INTRODUCTION
Viruses, bacteria, fungi, protistans and digenean metazoans are the main agents causing molluscan diseases, the protistans considered to cause epizootics in commercially exploited bivalve species (Lauckner, Reference Lauckner and Kinne1983; Figueras & Novoa, Reference Figueras and Novoa2011). These parasites can affect reproduction and even survival of their hosts, representing a substantial risk for their fisheries and culture, causing severe economic losses. Baseline information on the health status of exploited populations is needed prior to mass mortalities occurring, in order to facilitate the interpretation of the cause of an eventual disease outbreak, allowing the adoption of practices and techniques of culture leading to limited spread and impact of the disease (Figueras & Novoa, Reference Figueras and Novoa2011).
The southern geoduck P. abbreviata Valenciennes, 1839 (Hiatellidae) is endemic from the South-western Atlantic (SWA); this species is a large, long-living bivalve which occurs between 23°S and 48°S in the SWA from the low intertidal to at least 75 m depth (Ciocco, Reference Ciocco2000; Morsan & Ciocco, Reference Morsan and Ciocco2004). Panopea abbreviata represents a valuable fishery resource. In recent years, its Atlantic populations have been exploited and harvested by artisanal fishing (Morsan & Ciocco, Reference Morsan and Ciocco2004; Morsan et al., Reference Morsan, Zaidman, Ocampo-Reinaldo and Ciocco2010). Its fishery was encouraged by the high prices of its congener Panopea abrupta, which is one of the most valuable commercial shellfish species harvested in British Columbia and currently supports a commercial fishery in Alaska, British Columbia and Washington (Bradbury et al., Reference Bradbury, Sizemore, Rothaus and Ulrich2000).
Previous basic knowledge on the biology of P. abbreviata deals with information on the gametogenic cycle in Patagonian gulfs, population structure, mortality and inter-population comparisons of growth rates, and harvesting, which represents valuable information with view to the management of P. abbreviata fisheries (Van der Molen et al., Reference Van der Molen, Kroeck and Ciocco2007; Morsan et al., Reference Morsan, Zaidman, Ocampo-Reinaldo and Ciocco2010). Nevertheless, little is known about its health status. In recent years, studies regarding the distribution and reproduction of the nemertean Malacobdella arrokeana Ivanov, Bigatti, Penchaszadeh & Norenburg, Reference Ivanov, Bigatti, Penchaszadeh and Norenburg2002 (Bdellonemertea) affecting the Patagonian populations of Panopea abbreviata have been reported by Martorelli et al. (Reference Martorelli, Ciocco and Bazterrica2003) and Teso et al. (Reference Teso, Bigatti, Ciocco and Penchaszadeh2006). Vázquez et al. (Reference Vázquez, Bigatti, Ituarte and Cremonte2009) described the histopathological effect of the attachment of this nemertean on the mantle tissues of the pallial cavity. The parasitic green alga Coccomyxa parasitica Stevenson & South, Reference Stevenson and South1974 (Coccomyxaceae) was identified based on morphological (transmission electron microscopy) and molecular information by Vázquez et al. (Reference Vázquez, Rodriguez, Ituarte, Klaich and Cremonte2010). Brusa et al. (Reference Brusa, Vázquez and Cremonte2011) described Paravortex panopea Brusa, Vázquez & Cremonte, Reference Brusa, Vázquez and Cremonte2011 (Grafillidae), a turbellarian species inhabiting the intestine lumen. Nevertheless, up to now, no study regarding the health status of P. abbreviata from a histopathological point of view has been carried out.
The aim of the present work was to perform a histopathological study of the P. abbreviata populations in Northern Patagonian gulfs (Argentina). In addition, we investigate, using Generalized Linear Model analysis, how the presence of parasites and the parasitic abundance are influenced by some environmental and biological variables (water temperature, sex, gonad developmental stages, shell length and condition index of the geoduck).
MATERIALS AND METHODS
Study area and sample collection
The San José Gulf (SJG) is a small, shallow, semi-enclosed water body (817 km2; mean depth 30 m) located on the north coast of Argentine Patagonia. It opens to the north into the much larger San Matías Gulf (SMG) (18,000 km2) through a narrow (6.9 km) mouth (Amoroso et al., Reference Amoroso, Parma, Orensanz and Gagliardini2011). During 2007 a total of 150 geoducks of 80 ± 11.87 mm maximum length (mean ± SD) were sampled; 120 specimens were collected seasonally (30 each season) at Fracasso Beach (42°25′S 64°07′W), SJG and 30 specimens were collected during a single sampling occasion at Puerto Lobos in the austral summer (42°00′S 65°05′W), SMG, in order to make an accurate comparison between the two populations (Figure 1). The clams were collected by scuba diving at about 15 m depth, transported to aquaria with aerated seawater and maintained up to 24 h until processing.
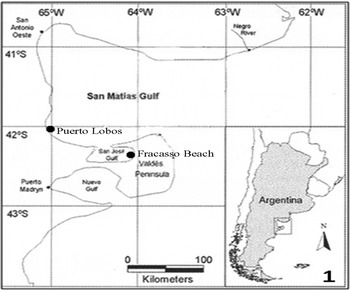
Fig. 1. Panopea abbreviata sampling sites in northern Patagonian gulfs: Puerto Lobos (San Matías Gulf) and Fracasso Beach (San José Gulf), Argentina.
Histological processing
Maximum shell length of each specimen was measured on the right valve. Clams were opened carefully by cutting the adductor muscles and in each clam, the total number and location of individuals of Malacobdella arrokeana were recorded. The soft parts were carefully removed from valves and separately weighed to calculate the condition index ((wet soft part weight/valve weight) × 100) (Lucas & Benninger, Reference Lucas and Benninger1985). Soft parts of all specimens were inspected for the presence of epibionts and symbionts and were then fixed in Davidson's solution (Shaw & Battle, Reference Shaw and Battle1957) for 24 h and stored in 70% ethanol. A transverse 5 mm thick slice, containing gill, digestive gland, intestine, mantle, nephridia and gonad was taken from each geoduck. Tissues were embedded in paraffin, sectioned at 5 μm thick, and stained with haematoxylin and eosin. Histological sections were examined under a light microscope for presence of parasites and pathological alterations. For each geoduck from San José Gulf, sex and gonad stage were recorded; for this, a 6 stage gametogenic scale proposed by Van der Molen et al. (Reference Van der Molen, Kroeck and Ciocco2007) was used: 1: early active, 2: late active, 3: ripe, 4: spawning, 5: spent, 6: undifferentiated.
Statistical analyses
Prevalence (P) and Mean Intensity (MI) of parasites were calculated according to Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). The intensity of infection was estimated by counting the number of parasites in each histological section. Mean abundance (MA) was calculated as the sum of all parasites in each examined host divided by the total number of hosts examined.
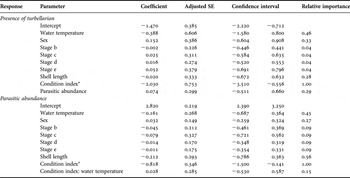
To evaluate the variables affecting the presence and abundance of parasites (response variables) in geoducks from San José Gulf, Generalized Linear Models (GLMs) were applied. The type of data analysed in this paper, as presence/absence of parasite (binary response) and abundance of parasites (count data), are problematic when applying statistical parametric or non-parametric tests. Another problem is that many observations are zeros and the response variables can be affected by many explanatory variables. GLMs are appropriate when the variance is not constant, and/or when the errors are not normally distributed (Crawley, Reference Crawley2007). The presence/absence of the most prevalent parasites model was fitted with a binomial error structure with a logit link function, and abundance of parasites model was fitted with a Poisson distribution with a log link function. The GLM regarding the presence of a particular parasite as the response variable included the following predictor variables: water temperature, sex, gonad stage, shell length, condition index and parasitic abundance. The GLM regarding the parasitic abundance as the response variable also included two simple interactions, one with the gonad stages and the condition index and the other between water temperature and the condition index of the geoduck. The Akaike's information criterion (AIC) was used to determine the best model for the analysed dataset. Model selection was performed with an IT approach using the AIC and model averaging (Grueber et al., Reference Grueber, Nakagawa, Laws and Jamieson2011). Because the response variables were over-dispersed, we calculated an AIC modified by the principle of quasi-likelihood or QAIC, and a version of QAIC for small sample sizes, QAICc. From the AICc differences (Di), where Di = AICCi − AICCmin, Akaike weights (wi) were obtained for all candidate models. For each dataset, the models were ranked by their ‘wi’ values. The model with the highest ‘wi’ was considered the one with the best support. Model averaging was calculated using candidate models, which together account for the 95% confidence level. The top model set was averaged using a zero method (Symonds & Moussalli, Reference Symonds and Moussalli2011), where the best AIC model was not strongly weighted. Global model was performed in R (R Development Core Team, 2011) and the standardized function to input variables is available within the ‘arm’ (Data Analysis Using Regression and Multilevel/Hierarchical Models) package (Gelman et al., Reference Gelman, Su, Yajima, Hill, Pittau and Kerman2009). Model selection and averaging were calculated with the MuMIn package (Barton, Reference Barton2009). The predictor variables in the top models were reported with their relative importance weights, model-averaged parameter estimates, unconditional SE and 95% confidence intervals. Results were expressed in terms of odds ratios. Odds were calculated as the exponential of the coefficient of each parameter corresponding to the averaging model.
Additionally, a Kruskal–Wallis test was applied to compare the significance of the differences between the condition index and the season during a year as well as the gonad stage of P. abbreviata and the condition index.
RESULTS
A summary of the data of the main characteristics of Panopea abbreviata populations and the mean parasitic abundance by season, as well as the results of histological examinations (prevalence and mean intensity of infection for all parasites) are presented in Tables 1 and 2 respectively.
Table 1. Main characteristics of Panopea abbreviata: sex ratio (F: female, M: male), predominant gonad stage (percentage of geoducks in the gonad stage in parentheses), maximum shell length range (in mm), condition index (mean followed by range in parentheses) and parasitic abundance (mean followed by range in parentheses) given by season during 1 year at San José Gulf and from the austral summer at San Matías Gulf (*), northern Patagonia, Argentina.
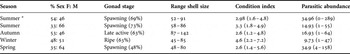
Table 2. Prevalence (P) (%) and intensity (I) (mean followed by range in parentheses) of parasites on Panopea abbreviata and their sites of infection given by season during 1 year at San José Gulf and from austral summer at San Matías Gulf (*), northern Patagonia, Argentina.

The condition index of P. abbreviata varied significantly among seasons (Kruskal–Wallis, H=42.34, P<0.0001), recording the highest value during the austral winter (July). The geducks were in ripe and spawning condition for most of the year, although higher proportions occurred during winter and summer, respectively (Table 1). Significant differences in the condition indices related to gonad stage of the geoducks were observed (Kruskal–Wallis, H=38.02, P<0.0001), recording the highest values of condition index in ripe geoducks, corresponding to the cold season.
The two studied populations of P. abbreviata were parasitized by prokaryotic microrganisms, observed as intracellular inclusions in the digestive gland epithelium (Figure 2) and gills; ciliates in gills (Figure 3) and labial palps; Porospora-like gregarines (Apicomplexa) in the mantle connective tissue (Figure 4); the turbellarian Paravortex panopea (Graffillidae) in the intestine lumen (Figure 5); the nemertean Malacobdella arrokeana (Bdellonemertea) in the mantle cavity (Figure 6); and the intracellular green alga Coccomyxa parasitica in the connective tissue of the distal end of the siphon (Figure 7).
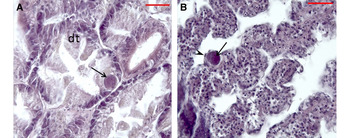
Fig. 2. Histological sections (H & E) of the geoduck Panopea abbreviata. (A) Prokaryotic inclusions (arrow) in the digestive tubule epithelium, (B) Prokaryotic inclusions (arrow) in gill filaments encapsulated by haemocytes (arrow head). dt: digestive tubule.
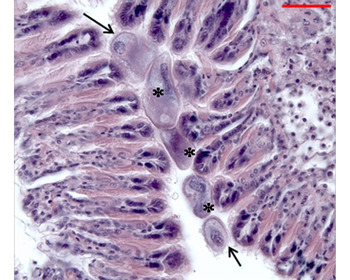
Fig. 3. Ciliates (arrows) between gill filaments and adhered to the epithelium of the gill filaments (*).
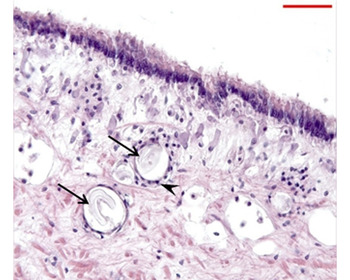
Fig. 4. Sporozoites of Porospora-like gregarine (arrows) in the mantle connective tissues encapsulated by haemocytes (arrowhead).
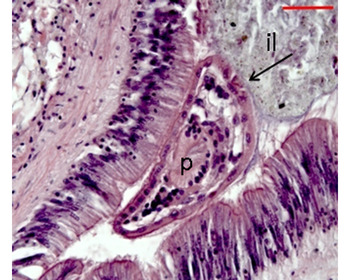
Fig. 5. Turbellarian Paravortex panopea (arrow) in the intestinal lumen. p: pharynx, il: intestinal lumen.
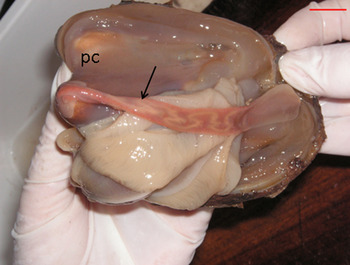
Fig. 6. Nemertean Malacobdella arrokeana (Malacobdellidae) (arrow) in the pallial cavity (pc) of Panopea abbreviata. Scale bar: 1 cm.
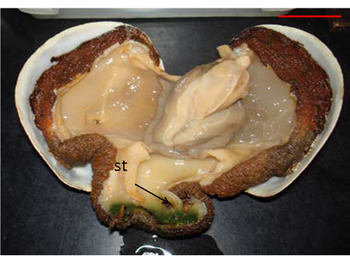
Fig. 7. Intracellular alga Coccomyxa parasitica (arrow) in the siphonal tissues (st) of Panopea abbreviata. Scale bar: 2 cm.
The highest values of prevalence and intensity of infection for most of the parasite species in geoducks from San José Gulf were recorded during the late austral spring (November), except for Porospora-like gregarines, which were more prevalent during the austral summer. The highest prevalence was shown by Malacobdella arrokeana (98.3%), followed by ciliates (79%), Coccomyxa parasitica (51%), prokaryotic inclusions (32.2%) and Paravortex panopea (27.1%). Porospora-like gregarines showed the lowest prevalence values (15.2%). However, in geoducks from San Matías Gulf, the highest prevalence (75%) and intensity values were found for Porospora-like gregarines, recording a maximum of 279 organisms per histological section.
The following description of the histological observations and the parasitological indices are reported for geoducks from San José Gulf. The histological study of the digestive gland infected by rounded intracellular basophilic colonies of prokaryotic microrganisms (23 ± 3.79 μm of length) revealed, in some cases, a hypertrophy of the host cell (Figure 2A), as well as an encapsulation by haemocytes in gill filaments (Figure 2B). The maximum intensity was 47 prokaryotic microrganism colonies per histological section (Table 2).
Ciliates were recorded mainly on the gill filaments and often on the surface of labial palps. These protozoans had an oval or rounded shape, a surface densely ciliated, and a large polymorphic macronucleus. The relationship to the gill epithelium seemed to be superficial and no particular host response was apparent (Figure 3). The maximum intensity was 147 ciliates per histological section (Table 2).
Porospora-like gregarines, as naked vermiform sporozoites, were found inhabiting the connective tissue of the mantle. The host response was shown by a haemocytic encapsulation of each sporozoite (45.58 ± 10.4 μm wide) (Figure 4). The maximum intensity was five parasites per histological section (Table 2).
The turbellarian Paravortex panopea Brusa, Vázquez & Cremonte, Reference Brusa, Vázquez and Cremonte2011 (Grafillidae) was found in the intestinal lumen. It was characterized by its ciliated epidermis, ocelli and muscular pharynx. No evidence of direct physical damage was observed, although fully gravid specimens nearly occluding the intestine lumen were often found (Figure 5). One or two individuals were frequently observed per histological section, although the maximum intensity was 14 (Table 2).
The nemertean Malacobdella arrokeana was found in the pallial cavity attached to the mantle, usually only one adult individual per geoduck, with overall prevalence of 98% (Figure 6). Only 8% of the geoducks were found also hosting young nemerteans (up to 8 individuals) attached to the gills (Table 2).
The green alga Coccomyxa parasitica was found in the siphonal tissues in the 51% of the geoducks examined, affecting no more than 3 cm of the tip of siphon (Figure 7).
Regarding the variables affecting the presence of the most prevalent parasites, the only significant results were recorded for the turbellarians. Model analysis on the presence of the turbellarian P. panopea resulted in five candidate's models with ΔAIC<2 of the best model (Table 3). Condition index emerged as the most robust predictor variable, with a relative importance weight of 1.00 and 95% confidence interval bounded away from zero (Table 3). The condition index and the presence of turbellarians showed a negative relationship (Table 3), indicating that the geoducks hosting these flatworms had a poorer condition (Figure 8).
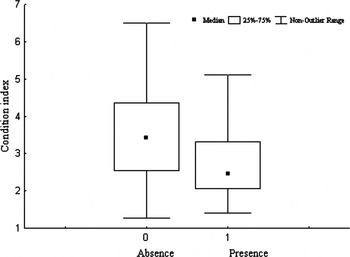
Fig. 8. Relationship between the absence (0) – presence (1) of Paravortex panopea turbellarian and Panopea abbreviata condition index.
Table 3. Predictor variables from top models for each response variable in Panopea abbreviata from San José Gulf, Argentina. Coefficient estimates, their unconditional standard error (SE), 95% confidence interval (CI) and relative importance weights (w(i)) after model averaging are shown for each variable. Stage a was the reference category *Variables have a 95% confidence interval bounded away from zero (significant results).
In addition, model analysis on parasitic abundance resulted in six candidate's models with △QAIC<2 of the best model (Table 3). The condition index and the abundance of parasites in P. abbreviata showed a negative relationship (Table 3), showing a low condition index with increasing the number of parasites (Figure 9). The geoduck shell length had a lower relative importance weight (0.74) and the confidence interval included zero (Table 3), indicating that there is little evidence to consider that the parasitic abundance would be affected by this predictor variable. The mean parasitic abundance did not vary significantly by season, although the most parasitized geoducks were recorded during the late austral spring (November) (Figure 10).
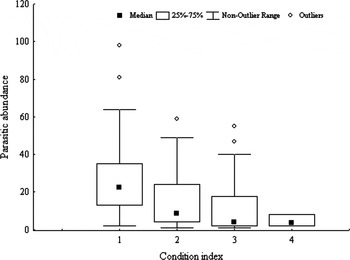
Fig. 9. Abundance of parasites in Panopea abbreviata from San José Gulf population vs the condition index.
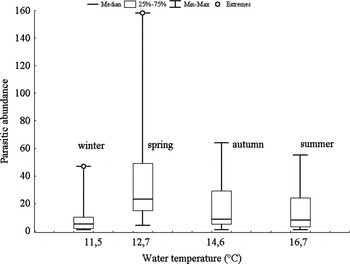
Fig. 10. Abundance of parasites in Panopea abbreviata from San José Gulf population vs the mean seawater temperature corresponding to the different seasons.
DISCUSSION
This paper is the first histopathological study of the southern geoduck Panopea abbreviata. The two populations of P. abbreviata studied were free of severe pathogens.
Prokaryotic inclusions were reported from different tissues of diverse bivalve species (Lauckner, Reference Lauckner and Kinne1983), also in Panopea abrupta (Bower & Blackbourn, Reference Bower and Blackbourn2003), in which the colonies were located in the gills and labial palps. Heavy infections of intracellular colonies of these organisms could be potentially pathogenic since infections by prokaryote-like colonies have been associated with mass mortalities in some bivalves such as scallops (Gulka et al., Reference Gulka, Chang and Marti1983; Le Gall et al., Reference Le Gall, Chagot, Mialhe and Grizel1988), clams (Norton et al., Reference Norton, Shepherd, Abdon-Naguit and Lindsay1993; Villalba et al., Reference Villalba, Carballal, López, Cabada, Corral and Azevedo1999) and abalone snails (Friedman et al., Reference Friedman, Thomson, Chun, Haaker and Hedrick1997). In the present study, the prokaryote inclusions were found mainly in the digestive gland, often causing the hypertrophy of the host cell and haemocytic encapsulation of the pathogens when present in the gill filaments; however, they always occurred in low intensities of infection.
The role of the ciliates is controversial, since they are common inhabitants of the bivalve gills (Lauckner, Reference Lauckner and Kinne1983; Bower et al., Reference Bower, McGladdery and Price1994), and in high intensities they are considered pathogenic (Elston et al., Reference Elston, Cheney and Frelier1999; Scarpa et al., Reference Scarpa, Ford, Smith and Bushek2006) because they cause inflammatory processes in gills, possibly interfering with laminar water flow on the surface of the gill lamellae. In this study, the ciliates were observed predominantly adhered to the epithelial cells of gills and between the gill filaments, without causing any apparent pathology.
The apicomplexan Porospora-like gregarine was observed inducing a focal inflammatory response in the form of a haemocytic encapsulation; this suggests a strong host response. Nevertheless, the intensity of infection was low and no evidence of disease generated by the presence of the Porospora-like gregarine was found. In P. abrupta, an unknown apicomplexan protozoan was observed in the connective tissue of the labial palps and muscular portion of mantle, as well as in the gills, but with very low prevalence and intensity of infection, without significant health effects (Bower & Blackbourn, Reference Bower and Blackbourn2003). In the present study, Porospora-like gregarines seemed to have high specificity for the site of infection, since they occurred only in the connective tissue of the mantle.
Turbellarians of the genus Paravortex are commonly reported obstructing the intestinal lumen, however without causing severe effects in bivalves (Jennings, Reference Jennings1971; Brusa & Ponce de León, Reference Brusa and Ponce de León2006). Paravortex panopea Brusa, Vázquez and Cremonte, Reference Brusa, Vázquez and Cremonte2011 (Grafillidae) was described from the intestinal lumen of P. abbreviata and Enis macha (Pharidae) populations in San José Gulf (Argentina) (Brusa et al., Reference Brusa, Vázquez and Cremonte2011). In the present study, gravid specimens of P. panopea were found occupying a great part of the transverse section of the intestinal lumen with no evidence of direct physical damage. Its presence seemed to play a detrimental effect evidenced in the lower condition index of the infected geoducks. This effect could be due to direct feeding on the intestinal tissue or host's ingesta by the turbellarians (Jennings, Reference Jennings1988). Jennings (Reference Jennings1997) pointed out that two entosymbiotic species of Paravortex migrate from the host's intestine (P. scrobiculariae) or stomach (P. cardii) into the digestive gland of the host, where they ingest the partly digested food of the host and some of the heterolysosomes being discharged from disintegrating cells in the digestive gland. Moreover, histology underestimates the actual number of turbellarians in a host because only a section of the intestinal tract is inspected (Brusa et al., Reference Brusa, Vázquez and Cremonte2011). Therefore, the prevalence and mean intensities of infection observed in the present study by histology (one section about 5 μm thick) were probably low, making the true values likely higher than those reported here.
The nemerteans of the family Malacobdellidae comprise a single genus, Malacobdella, with six known species inhabiting the pallial cavity of bivalves (Ivanov et al., Reference Ivanov, Bigatti, Penchaszadeh and Norenburg2002; Jensen & Sadeghian, Reference Jensen, Sadeghian and Rhode2005). They are usually considered as entocommensals, however, Sundet & Jobling (Reference Sundet, Jobling, Grey and Christiansen1985) supported their parasitic life style on the basis of the slight but significant diminution in the growth rate and a lower condition index of parasitized bivalves caused by the nemertean. Vázquez et al. (Reference Vázquez, Bigatti, Ituarte and Cremonte2009) reported in P. abbreviata, a mechanical alteration of the area of the inner mantle epithelium at the point of attachment of M. arrokeana determined by the vacuum force produced by the sucker to secure the nemertean to the host within the pallial cavity. Although, from the histological point of view, the absence of hyperplasia and metaplasia of the mantle epithelium showed that the presence of the nemertean would be harmless, and only a moderate host inflammatory process in the connective tissue between inner and outer mantle epithelium in the adjacencies of the attachment of the nemertean indicated a minor injury for the bivalve host (Vázquez et al., Reference Vázquez, Bigatti, Ituarte and Cremonte2009). The high prevalence recorded in the present study agrees with that reported by Ivanov et al. (Reference Ivanov, Bigatti, Penchaszadeh and Norenburg2002) and Teso et al. (Reference Teso, Bigatti, Ciocco and Penchaszadeh2006).
Coccomyxa parasitica was reported in Mytilus chilensis from Patagonia, Argentina (Boraso de Zaixso & Zaixso, Reference Boraso de Zaixso and Zaixso1979; Bala, Reference Bala1995) and from Malvinas (Falkland) Islands (Gray et al., Reference Gray, Lucas, Seed and Richardson1999). Vázquez et al. (Reference Vázquez, Rodriguez, Ituarte, Klaich and Cremonte2010) identified this parasitic green alga in the connective tissue at the tips of the siphon of P. abbreviata, describing a severe inflammatory response by the host in form of a haemocytic infiltration and encapsulation of alga colonies. Moreover, the authors reported a lowering in the condition index on infected geoducks. Although the histopathological effect of Coccomyxa parasitica on the clams was not examined in the present study, the relatively high prevalence of infection (especially in the spring) did not seem to independently affect the condition index of the clams in a statistically significant manner.
Whilst the condition index is often used as an indicator of the metabolic condition of a bivalve, its relationship with the amount of glycogen stored or the amount of gametes produced having been determined (Gabbott & Stephenson, Reference Gabbott and Stephenson1974; Mann, Reference Mann, Thorp and Gibbons1978; Soniat & Ray, Reference Soniat and Ray1985), this parameter is also employed to monitor the effect of pollutants and diseases (Crosby & Gale, Reference Crosby and Gale1990). It is possible that reproductive stages of geoducks have some impact on the condition index, since the highest values were recorded in the maximum percentages of clams in ripe condition, where the high energy content was directed to gametogenesis. Likewise, the parasitism suggested a detrimental effect on the physiological condition of P. abbreviata, since condition index decreased with increasing the abundance of parasites (Tables 1 and 3). This effect is probably associated with the energy cost of the cellular defence mechanism shown, in some cases, by the haemocytic infiltration and encapsulation process. Moreover, P. panopea turbellarians were also associated with a lower condition index of the geoduck, possibly due to the sequestering of nutrients from the host intestinal contents. These results suggest that the parasitic abundance has an adverse effect on the health of P. abbreviata, in some way obscured by the fact that the greatest mean abundance of parasites was found mainly in spring, at the same time as the lowering of condition index expected at one peak of the spawning period of bivalves. Nevertheless, none of the parasites found in this study is considered as a severe pathogen, since none caused significant damage to geoducks and none appears to be a problem to the fishery since they are not considered as notifiable pathogens by the World Organization for Animal Health (OIE, 2013).
ACKNOWLEDGEMENTS
The authors express their gratitude to Ricardo Vera and Hormiga Diaz†, for the assistance in the field and to Norma Bustos for the histological sections. We also thank the Administración Area Natural Protegida Península Valdés for its cooperation. All authors are members of CONICET.
FINANCIAL SUPPORT
The present study was funded by PIP CONICET 6263 and ANPCyT (PICT 2013-1702) to Florencia Cremonte and by ANPCyT (PICT 2013-2582) to Nuria Vázquez.















