INTRODUCTION
The mussel Brachidontes rodriguezii (d'Orbigny, 1846) dominates intertidal rocky coasts and is distributed from Uruguay to north Patagonia along the Argentinean coast (Penchaszadeh, Reference Penchaszadeh1973; Adami et al., Reference Adami, Pastorino and Orenzans2013; Arribas et al., Reference Arribas, Bagur, Klein, Penchaszadeh and Palomo2013; Torroglosa & Giménez, Reference Torroglosa and Giménez2015). Mussels constitute diverse assemblages of several species (Scelzo et al., Reference Scelzo, Elías, Vallarino, Charrier, Lucero and Alvarez1996; Vallarino et al., Reference Vallarino, Rivero, Gravina and Elías2002; Adami et al., Reference Adami, Tablado and López Gappa2004; Calcagno et al., Reference Calcagno, Curelovich, Fernández, Thatje and Lovrich2012), becoming an ecosystem engineer (Borthagaray & Carranza, Reference Borthagaray and Carranza2007; Carranza et al., Reference Carranza, Defeo, Beck and Castilla2009; Arribas et al., Reference Arribas, Donnarumma, Palomo and Scrosati2014), so it is an important component of benthic intertidal coast communities. Brachidontes rodriguezii is commonly found in hard substrates of rocky coasts, but as a consequence of the introduction of artificial hard substrates (e.g. fishery piers and seawalls), it is also found in sandy coasts. These artificial hard substrates (vertical surfaces mostly) comprise a novel habitat along the coastline that might introduce changes into local and regional biodiversity modifying patterns of dispersal, establishment and spread of species (Bulleri & Chapman, Reference Bulleri and Chapman2010; Martins et al., Reference Martins, Jenkins, Neto, Hawkins and Thompson2015). To establish and colonize new habitats, species need a successful reproductive strategy (Mackie, Reference Mackie1991; Barber et al., Reference Barber, Fajans, Baker and Baker2005). Size at first maturity together with the reproductive cycle constitutes reproductive aspects that maximize reproductive effort of species (Todd, Reference Todd, Moore and Seed1985; Gage, Reference Gage1995). In addition, size at first maturity is useful to determine measures for adequate natural resource management (Chung, Reference Chung2007, Reference Chung2008), spatial monitoring (Camacho-Mondragón et al., Reference Camacho-Mondragón, Arellano-Martínez and Ceballos-Vázquez2012) and temporal changes associated with commercial exploitation (Giménez & Penchaszadeh, Reference Giménez and Penchaszadeh2003; Torroglosa & Giménez, Reference Torroglosa and Giménez2010). Brachidontes rodriguezii is a small mytilid (35 mm shell length) that often forms multi-layer beds on vertical intertidal substrates reaching high densities (170 000 ind. m−2) (Penchaszadeh, Reference Penchaszadeh1973: Gutiérrez et al., Reference Gutiérrez, Palomo, Bagur, Arribas and Soria2015). Some aspects of the reproductive biology of the species are known; B. rodriguezii recruits continuously during the year, with peaks during summer and autumn (Penchaszadeh, Reference Penchaszadeh1973; Adami et al., Reference Adami, Tablado and Sodor2008). The spawning peak occurs at the end of summer, however, mature males and females were recorded all year round suggesting minor spawning events that explain the continuous recruitment (Torroglosa, Reference Torroglosa2015). Knowledge of the reproductive cycle and the spawning season, together with size at first maturity will provide necessary information for further determination of recruitment rate during the spawning period, and the impacts on the structure population of B. rodriguezii in the south-western Atlantic Ocean. The objectives of this study were to characterize the gonad development according to shell length and to estimate the size at first maturity of B. rodriguezii in Villa Gesell.
MATERIALS AND METHODS
Specimens of Brachidontes rodriguezii were collected by hand from the basal area of the pier pilings in a sandy beach at Villa Gesell (37°16′S 56°53′W). Sampling was carried out on middle intertidal at 20 cm below the sea level at low tide during two consecutive reproductive seasons (Torroglosa, Reference Torroglosa2015): the period December 2011–February 2012 and December 2012–February 2013.
The shell length (SL) of all individuals was measured with stereomicroscope or a digital caliper to the nearest 0.01 mm. Individuals with SL < 8 mm were decalcified in Jenkins solution for 96 h. Tissues were removed from shells and fixed in Bouin for 12 h. They were then dehydrated in alcohol, embedded in metacrylate resin and sectioned at 5 µm. The sections were stained with haematoxylin and eosin (H & E) (Howard et al., Reference Howard, Lewis, Keller and Smith2004). Individuals were considered sexually mature when gonads were completely developed in both mantle lobes and the visceral mass.
The percentages of individuals arranged in 1 mm size class were plotted against SL and data fitted using a non-linear modelling procedure to the logistic equation (Roa et al., Reference Roa, Ernst and Tapia1999): P m = [1/1 + exp (-a (SL – SL0)] 100. Where Pm is the proportion of mature males and females, SL0 and a (slope of the logistic function) were constants. The parameters for the equation were estimated by using the Solver function in the Excel statistical package (Microsoft® Excel 2007). The logistic function was fitted to data on maturity status at shell length using the least-squares method. Each size class comprised between 20 and 27 individuals. The shell length at which 50% of individuals exhibited mature gonads was considered the size at first maturity (SL50) for the population (Roa et al., Reference Roa, Ernst and Tapia1999). To evaluate sex ratio, chi-square test (χ2) was used to assess significant differences between two random samples (N = 100) taken each reproductive season (shell length between 8.10–28.30 mm).
RESULTS
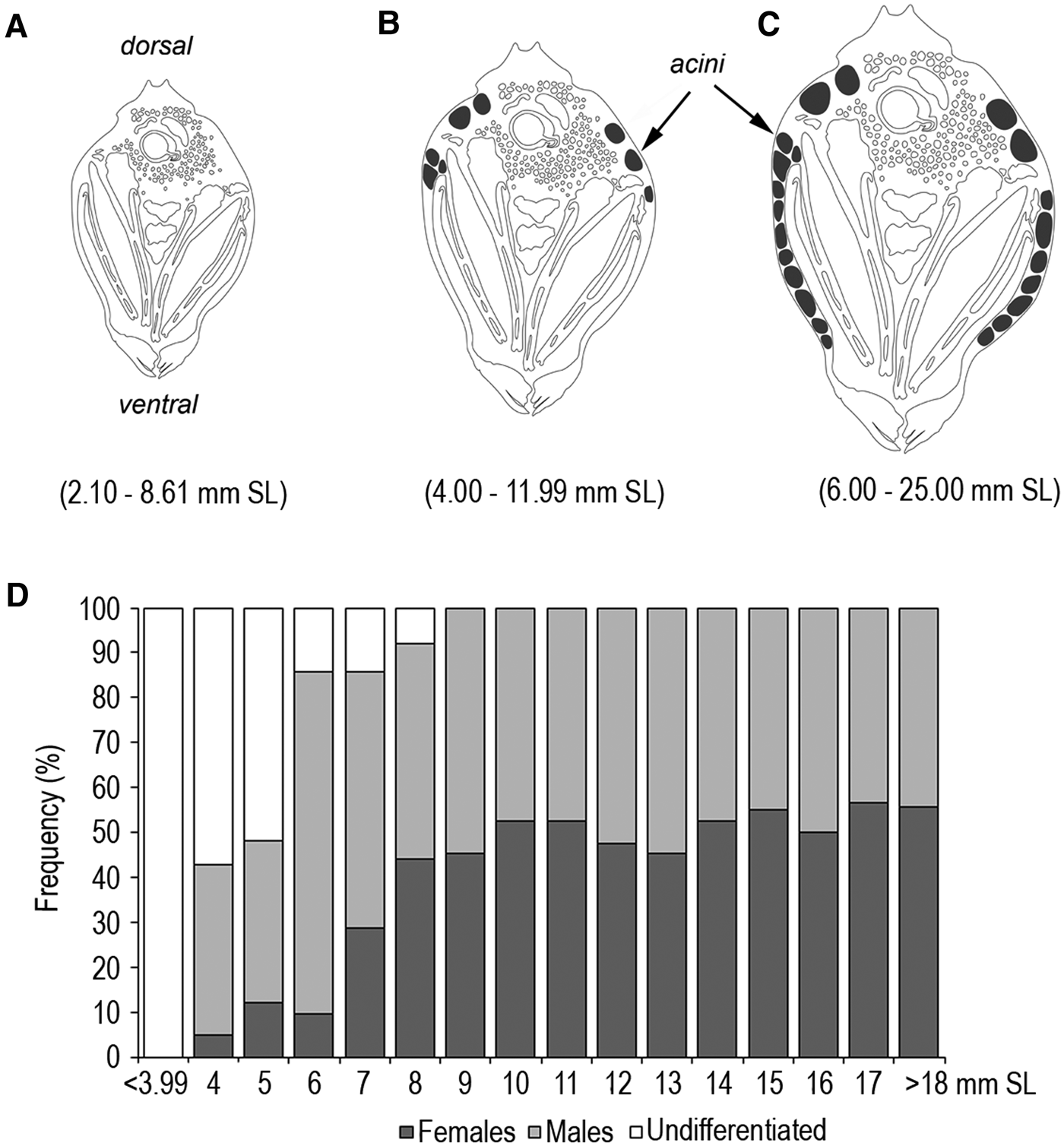
A total of 355 mussels with shell lengths ranging from 2.10 to 25.50 mm were studied. Sex ratio for B. rodriguezii was not significantly different from 1:1 (χ2, P > 0.05). The histological examination showed sexually immature individuals with undeveloped gonads with shell length (SL) < 3.99 mm. The tubules of the digestive gland were distributed entirely in the dorsal region adjacent to the stomach and the digestive diverticula. In both mantle lobes there was connective tissue. For some individuals with shell length between 4.00 and 4.75 mm SL, adjacent to the epithelium of the mantle, there were groups of cells (clusters) undergoing mitosis (Figure 1) suggesting an early development stage of undifferentiated gonad. These individuals were considered undifferentiated because no sex determination was possible. For individuals with shell length > 5.00 mm, the observation of these clusters was in low proportion.

Fig. 1. Brachidontes rodriguezii sexually immature (4.45 mm SL) with early stages of gametogenesis. Between the digestive gland and the epithelium of the mantle, there were clusters of cells undergoing mitosis (arrowhead) within acini. arrow, acinus wall cells; dg, digestive gland; mfe, epithelium of the mantle. Scale bar: 20 µm.
The histological examination showed differentiated gonads, allowing differentiation between immature males and females. The gonad consisted of acini distributed dorsal and laterally to the digestive gland and stomach (Figure 2A, B). The development of the connective tissue next to the gonad and into both mantle lobes was reduced. Immature males also exhibited few acini with early spermatogenic stages: spermatogonia, spermatocytes and spermatids (Figure 2C). This early development condition for males was registered between 4.00 and 7.38 mm SL. In immature females there were a few acini with early stages of oogenesis: oogonia, previtellogenic and early vitellogenic oocytes (Figure 2D). This early development condition for females was registered between 4.97 and 6.46 mm SL. The minimum SL with spermatozoa within acini was 6.00 mm SL, while the minimum SL with vitellogenic oocytes (mature oocytes) was 5.75 mm SL. However, the criteria for maturity included (besides the presence of mature gametes) acini increased in size and number and the decrease of the connective tissue. Mature males and females showed a well-developed gonad where the acini were distributed into the visceral mass next to the epithelium of the mantle and the tubules of the digestive gland. Also both mantle lobes were full of acini while the connective tissue was completely reduced. Gonads of mature males consisted of acini almost entirely filled with spermatozoa (Figure 2E) while mature females exhibited growing (early vitellogenic) and vitellogenic oocytes (Figure 2F). The first record of maturity according to the histological criteria described above was at 6.00 mm SL for males and 6.99 mm SL for females.

Fig. 2. Transverse sections of immature B. rodriguezii. (A) Detail of male gonad (4.60 mm SL) with spermatogonia within acini next to the digestive gland. (B) Detail of a female gonad (4.97 mm SL) with aggregated oogonia. (C) Male gonad showing spermatocytes and spermatids (7.60 mm SL). (D) Female gonad showing ogonia, previtellogenic oocytes, and early vitellogenic oocytes (8.00 mm SL). (E) Mature male with gonad completely expanded into the mantle lobe full with spermatozoa in lumen (*) of the acini. (F) Female gonad with early vitellogenic and vitellogenic oocytes, arrowhead indicated wall of the acini. dg, digestive gland; evo, early vitellogenic oocytes; mfe, epithelium of the mantle; oo, oogonia; pvo, previtellogenic oocytes; spc, spermatocytes; spg, spermatogonias; spm, spermatids; vo, vitellogenic oocyte. Scale bars = A–D, 20 µm; E–F = , 50 µm.
The record of gametogenic activity according to shell length was summarized graphically based on histological characterization (Figure 3A–C). The proportion of mussels with different maturation conditions in each size class showed a high proportion of undifferentiated individuals with undeveloped gonads at smaller sizes; the proportion of males increased faster with respect to the size than females until both reached maturity and sex ratio showed no differences between males and females (Figure 3D).

Fig. 3. Development of the gonad of B. rodriguezii as shell length increased. (A) Sexually immature and undifferentiated individuals. (B) Immature differentiated with an early development of gonad (shading). (C) Sexually mature. Between parentheses: shell length intervals for each stage. (D) Frequency of undifferentiated and differentiated individuals (males and females).
Size at first maturity
For differentiated individuals, males and females, the parameters of the logistic equation were: a = 1.07 and SL0 = 8.1 for males and f = 0.90 (∑dif2 0.01) and SL0 = 7.1 (∑dif2 0.16) for females. The value of the slope of the logistic function was higher in females than in males. The size at first maturity estimated was found to be 8.13 mm for males and 7.05 mm for females. For shell length larger than 14.00 mm all males were mature while all females larger than 13.00 mm were sexually mature (Figure 4).

Fig. 4. Brachidontes rodriguezii. The proportion of mature females and males as a function of shell length (SL) modelled with a logistic function: the model fitted (females: continuous line and males: dotted line) (N = 270).
DISCUSSION
According to the results of this study, the development of gonads in B. rodriguezii occurred together with the start of gametogenic activity and size of gonads increased with shell length. The first acini were located laterally and dorsally to the digestive gland; while maturation progressed, the acini of the gonad increased their size and number and changed its distribution, reaching both mantle lobes. While development of gonad tissue occurred the connective tissue tended to become less abundant. Mature males and females exhibited a well-developed gonad which extended from the dorsal region of the visceral mass to both mantle lobes with mature gametes within acini. Our observation about gonad development coincided with descriptions made by Allen (Reference Allen1962) for Brachidontes recurves; the gonad is a branched organ with ducts which end in follicles. The early development of gonadal tissue begins in the mantle and spreads to the mesosoma. When individuals reach maturity the gonad occupies the entire mantle, mesosoma and penetrates into the foot and is adjacent to the stomach and digestive tubules. The morphology of gonadal tissue of B. rodriguezii resembles those mentioned for Perna viridis, Brachidontes exustus (Barber et al., Reference Barber, Fajans, Baker and Baker2005) and Mytilus galloprovincialis (Suárez et al., Reference Suárez, Alvarez, Molist and San Juan2005).
According to Stearns (Reference Stearns1992) females of species with external fertilization tend to delay maturity so females become mature at bigger sizes than males. Our results showed that B. rodriguezii males reached gonadal maturity at slightly shorter shell length than females, but 50% of the female population reached gonadal maturity at a smaller size than males: 7.05 mm for females and 8.13 mm shell length for males. The steep slope of the logistic function estimated for females suggested a faster transition from immature to mature population in females than in males.
Many studies showed that reproductive seasonality is related to temperature and food intake (Giese & Pearse, Reference Giese, Pearse, Giese and Pearse1974; Lubet et al., Reference Lubet, Gimázane and Prunus1981; Suárez et al., Reference Suárez, Alvarez, Molist and San Juan2005; Fearman & Moltschaniwskyj, Reference Fearman and Moltschaniwskyj2010). Franz (Reference Franz1996) studied gametogenic activity in Geukensia demissa exposed to a gradient of wave exposition and observed that individuals exposed to immersed condition during prolonged periods of time reached maturity at shorter sizes than individuals exposed to aerial conditions. Intertidal coast organisms are able to obtain food and oxygen and to avoid desiccation (stress conditions are reduced) only during the period of immersion (Petes et al., Reference Petes, Menge and Murphy2007, Reference Petes, Menge and Harris2008). Previous studies for a population of B. rodriguezii inhabiting a rocky coast, in the high level of the middle intertidal, reported a size of first maturity between 6.2 and 7 mm shell length (Nugent-Rincón, Reference Nugent-Rincón1989). In our study specimens were collected from the lower zone of pier pilings (middle intertidal) where mussels were under water even in low tides, suggesting an intake of resources and more appropriate conditions to grow and develop. Delgado & Defeo (Reference Delgado and Defeo2007) observed that Donax hanleyanus delayed maturity in beaches where individuals were able to allocate more resources for reproduction and somatic growth.
Size at first maturity together with the reproductive cycle constitute reproductive aspects that maximize reproductive effort of species (Todd, Reference Todd, Moore and Seed1985; Gage, Reference Gage1995). Size at first maturity could be useful in determining measures for adequate natural resource management (Chung, Reference Chung2007, Reference Chung2008) and spatial monitoring (Camacho-Mondragón et al., Reference Camacho-Mondragón, Arellano-Martínez and Ceballos-Vázquez2012). The mussel B. rodriguezii lacks fishery or artisanal exploitation at present, although it is considered an ecosystem engineer encouraging species to the landscape (Borthagaray & Carranza, Reference Borthagaray and Carranza2007; Carranza et al., Reference Carranza, Defeo, Beck and Castilla2009; Arribas et al., Reference Arribas, Donnarumma, Palomo and Scrosati2014); a key species that structures the intertidal benthic community from Buenos Aires Province to north Patagonia (Adami et al., Reference Adami, Tablado and Sodor2008; Arribas et al., Reference Arribas, Bagur, Klein, Penchaszadeh and Palomo2013), hence the importance of understanding as many population aspects as possible with special regard to reproductive biology. Further studies should focus on evaluating changes in settlement and population structure according to substrate and environmental conditions, due to the progressive increase in the use of man-made structures and its impact in coastal areas and biodiversity.
FINANCIAL SUPPORT
This work was partially supported by Universidad de Buenos Aires (grant UBACyT 086) and the Banco Interamericano de Desarrollo (grant PICT 1159).