the number of hosts parasitized by a parasitoid in a lifetime without supplementary food). For each soybean cultivar by host instar treatment 30 replicates were run.
Statistical analyses
Since our focus was to extrapolate from this study the effects of resistant soybean cultivars on variability of parasitoid life history traits, we used mixed effects model to analyze random effects for soybean cultivars to make predictions of unobserved population effects, by treating the soybean cultivar as a random effect variable, and including host body weight as a covariate (fixed effect variable) to adjust for its effect. The data for the 2nd and 4th instar hosts were analyzed separately to obtain estimates of the random effects (intercept variation) for each instar host. We used a linear mixed model to analyze emerging wasp hand tibia length (numerical data, Gaussian distribution), and a generalized linear mixed-effects model to analyze survival success (binary data, logit-binomial link distribution), developmental time, and fecundity (both count data, log-poisson link distribution). Individual-level variability was used to account for overdispersion if necessary (Bolker et al., Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2009). We made predictions for soybean cultivar effects from the conditional standard deviation (Bates, Reference Bates2010). We performed ‘lme4’ package to use mixed effects models for the analyses with the statistical program R 3.1.0 (R Development Core Team, 2014).
Results
The development time of offspring parasitoids in the 2nd and 4th instar host larvae was summarized in fig. 1. It varied slightly in both the 2nd (variance = 0.007; model fit: log-likelihood = −17.1, deviance = 34.2, AIC = 40.2, BIC = 50.7, df. resid = 240) and 4th instar hosts (variance = 0.010; model fit: log-likelihood = −11.4, deviance = 22.7, AIC = 28.7, BIC = 39.2, df. resid = 242) across soybean cultivars (fig. 2). Survival to adulthood in the 2nd and 4th instar host larvae was summarized in fig. 3. It varied greatly across soybean cultivars, but more in the 4th (variance = 1.158; model fit: log-likelihood = −107.6, deviance = 215.2, AIC = 221.2, BIC = 231.6, df. resid = 240) than 2nd (variance = 0.819; model fit: log-likelihood = −138.9, deviance = 277.8, AIC = 283.8, BIC = 294.3, df. resid = 242) instar host larvae (fig. 4). The hind tibia length of offspring adults was summarized in fig. 5, varying slightly in both the 2nd (variance = 0.011; model fit: log-likelihood = −157.7, deviance = −311.3, AIC = −307.4, BIC = −295.3, df. resid = 152) and 4th (variance = 0.007; model fit: log-likelihood = 206.0, deviance = −411.7, AIC = −404.1, BIC = −391.3, df. resid = 177) instar hosts across soybean cultivars (fig. 6). Lifetime fecundity of parasitoid offspring from attacking the 2nd and 4th instar host larvae was summarized in fig. 7, varying substantially, but bigger in the 2nd (variance = 1.753; model fit: log-likelihood = −377.6, deviance = 755.1, AIC = 761.1, BIC = 770.2, df. resid = 152) than 4th (variance = 0.583; model fit: log-likelihood = −219.8, deviance = 439.6, AIC = 447.6, BIC = 460.3, df. resid = 177) instar larvae across soybean cultivars (fig. 8).
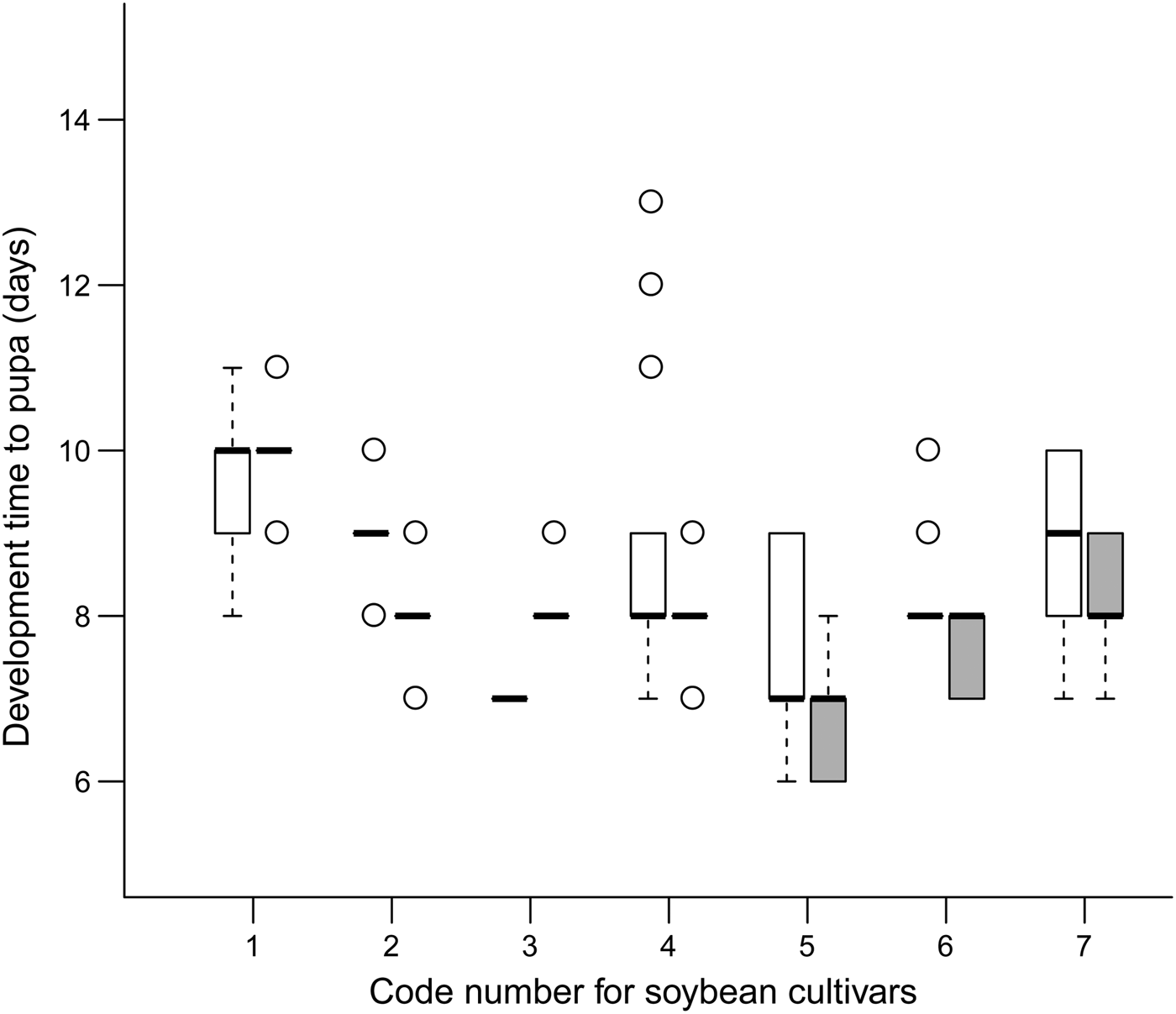
Fig. 1. Egg-to-pupa development time (medians and interquartile ranges) of offspring parasitoids in the 2nd (empty bar) and 4th instar (filled bar) S. litura larvae across soybean cultivars.
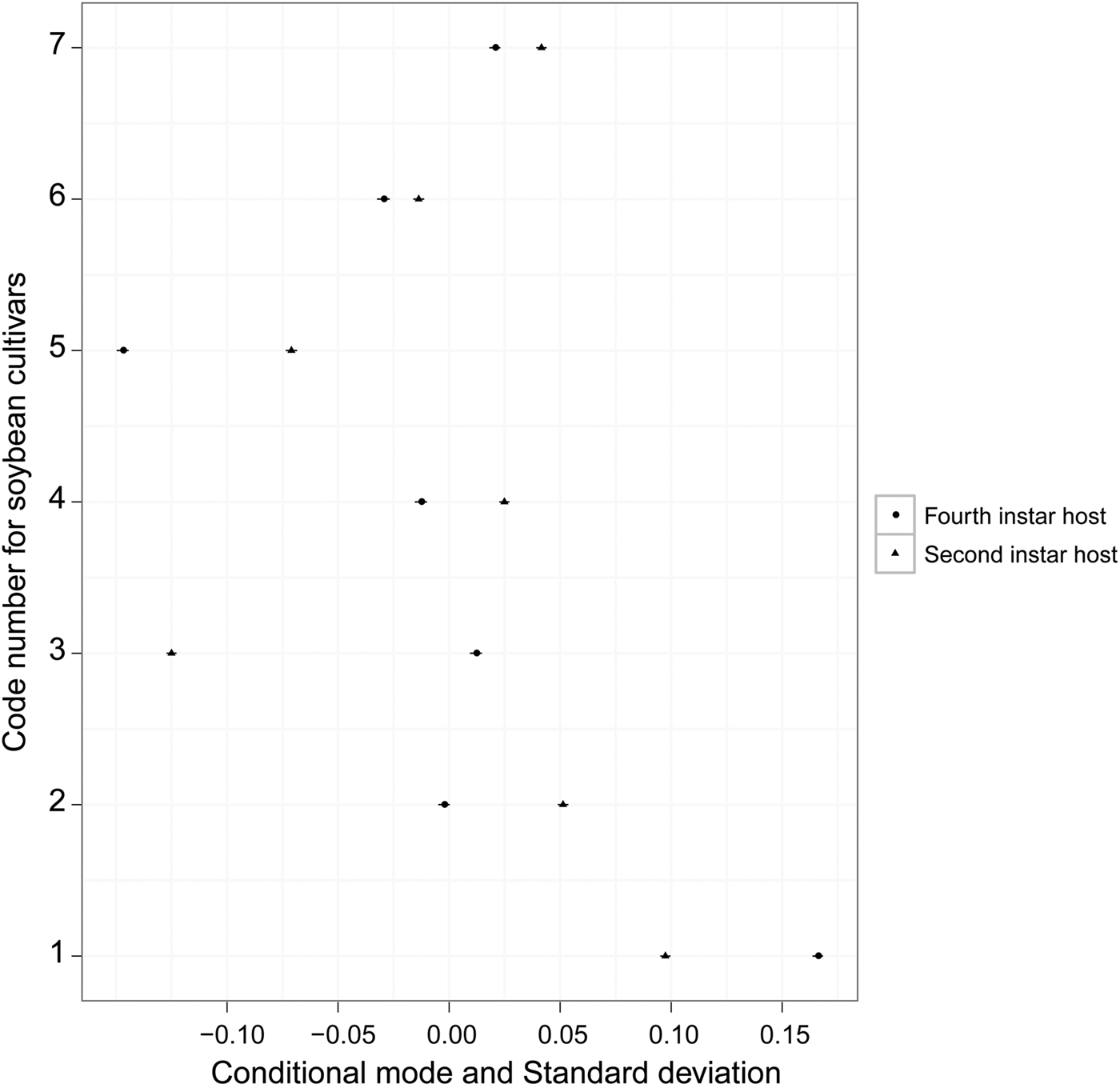
Fig. 2. 95% prediction intervals (conditional modes ± standard deviation) on the random effects of resistant soybean cultivars on egg-to-pupa development time from attacking the 2nd and 4th instar hosts.

Fig. 3. Developmental survival rate to adult of offspring parasitoids in the 2nd (empty bar) and 4th (filled bar) instar host larvae across soybean cultivars.

Fig. 4. 95% prediction intervals (conditional modes ± standard deviation) on the random effects of resistant soybean cultivars on developmental success to adult from attacking the 2nd and 4th instar hosts.
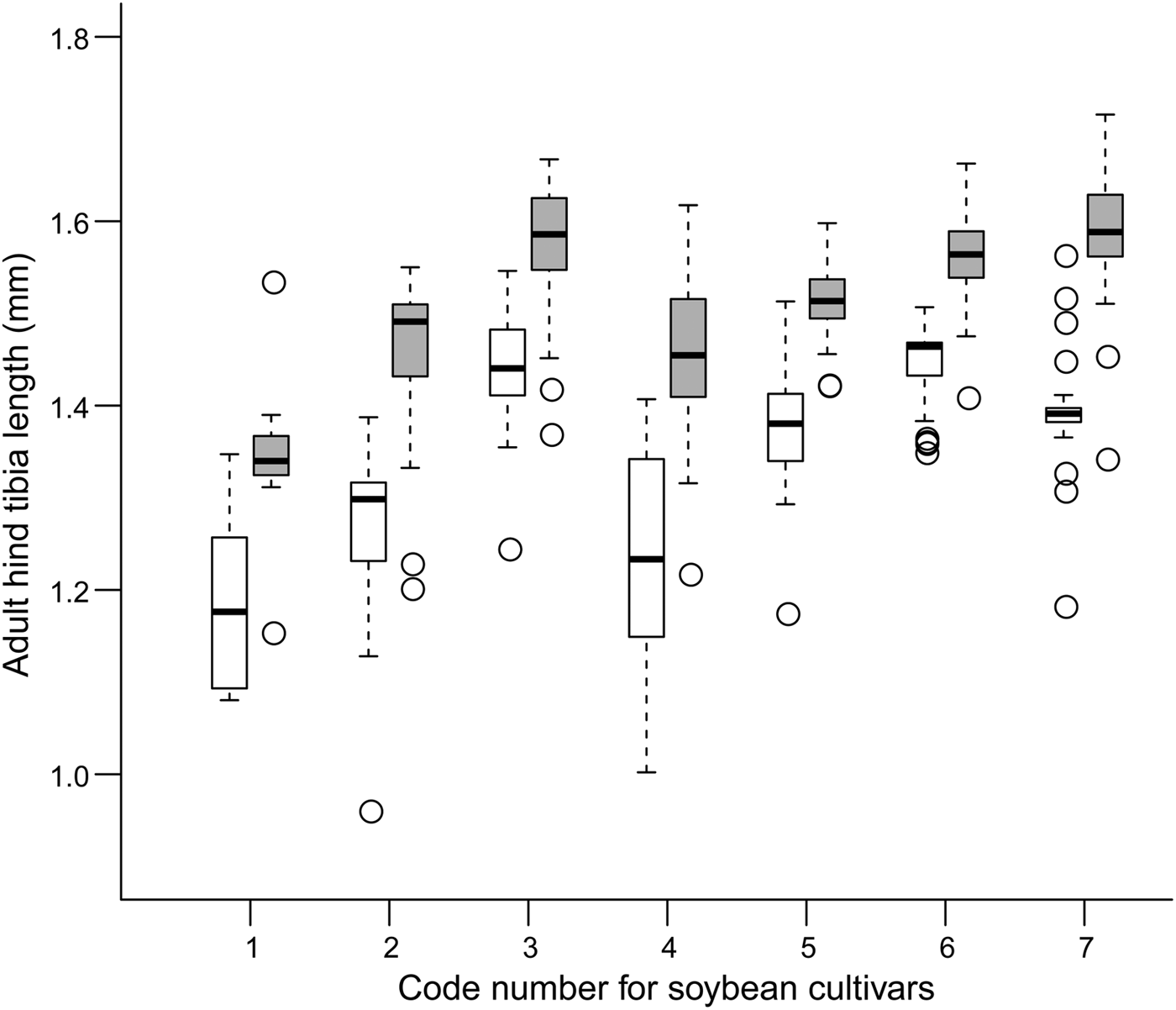
Fig. 5. Adult body size (medians and interquartile ranges) of offspring parasitoids in the 2nd (empty bar) and 4th instar (filled bar) host larvae across soybean cultivars.
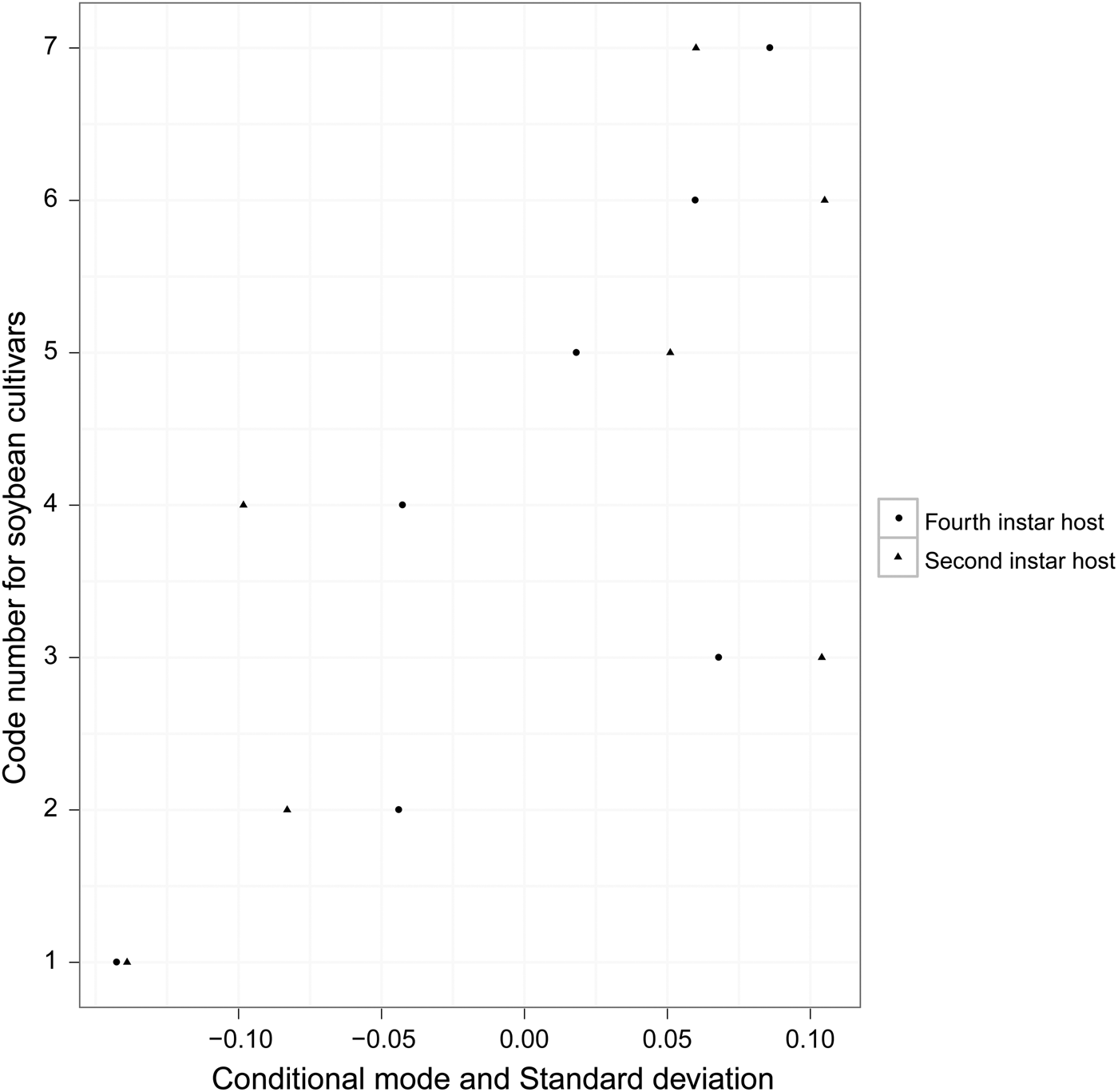
Fig. 6. 95% prediction intervals (conditional modes ± standard deviation) on the random effects of resistant soybean cultivars on adult body size of offspring parasitoids from attacking the 2nd and 4th instar hosts.
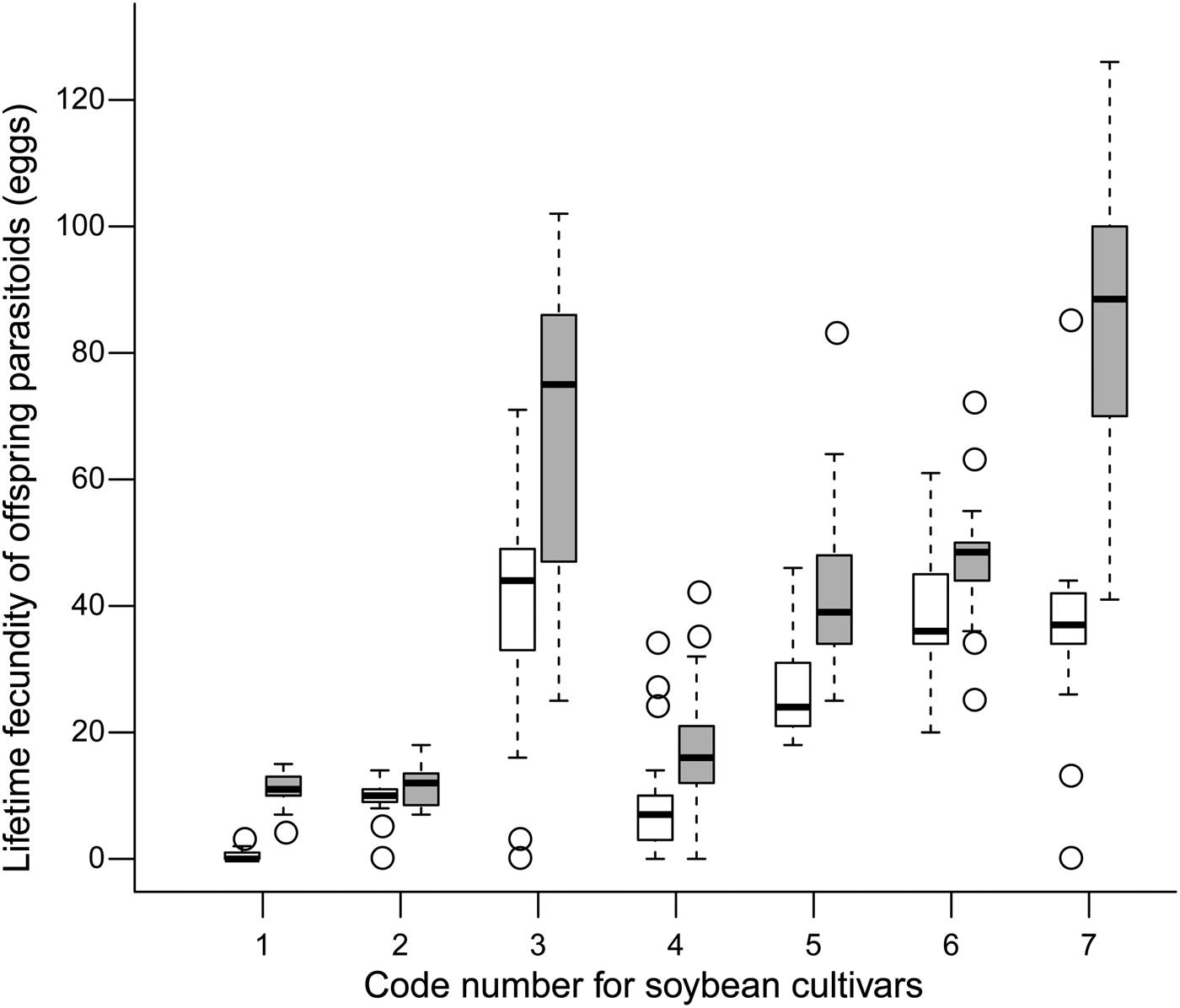
Fig. 7. Lifetime fecundity (medians and interquartile ranges) of offspring parasitoids from the 2nd (empty bar) and 4th instar (filled bar) S. litura larvae across soybean cultivars.

Fig. 8. 95% prediction intervals (conditional modes ± standard deviation) on the random effects of resistant soybean cultivars on lifetime fecundity of offspring female parasitoids from attacking the 2nd and 4th instar hosts.
Discussion
Our study showed that soybean cultivars varying in resistance to S. litura larvae had strong effects on variability in survival and lifetime fecundity of M. pulchricornis offspring parasitoids. These results suggest that parasitoid developmental survival and fecundity may be more flexible than other life history traits in response to plant cultivars at varying resistance levels to the host.
Survival and fecundity are major components of fitness in parasitoids (Godfray, Reference Godfray1994). A wealth of studies demonstrates negative effects of plant cultivars or species resistant to insect hosts on parasitoids attacking them in developmental and reproductive performances (reviewed in Ode, Reference Ode2006). Many diverse mechanisms have been proposed by which variation in plant quality can influence the performances of parasitoids (Hunter, Reference Hunter2003). As the performance of the host and its parasitoid are often positively correlated (Benrey et al., Reference Benrey, Callejas, Rios, Oyama and Denno1998; Harvey et al., Reference Harvey, van Dam and Gols2003; Sznajder & Harvey, Reference Sznajder and Harvey2003; Gols & Harvey, Reference Gols and Harvey2009), constitutive plant secondary compounds that are detrimental to many herbivores can also be detrimental to their parasitoids, especially so if the parasitoids are generalists (Hare, Reference Hare, Tscharntke and Hawkins2002). In our study, marked variation in survival success of the parasitoid offspring is believed to be caused by host mortality, which varied highly across soybean cultivars tested in this study (unpublished data). A previous study documented that S. litura host larvae are more likely to fail to complete their development on highly resistant soybean genotypes (Wu et al., Reference Wu, Wu, Wu, Wang, Gai and Yu2006). In a study by Orr & Boethel (Reference Orr and Boethel1986), developmental mortality of Pseudoplusia includes caterpillars fed a susceptible soybean cultivar was 10%, but increased to 70% when it fed on a resistant cultivar. The negative impact of pest-resistant plants on parasitoid fecundity can even occur on the 4th trophic level. Orr & Boethel (Reference Orr and Boethel1986) found that a resistant soybean genotype negatively affected the lifetime fecundity of Telenomus podisi, a 4th trophic level scelionid egg parasitoid of the predatory pentatomid Podisus maculiventris.
Adult body size is generally believed to be closely correlated with fitness in parasitoids, as the size of the adult parasitoid is largely determined by the amount and quality of food consumed as a larva (Godfray, Reference Godfray1994), with numerous studies indicating that parasitoid body size is influenced by plant quality of the insect host (Hunter, Reference Hunter2003; Ode, Reference Ode2006). Our results from this study, however, showed that adult body size (hind tibia length) of parasitoid offspring did not vary obviously in response to soybean cultivars varying in resistance levels to the host, suggesting that M. pulchricornis body size is not in line with its fecundity in response to soybean resistant genotypes. Differential reactions among life history traits to host plant chemistry were also found in other studies. For example, a series of studies examining the toxicity of nicotine to the specialist parasitoid Cotesia congregate found that the parasitoid suffered an increase in larval mortality when nicotine was added to the artificial diet of the tobacco hornworm, Manduca sexta, but parasitoid adult body size was unaffected (Thurston & Fox, Reference Thurston and Fox1972; Barbosa et al., Reference Barbosa, Saunders, Kemper, Trumbule, Olechno and Martinat1986, Reference Barbosa, Gross and Kemper1991). Thorpe & Barbosa (Reference Thorpe and Barbosa1986) documented similar effects of two varieties of tobacco with different nicotine content on C. congregate. Some evidence shows that parasitoids, depending on resource availability during development, can adjust relative resource allocation between somatic maintenance and reproduction (egg production) to suit the life history imposed by their body size (Olson & Andow, Reference Olson and Andow1998; Thorne et al., Reference Thorne, Pexton, Dytham and Mayhew2006).
Our study further found that developmental survival and fecundity of M. pulchricornis offspring did not correspond with each other in variability in response to soybean resistant cultivars when attacking S. litura 4th as compared with attacking the 2nd instar larvae. Higher variability in survival was detected when attacking S. litura 4th than 2nd instar hosts, but the reverse was found for lifetime fecundity. The differential performance of parasitoids attacking different aged hosts could be explained that parasitoid developmental survival was more severely affected when attacking host larvae at later than earlier stages, as later-stage host larvae feeding on resistant plants had higher mortality than younger ones. Yet, realized fecundity of surviving parasitoids was higher when attacking hosts at earlier stages. This may be because young host larvae have greater growth potential than older ones for koinobiont parasitoids, as the host continues to grow after being parasitized (Sequeira & Mackauer, Reference Sequeira and Mackauer1992; Harvey et al., Reference Harvey, Harvey and Thompson1994, Reference Harvey, Sano and Tanaka2010; Mackauer et al., Reference Mackauer, Sequeira, Otto, Bauer, Dettner and Völkl1997; Harvey, Reference Harvey2000, Reference Harvey2005; Harvey & Strand, Reference Harvey and Strand2002; Li & Mills, Reference Li and Mills2004).
We conclude that the parasitoid M. pulchricornis exhibits wider variability in survival and lifetime fecundity than in developmental time and adult body size in response to soybean cultivars at variable levels of resistance to the host S. litura. Furthermore, parasitoid survival varies more in attacking the 4th than 2nd instar larval hosts but the reverse is true for lifetime fecundity. Our study provides further evidence supporting that plant resistance to herbivorous hosts have variable effects on different life history traits of higher trophic level parasitoids.
Acknowledgements
We thank Yi Lu and Liangxia Lu for their help in the experiments, and Michael Garvey for valuable suggestions on earlier versions of this manuscript. This study was supported by the Special Foundation for Agro-Scientific Research in the Public Interest (201103002) and Natural Science Fund of China (NSFC 31570389).











