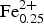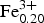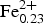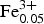Introduction
The Hatrurim Formation (also known as the ‘Mottled Zone’) is a unique complex of pyrogenic rocks involving various larnite, spurrite and other Ca-rich rock types from solid-state metamorphic rocks to paralavas. Previously, the mineral assemblages of the Hatrurim Formation were interpreted to indicate maximum temperatures of combustion metamorphism of 1000°C to 1100°C (Gross, Reference Gross1977). The Hatrurim Formation has become a ‘mineralogical Mecca’ due to numerous new minerals being found in these unique rocks. In general, these discoveries were initiated due to the now famous works of Sh. Gross and Y. Bentor (Bentor et al., Reference Bentor, Gross and Heller1963; Gross, Reference Gross1977 and others). In last decade more than 20 new minerals have been found within the Hatrurim Formation: barioferrite; shulamitite; murashkoite; zadovite; gurimite; vapnikite; nabimusaite; aradite; gazeevite; fluormayenite; fluorkyuygenite; negevite; halamishite; zuktamrurite; silicocarnotite; flamite; tululite and others (Murashko et al., Reference Murashko, Chukanov, Mukhanova, Vapnik, Britvin, Krivovichev, Polekhovsky and Ivakin2011; Sharygin et al., Reference Sharygin, Lazic, Armbruster, Murashko, Wirth, Galuskina, Galuskin, Vapnik, Britvin and Logvinova2013; Galuskin et al., Reference Galuskin, Galuskina, Kusz, Armbruster, Marzec, Dzierżanowski and Murashko2014; Reference Galuskin, Gfeller, Armbruster, Galuskina, Vapnik, Dulski, Murashko, Dzierżanowski, Sharygin, Krivovichev and Wirth2015a–Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Wlodyka, Dzierżanowski and Murashkoc; Reference Galuskin, Galuskina, Gfeller, Krueger, Kusz, Vapnik, Dulski and Dzierzanowski2016; Reference Galuskin, Gfeller, Galuskina, Armbruster, Krzatala, Vapnik, Kusz, Dulski, Gardocki, Gurbanov and Dzierżanowski2017; Galuskina et al., Reference Galuskina, Vapnik, Lazic, Armbruster, Murashko and Galuskin2014; Reference Galuskina, Galuskin, Pakhomova, Widmer, Armbruster, Krueger, Grew, Vapnik, Dzierzanowski and Murashko2017a,Reference Galuskina, Galuskin, Prusik, Vapnik, Juroszek, Jeżak and Murashkob; Britvin et al., Reference Britvin, Murashko, Vapnik, Polekhovsky and Krivovichev2015; Sokol et al., Reference Sokol, Seryotkin, Kokh, Vapnik, Nigmatulina, Goryainov, Belogub and Sharygin2015; Khoury et al., Reference Khoury, Sokol, Kokh, Seryotkin, Nigmatulina, Goryainov, Belogub and Clark2016). Most of these were identified in larnite and spurrite rocks and Ca-rich paralavas; and some could be used as indicator minerals to estimate the history of the rock formation. For example, shulamitite (+ Fe-perovskite) from the Hatrurim Basin larnite rocks indicates crystallisation under high temperatures (1150–1170°C) and low pressures (high-T-region of the spurrite–merwinite facies) (Sharygin et al., Reference Sharygin, Sokol and Vapnik2008a; Reference Sharygin, Lazic, Armbruster, Murashko, Wirth, Galuskina, Galuskin, Vapnik, Britvin and Logvinova2013). Coexistence of flamite and larnite in the Hatrurim Ca-Al-rich paralavas reveals a specific cooling history (quenching of melt) during their solidification (Sokol et al., Reference Sokol, Seryotkin, Kokh, Vapnik, Nigmatulina, Goryainov, Belogub and Sharygin2015). Estimating the formation temperatures is possible for the Hatrurim Formation Ca-rich silica-undersaturated rocks, but remains problematic for more silicic plagioclase-clinopyroxene hornfels and paralavas, due to the lack of indicator minerals. Investigation of different plagioclase-clinopyroxene paralavas has indicated minimal melting temperatures of >1100°C (Vapnik et al., Reference Vapnik, Sharygin, Sokol, Shagam and Stracher2007), whereas the temperature regime of the same hornfels (solid phase transformation) remains enigmatic.
Corundum and hematite are rarely associated in terrestrial rocks, but sometimes occur in Al-rich metamorphic rocks after bauxites and laterites (Feenstra et al., Reference Feenstra, Samann and Wunder2005 and references therein). Exsolution of hematite in gem-quality corundum of metamorphic or magmatic origin and reverse exsolution structure (corundum in hematite or another Fe-oxide minerals) are also rare phenomena (Feenstra et al., Reference Feenstra, Samann and Wunder2005 and references therein; Kulikova and Varlamov, Reference Kulikova and Varlamov2006; Izokh et al., Reference Izokh, Smirnov, Egorova, Tran, Kovyazin, Ngo and Kalinina2010). Fe-rich corundum, hibonite, dorrite-like minerals, Mn-Al-rich hematite and magnesioferrite have been found as a spongy aggregate in vugs from one of the ‘metacarbonate nuts’ of a burnt dump of the Kalinin coal mine, Donets coal basin, southeast Ukraine (Sharygin, Reference Sharygin2010). The Fe-rich corundum + Al-rich hematite + spinel + hibonite assemblage in the Hatrurim plagioclase-clinopyroxene rock was described briefly in a previous publication (Sharygin et al., Reference Sharygin, Vapnik and Sokol2008b). The present paper provides more details of the rock mineralogy, with implications for formation conditions of the specific Fe–Al–Mg-oxide assemblage.
The Hatrurim Basin and the Hatrurim Formation: geological background
The Hatrurim Formation (Mottled Zone, MZ) complexes are located in the vicinity of the Dead Sea Transform (Gross, Reference Gross1977; Novikov et al., Reference Novikov, Vapnik and Safonova2013), which is a prominent shear zone separating the Arabian plate from the Sinai microplate (Supplementary Fig. S1, based on data from Bentor and Vroman, Reference Bentor and Vroman1960; Bentor et al., 1965; Picard and Golani, Reference Picard and Golani1965; Nissenbaum and Goldberg, Reference Nissenbaum and Goldberg1980; Burg et al., Reference Burg, Starinsky, Bartov and Kolodny1991, Reference Burg, Kolodny and Lyakhovsky1999; Gardosh et al., Reference Gardosh, Kashai, Salhov, Shulman, Tannenbaum, Niemi, Ben-Avraham and Gat1997, Reference Gardosh, Druckman, Buchbinder and Rybakov2008; Shen et al., Reference Shen, Bartov, Rosensaft and Weissbort1998; Hall et al., Reference Hall, Krasheninnikov, Hirsch, Benjamini and Flexer2005; Sokol et al., Reference Sokol, Novikov, Vapnik and Sharygin2007, Reference Sokol, Novikov, Zateeva, Vapnik, Shagam and Kozmenko2010, Reference Bentor and Vroman2014c; Hirsch et al., Reference Hirsch, Burg and Avni2010). The Dead Sea Transform system consists of six major, left-stepping strike-slip faults with deep rhomb-shaped depressions between each fault pair, the largest one is the Dead Sea basin. This depression is boarded by uplifted margins and filled with up to 10 km thick Miocene to Holocene sediments (Garfunkel, Reference Garfunkel, Niemi, Ben-Avraham and Gat1997; Maercklin, Reference Maercklin, Haberland, Ryberg, Weber and Bartov2004). Along the eastern shore Lower Cretaceous to Cambrian Nubian sandstones are exposed, whereas the western margin is composed of a Permian to Cretaceous sedimentary sequence ~4 km thick. The Upper Cretaceous – Lower Tertiary marine sediments cover the area almost continuously and consist of limestone, chalk (including bituminous varieties), and dolomite with marl, phosphorite and chert intercalations (Zeigler, Reference Zeigler2001; Hall et al., Reference Hall, Krasheninnikov, Hirsch, Benjamini and Flexer2005). The widths of Dead Sea Transform-related damage zones, consisting of minor fault fractures and fracture networks, range from metres to several hundreds of metres, and control the ascending fluid flow from subsurface (Gardosh et al., Reference Gardosh, Kashai, Salhov, Shulman, Tannenbaum, Niemi, Ben-Avraham and Gat1997; Gvirtzman and Stanislavsky, Reference Gvirtzman and Stanislavsky2000; Sokol et al., Reference Sokol, Kozmenko, Smirnov, Sokol, Novikova, Tomilenko, Kokh, Ryazanova, Reutsky, Bul'bak, Vapnik and Deyak2014c). The ongoing activity appears as saline springs and methane outbursts from shallow wells, as well as famous asphalt floating blocks and ‘springs’ in the southern Dead Sea (Nissenbaum and Goldberg, Reference Nissenbaum and Goldberg1980).
Tectonically, shores of the Dead Sea basin are composed of a series of step-faulted blocks (Garfunkel, Reference Garfunkel, Niemi, Ben-Avraham and Gat1997). The south-western shoulder of the rift within the Masada-Zohar block is prominent due to hydrocarbon accumulations, namely small commercial gas fields (Zohar, Kidod and Haqanaim) as well as non-commercial heavy oil (at Gurim-1 and -2) and light oil (Zuk-Tamrur-1) (Gardosh et al., Reference Gardosh, Kashai, Salhov, Shulman, Tannenbaum, Niemi, Ben-Avraham and Gat1997). The Hatrurim Basin (11.3 km × 7.3 km, 47.8 km2, Bentor and Vroman, Reference Bentor and Vroman1960), one of the largest Mottled Zone complexes, is also located within the Masada-Zohar block (31°12′N, 35°16′E) (Fig. S1 A,B).
The specific non-stratigraphic unit of the MZ rocks lies at the top of the Upper Cretaceous – Lower Tertiary section of the area and reaches depths from 30–40 to 120 m below the surface (Fleischer and Varshavsky, Reference Fleischer and Varshavsky2002). The term ‘MZ sequence’ involves diverse sedimentary rocks, which underwent post-depositional alteration under different conditions. The section consists mainly of unevenly distributed brecciated sediments (mainly chalk enclosing phosphorite, chert and marl), which have undergone extensive low-temperature hydrothermal alteration and are cut by abundant veinlets containing calcite, aragonite, gypsum, various hydrated Ca-silicates, zeolites and ettringite. The uppermost part of the same section includes numerous foci of diverse anhydrous high- to ultrahigh-temperature combustion metamorphic rocks.
The conical hills are a characteristic feature of the geomorphology of the Hatrurim Basin. The low and middle parts of these hills are represented by the middle-temperature combustion metamorphism rocks: spurrite marbles (T ~700–800°C). Relatively fresh marbles (rare) as well as their hydrated varieties (common) up to 4 m thick cover large areas and rim a set of circle structures with diameters from a few hundred metres to a few kilometres (Fig. S1 C). The upper part consists typically of coarse-clastic breccias with lumps of marl, limestone, phosphorite and flint from strata below. Occurrences of the rocks of the so-called ‘olive unit’ (Gross, Reference Gross1977; Burg et al., Reference Burg, Starinsky, Bartov and Kolodny1991) are confined to the top of the hills. These highly-porous rocks are composed mainly of calcite and zeolites, which cemented the quartz sand and clasts of the sediments below. They commonly form specific swells and structures of ‘plugged land’ (Vapnik et al., Reference Vapnik, Sharygin, Sokol, Shagam and Stracher2007). The concentric swells (zones) are of high-temperature clinopyroxene-anorthite hornfels with paralava lenses and veinlets of the same mineral composition. The zones of calcination and metamorphism of parent marly sediments produce hornfels. The depth of these hornfel zones does not exceed 3 m. A supply channel now filled with brecciated sediments with carbonate and/or zeolite cement has been observed for some bodies. They form sharp downward cones 12–20 m deep (Vapnik et al., Reference Vapnik, Sharygin, Sokol, Shagam and Stracher2007).
The high-temperature larnite-bearing rocks (T = 1000–1400°C) were observed at several levels of the Hatrurim Formation section, mainly in the northern and central parts of the Hatrurim Basin (Fig. S1). In the lower section within 20 m above its basement, they are from a few tens of centimetres to a few metres thick and neighbour bodies of gehlenite hornfels. Both larnite and gehlenite combustion metamorphism rocks transform easily into the so-called ‘pseudo-conglomerates’ by retrograde hydration and/or carbonation. The secondary products are mainly calcite, aragonite, gypsum, ettringite and minor Ca silicate-hydrates. Up section, larnitic rocks occur as isolated mottles (to 10 m across) among extensively altered varieties. At hilltops, monolithic larnite rocks make up separate isometric massive blocks, plates or cliff scarps, up to 50 m across and 10 m thick (Gross, Reference Gross1977, Reference Gross1984; Burg et al., Reference Burg, Starinsky, Bartov and Kolodny1991; Sharygin et al., Reference Sharygin, Sokol and Vapnik2008a, Reference Sharygin, Lazic, Armbruster, Murashko, Wirth, Galuskina, Galuskin, Vapnik, Britvin and Logvinova2013; Sokol et al., Reference Sokol, Kokh, Vapnik, Thiery and Korzhova2014b; Galuskina et al., Reference Galuskin, Gfeller, Armbruster, Galuskina, Vapnik, Dulski, Murashko, Dzierżanowski, Sharygin, Krivovichev and Wirth2015a–Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Wlodyka, Dzierżanowski and Murashkoc). Descriptions of individual bodies of these rocks at the Hatrurim Basin are given in many publications (e.g. Kolodny and Gross, Reference Kolodny and Gross1974; Gross, Reference Gross1977; Burg et al., Reference Burg, Starinsky, Bartov and Kolodny1991, Reference Burg, Kolodny and Lyakhovsky1999; Vapnik et al., Reference Vapnik, Sharygin, Sokol, Shagam and Stracher2007; Sharygin et al., Reference Sharygin, Sokol and Vapnik2008a; 2013; Sokol et al., Reference Sokol, Novikov, Zateeva, Sharygin and Vapnik2008; Reference Sharygin, Vapnik and Sokol2014b; Novikov et al., Reference Novikov, Vapnik and Safonova2013; Galuskina et al., Reference Galuskin, Gfeller, Armbruster, Galuskina, Vapnik, Dulski, Murashko, Dzierżanowski, Sharygin, Krivovichev and Wirth2015a,Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Wlodyka, Dzierżanowski and Murashkoc).
Many authors interpreted the Hatrurim Formation complexes as products of in situ combustion of low-calorific fuel, specifically, disseminated bituminous matter of marine chalk (Bentor et al., Reference Bentor, Gross and Heller1963; Burg et al., Reference Burg, Starinsky, Bartov and Kolodny1991; Reference Burg, Kolodny and Lyakhovsky1999; Khoury and Nassir, Reference Khoury and Nassir1982; Techer et al., Reference Techer, Khoury, Salameh, Rassineux, Claude, Clauer, Pagel, Lancelot, Hamelin and Jacquot2006). Other authors attributed combustion metamorphism events within the Hatrurim Formation complexes to local breakthrough and ignition of high-calorific hydrocarbon gases, mainly methane, which might be derived from mud volcanism (Gilat, Reference Gilat1998, Vapnik et al., Reference Vapnik, Sharygin, Sokol, Shagam and Stracher2007; Sharygin et al., Reference Sharygin, Sokol and Vapnik2008a,Reference Sharygin, Vapnik and Sokolb; Reference Sharygin, Lazic, Armbruster, Murashko, Wirth, Galuskina, Galuskin, Vapnik, Britvin and Logvinova2013; Seryotkin et al., Reference Seryotkin, Sokol and Kokh2012; Sokol et al., Reference Sokol, Novikov, Zateeva, Sharygin and Vapnik2008; Reference Sokol, Novikov, Zateeva, Vapnik, Shagam and Kozmenko2010; Reference Sokol, Kokh, Vapnik, Thiery and Korzhova2014b; Novikov et al., Reference Novikov, Vapnik and Safonova2013). The mud volcanism is spatially and structurally related to the neotectonic movements, folds and deformation zones, and may be considered as a response to the development of the Red Sea Fault and Levantine Transform Fault (Novikov et al., Reference Novikov, Vapnik and Safonova2013). According to 40Ar/39Ar radiometric age determination of different MZ rocks (Gur et al., Reference Gur, Steinitz, Kolodny, Starinsky and McWilliams1995; Sokol et al., Reference Sokol, Gaskova, Kozmenko, Kokh, Vapnik, Novikova and Nigmatulina2014a) the processes of fluid impact and further gas ignition occasionally occurred in the 7–0.5 Ma range, which corresponds to the main (most active) stage of the opening of the Dead Sea rift (Maercklin, Reference Maercklin, Haberland, Ryberg, Weber and Bartov2004). Emissions of hydrocarbon gases in the geological past left an imprint in abundant foci of high- (800–1100°С) and ultrahigh-temperature (1200–1500°С) combustion metamorphism of sediments at the Hatrurim Basin and other localities (Vapnik et al., Reference Vapnik, Sharygin, Sokol, Shagam and Stracher2007; Sokol et al., Reference Sokol, Novikov, Zateeva, Sharygin and Vapnik2008, Reference Sokol, Kozmenko, Kokh and Vapnik2012; Reference Sokol, Kokh, Vapnik, Thiery and Korzhova2014b; Sharygin et al., Reference Sharygin, Sokol and Vapnik2008a,Reference Sharygin, Vapnik and Sokolb; Reference Sharygin, Lazic, Armbruster, Murashko, Wirth, Galuskina, Galuskin, Vapnik, Britvin and Logvinova2013; Seryotkin et al., Reference Seryotkin, Sokol and Kokh2012). On exposure to air, methane reacted with atmospheric oxygen and ignited. Burning of the high-calorific fossil fuel released enough heat to maintain combustion metamorphism alteration and local melting of marly sediments. Burned carbonate rocks transformed into diverse calcium-rich metamorphic rocks with a typical clinker mineralogy (Sharygin et al., Reference Sharygin, Sokol and Vapnik2008a), whereas marly sediments were melted at the same temperature, forming clinopyroxene-bearing paralavas (Vapnik et al., Reference Vapnik, Sharygin, Sokol, Shagam and Stracher2007).
Regardless of different opinions their on genesis, all metamorphic rocks of the Hatrurim Basin and other MZ localities are considered to be the products of the high-temperature (900–1200°C) solid-state reactions during organic matter combustion, sometimes with further local melting events (1250–1500°C) (Bentor et al., Reference Bentor, Gross and Heller1963; Kolodny, Reference Kolodny and Skalny1979; Matthews and Gross, Reference Matthews and Gross1980; Sokol et al., Reference Sokol, Maksimova, Nigmatulina, Sharygin and Kalugin2005; Reference Sokol, Novikov, Vapnik and Sharygin2007, Reference Sokol, Novikov, Zateeva, Sharygin and Vapnik2008; Reference Sokol, Novikov, Zateeva, Vapnik, Shagam and Kozmenko2010; Reference Sokol, Kokh, Vapnik, Thiery and Korzhova2014b; Zateeva et al., Reference Zateeva, Sokol and Sharygin2007; Vapnik et al., Reference Vapnik, Sokol, Murashko and Sharygin2006; Reference Vapnik, Sharygin, Sokol, Shagam and Stracher2007; Sharygin et al., Reference Sharygin, Sokol and Vapnik2008a,Reference Sharygin, Vapnik and Sokolb; Reference Sharygin, Lazic, Armbruster, Murashko, Wirth, Galuskina, Galuskin, Vapnik, Britvin and Logvinova2013; Geller et al., Reference Geller, Burg, Halicz and Kolodny2012, Wang et al., Reference Wang, Anovitz, Burg, Cole, Allard, Jackson, Stack and Rother2013; Kokh et al., Reference Kokh, Sokol, Sharygin, Stracher, Prakash and Sokol2014; Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Wlodyka, Dzierżanowski and Murashko2015c; Galuskina et al., Reference Galuskina, Galuskin, Pakhomova, Widmer, Armbruster, Krueger, Grew, Vapnik, Dzierzanowski and Murashko2017a,b).
Analytical methods
Double-polished rock sections (~50 μm thick) were used for optical examination of a plagioclase-clinopyroxene rock in transmitted and reflected light. The identification of minerals was based on energy-dispersive spectra (EDS), back-scattered electron (BSE) images and elemental mapping (EDS system), using a JEOL 6380LA and TESCAN MIRA 3MLU scanning electron microscopes equipped with an INCA Energy 450 XMax 80 microanalysis system (Oxford Instruments Ltd.) at the V.S. Sobolev Institute of Geology and Mineralogy (IGM), Novosibirsk, Russia. The instruments were operated at an accelerating voltage of 20 kV and a probe current of 1 nA in low-vacuum (40–60 Pa) or high-vacuum modes. Energy-dispersive analysis was performed using a TESCAN MIRA 3MLU scanning electron microscope at an accelerating voltage of 20 kV, a probe current of 1 nA, and accumulation time of 20 s. The following simple compounds and metals were used as reference standards for most of the elements: SiO2 (Si and O); Al2O3 (Al); diopside (Mg and Ca); albite (Na); orthoclase (K); Ca2P2O7 (P); BaF2 (Ba and F); Cr2O3 (Cr); pyrite (S); CsRe2Cl6 (Cl); LaPO4 (La); CePO4 (Ce); SrF2 (Sr); metallic Ti, Fe, Mn, Zn, Ni, V and Y. Correction for matrix effects was done using the XPP algorithm, implemented in the software of the microanalysis system. Metallic Co served for quantitative optimization (normalization to probe current and energy calibration of the spectrometer).
Electron microprobe analyses (EMPA) in wavelength-dispersive (WDS) mode were performed for rock-forming and opaque minerals of the Hatrurim rock using a Camebax microprobe at IGM. The operating conditions were as follows: beam diameter of 1–2 μm, accelerating voltage of 20 kV, beam current of 20 nA (silicates) and 50–60 nA (opaque minerals), and counting time of 10 s (5 + 5). The following standards were used for opaque minerals: hematite (Fe); spessartine (Mn); diopside (Ca and Si); albite (Na); MgAl2O4 (Mg and Al); ZnFe2O4 (Zn); NiFe2O4 (Ni); ilmenite (Ti); V2O5 (V) and others. Correction for matrix effects was done using a PAP routine (Pouchou and Pichoir, Reference Pouchou, Pichoir and Armstrong1985). The precision of analysis for major elements was better than 2% relative. The detection limits for elements are (in ppm): Si 210; Ti 162; Cr 248; V 170; Al 142; Fe 157; Mn 135; Mg 162; Ca 110; Sr 202; Ce 245; Na 310; Ni 208; Zn 293; and Cu 210.
Raman spectroscopy was used to identify some of the minerals in the Hatrurim plagioclase-clinopyroxene rock. We used a LabRAM HR 800 mm (HORIBA Scientific Ltd.) spectrometer equipped with a CCD detector and coupled to an Olympus BX40 confocal microscope (×100 objective). A semiconductor laser emitting at 514.5 nm with a nominal power output of 50 mW was used for excitation. In each case, 20 spectra were recorded for 20 s each at a hole diameter of 100 μm and integrated. The spectra were recorded between 100 and 1200 cm–1, and the monochromator was calibrated using the 520.7 cm–1 Raman line of elemental Si.
Mineralogy and petrography of plagioclase-clinopyroxene rock
A plagioclase-clinopyroxene rock sample (5203) was collected from the ‘olive unit’ on the top of a hill from the central part of the Hatrurim Basin (Fig. S1 C). A brief description of the rock was given in Sokol et al. (Reference Sokol, Maksimova, Nigmatulina, Sharygin and Kalugin2005) and Sharygin et al. (Reference Sharygin, Vapnik and Sokol2008b). The specific feature of this rock is the abundance of gas vesicles (100–300 μm) and the presence of lenticular segregation with Fe–Al–Mg-oxide minerals (up to 1 cm) with a leucocratic outer zone (Figs 1, 2). In general, the host rock is equigranular and vesicular and composed mainly of plagioclase (An99) and zoned clinopyroxene (size of grains ≈ 30–50 μm) (Fig. 1). On the background of the plagioclase-clinopyroxene aggregate large crystals (300–600 μm) of a SiO2 polymorph (quartz and/or tridymite) are shown clearly (Fig. 1, Fig S2). Elongated SiO2 crystals and anhedral fluorapatite are riddled with inclusions of plagioclase and sometimes clinopyroxene (up to 10 μm). K-feldspar, fluorapatite, hematite, titanite, ‘hyalophane’ (Cn51–69), Ti-andradite, celestine, baryte, calcite, anhydrite and an unidentified Mn-hydroxide occur as minor and accessory minerals in the rock (Fig. S2–S3). The opaque mineral assemblage is hematite ± titanite, these grains also contain numerous inclusions of plagioclase. Mineral relations are shown in Fig. S2-S3 and compositions of minerals of the host rock are given in Tables S1, S3 (Supplementary material). The vesicles in the host rock, opaque segregation and leucocratic zone are partly or completely filled with low-temperature calcite, zeolite-supergroup minerals, quartz and rare bassanite (or anhydrite) (Fig. S2–S3, Table S2). Some minerals (clinopyroxene, Ti-andradite, fluorapatite, K-feldspar) occasionally form well-faceted crystals in the vesicles. Previously we classified this rock as plagioclase-clinopyroxene hornfels (Sharygin et al., Reference Sharygin, Vapnik and Sokol2008b). However, the abundance of vesicles allows it to be interpreted as paralava, which was formed after hornfels.

Fig. 1. General view of plagioclase-clinopyroxene rock with opaque mineral segregation with corundum (OS) and surrounding leucocratic zone (LZ), Hatrurim Basin, ordinary light. Host rock is intermediate between hornfels and paralava.

Fig. 2. Corundum-bearing opaque mineral segregation (OS) surrounded by leucocratic zone (LZ), plagioclase-clinopyroxene rock, Hatrurim Basin, BSE image. Symbols: An – fine-grained anorthite; Cpx – clinopyroxene; Qz – SiO2 polymorph (quartz-tridymite), Hem – hematite, Ap – fluorapatite, vs – vesicle.
The segregation with opaque phases (up to 1 cm, Fig. 2) contains intergrowths of Fe–Al–Mg-oxide phases (corundum, hematite, spinel, hibonite) in coarse-grained plagioclase (up to 1 mm). Clinopyroxene, fluorapatite and K-feldspar are minor-to-accessory and localised mainly in the interstices between plagioclase crystals. Individual Fe–Al–Mg-oxide intergrowths are up to 70 μm and the ratio of minerals in them is variable (Fig. 3). Fluid and crystal inclusions are common in corundum (Fig. 4); the latter are represented by plagioclase and rarely by hematite and calcite. The majority of spinels contain oriented exsolution structures of hematite (supported by Raman spectra), whereas homogenous grains are rare. Hematite is also inhomogeneous and encloses oriented μm-sized exsolved corundum and rare inclusions of unidentified Ti–Fe- and Ca–V–Fe-oxide phases (Fig. 3; Fig. S5).

Fig. 3. Mineral intergrowths in coarse-grained anorthite from the opaque segregation, plagioclase-clinopyroxene rock, Hatrurim Basin, BSE images. Symbols: Hem – hematite; Spl – spinel; Crn – corundum; Hib – hibonite; Cc – calcite; Ap – fluorapatite; and fi – fluid inclusions in corundum.

Fig. 4. Fluid and crystal inclusions in corundum from opaque mineral segregation, Hatrurim Basin, transmitted light. Spl – spinel; Hem – hematite; An – anorthite. Scale bar is 25 μm.
The outer leucocratic zone is represented by fine-grained feldspathic aggregate (10–30 μm, plagioclase ± K-feldspar) with coarse grains (up to 1 mm) of clinopyroxene, SiO2 polymorph and fluorapatite, which are riddled with plagioclase inclusions (Fig. 2, Fig. S3). The opaque minerals (hematite ± ‘hemoilmenite’ (Fe2+,Fe3+)(Ti,Fe3+)O3 ± titanite ± perovskite) are also abundant (Fig. 2, Figs S3–S4). The association of Cr-rich hematite and spinel occurs scarcely, mainly near the opaque segregation (Fig. S4).
The compositions of silicates and phosphates from the host rock, opaque segregation and leucocratic zone are rather similar: plagioclase is anorthite (An99); clinopyroxene is diopside-dominant (Di30–55Ess21–39Hd10–27Ts4–20Acm1–2); K-feldspar has a moderate content of BaO (up to 2.5 wt.%); fluorapatite is enriched in SiO2 (2.6–6.9 wt.%) and SO3 (0.0–1.7 wt.%), sometimes in V2O3 (up to 2.3 wt.%) and oxides of light-rare-earth elements (LREE 2O3 up to 0.5 wt.%), showing evolution to fluorellestadite. Clinopyroxene from the host rock is zoned, and the core-to-rim variation is directed towards the enrichment in the Fe2+- and Fe3+-rich end-members (hedenbergite and esseneite, respectively). Clinopyroxene in the leucocratic zone and segregation with opaque phases is yellowish indicating higher contents of esseneite. The composition of the above silicates and Fe-rich oxides is given in Tables S1–S4.
Note that a single opaque segregation with corundum has been observed in this investigation. Other rare opaque assemblages in vesicles or nearby in this rock reveals diverse mineral compositions: intergrowths of spinel, hematite, hibonite, orthorhombic FeAlO3 and a dorrite-like phase (Sharygin and Sokol, Reference Sharygin and Sokol2017); hematite + Al-rich magnetite + dorrite-like phase + esseneite ± hibonite ± melilite/andradite or hematite + spinel ± ilmenite (author's unpublished data). Corundum is absent in these assemblages.
Composition of oxide minerals in the corundum-bearing opaque segregation
Fe–Al–Mg-oxide phases in the opaque segregation are distinguished easily in transmitted and reflected polarised light. Corundum is colourless and contains abundant fluid inclusions; hematite is translucent and red; spinel is light brown and with abundant exsolution hematite; and hibonite is yellow to yellow-brown. The mineral relationships do not allow a clear crystallization sequence to be established, but, in general, corundum is an earlier phase than hibonite (Fig. 3, Fig. S5). All oxide phases seem to be formed before, or simultaneously with anorthite. The assemblage has particular mineral compositions, which are common to pyrometamorphic and combustion metamorphism phases (Reverdatto, Reference Reverdatto1970; Sokol et al., Reference Sokol, Maksimova, Nigmatulina, Sharygin and Kalugin2005; Grapes, Reference Grapes2011).
Hibonite
This mineral is a minor component within the intergrowths (Fig. 3). Most hibonites are Ti-rich (TiO2 > 8 wt.%) with average composition Ca0.95(Al9.13Fe3+1.25Ti0.77Mg0.64Fe2+0.22Si0.04)O19 (Table 1), expressed in simplified formula as CaAl9Fe3+(Mg,Fe2+)TiO19. It is obviously not similar to ‘ideal’ hibonite CaAl12O19. Compositional variations of the Hatrurim hibonite are given in Fig. 5. The weak negative correlations are indicated between Al and Si, Ti and Fe3+. In general, terrestrial and extraterrestrial hibonites (Agrell et al., Reference Agrell, Chinner and Rowley1999; Simon et al., Reference Simon, Davis and Grossman2001; Simon and Grossman, Reference Simon and Grossman2003; Ulianov et al., Reference Ulianov, Kalt and Pettke2005; Nagashima et al., Reference Nagashima, Armbruster and Hainschwang2010; Rajesh et al., Reference Rajesh, Arai, Santosh and Tamura2010; Sharygin, Reference Sharygin2010; Ananyev et al., Reference Ananyev, Konovalenko, Rastsvetaeva, Aksenov, Chukanov, Sapozhnikov, Zagorsky and Virus2011; Doyle et al., Reference Doyle, Schofield, Berry, Walker and Knight2014; and references therein) show moderate-to-high concentrations of Ti and highlight that classification of the magnetoplumbite group needs revision, especially for Ca–Al-compositions.

Fig. 5. Variations of Al vs. major elements (in atoms per formula unit) for hibonite from opaque segregation in plagioclase-clinopyroxene rock, Hatrurim Basin, Israel.
Table 1. Representative compositions of hibonite from opaque corundum-bearing segregation in plagioclase-clinopyroxene rock, Hatrurim Basin, Israel.

n = number of analyses (WDS); La2O3, Y2O3, K2O and ZrO2 are below detection limits (<<0.01 wt.%); n.a. = not analysed.
*Fe2O3 and FeO are calculated from charge balance.
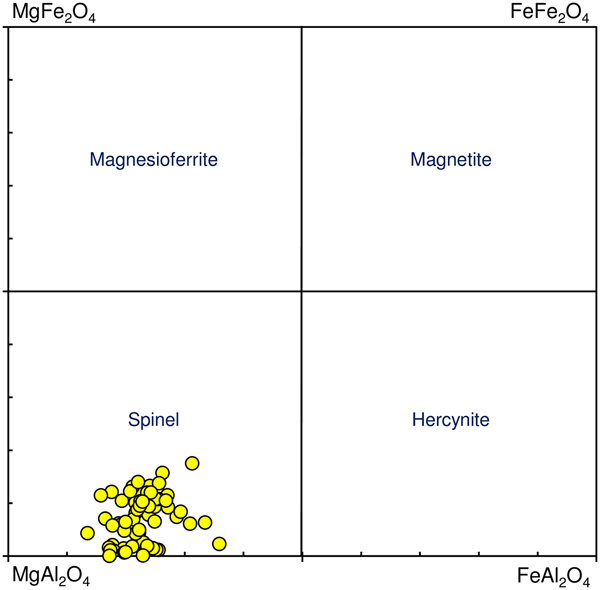
Spinel
According to the classification diagram (Fig. 6) all the minerals analysed in this group are spinel. However, individual grains or areas within the grains (homogeneous and with exsolution) differ in composition. Dominant spinel (with exsolution) has the average formula (Mg0.75![]() ${\rm Fe}_{{\rm 0}{\rm. 20}}^{2 +} $Ni0.03Zn0.01Mn0.01)(Al1.79
${\rm Fe}_{{\rm 0}{\rm. 20}}^{2 +} $Ni0.03Zn0.01Mn0.01)(Al1.79![]() ${\rm Fe}_{{\rm 0}{\rm. 20}}^{3 +} $Cr0.01)O4 (n = 40), whereas homogeneous species have (Mg0.77
${\rm Fe}_{{\rm 0}{\rm. 20}}^{3 +} $Cr0.01)O4 (n = 40), whereas homogeneous species have (Mg0.77![]() ${\rm Fe}_{{\rm 0}{\rm. 17}}^{2 +} $ Ni0.03Zn0.02Mn0.01)(Al1.94
${\rm Fe}_{{\rm 0}{\rm. 17}}^{2 +} $ Ni0.03Zn0.02Mn0.01)(Al1.94![]() ${\rm Fe}_{{\rm 0}{\rm. 05}}^{3 +} $Cr0.01)O4 (n = 24). This indicates evolution towards the magnesioferrite and magnetite end-members. Representative compositions of the Hatrurim spinel are given in Table 2. In general, both types of spinel are rich in NiO (up to 1.9 wt.) and ZnO (up to 1.4 wt.%); the content of other minor oxides is negligible (<0.15 wt.%). Thus, solid phase decay of an initial Fe-rich spinel with formation of hematite might be explained by the reaction of partial oxidation: 2(Mg0.5
${\rm Fe}_{{\rm 0}{\rm. 05}}^{3 +} $Cr0.01)O4 (n = 24). This indicates evolution towards the magnesioferrite and magnetite end-members. Representative compositions of the Hatrurim spinel are given in Table 2. In general, both types of spinel are rich in NiO (up to 1.9 wt.) and ZnO (up to 1.4 wt.%); the content of other minor oxides is negligible (<0.15 wt.%). Thus, solid phase decay of an initial Fe-rich spinel with formation of hematite might be explained by the reaction of partial oxidation: 2(Mg0.5![]() ${\rm Fe}_{{\rm 0}{\rm. 5}}^{2 +} $)(Al1.0
${\rm Fe}_{{\rm 0}{\rm. 5}}^{2 +} $)(Al1.0![]() ${\rm Fe}_{{\rm 1}{\rm. 0}}^{3 +} $)O4 + 0.25O2 → MgAl2O4 + 1.5Fe2O3.
${\rm Fe}_{{\rm 1}{\rm. 0}}^{3 +} $)O4 + 0.25O2 → MgAl2O4 + 1.5Fe2O3.

Fig. 6. Classification diagram for spinel from opaque segregation in plagioclase-clinopyroxene rock, Hatrurim Basin, Israel.
Table 2. Representative compositions (wt.%) of spinel from opaque segregation in plagioclase-clinopyroxene rock, Hatrurim Basin, Israel.

n = number of analyses (WDS); hom = homogeneous spinel; decay = spinel with solid phase decay structure; n.a. = not analysed; b.d.l = below detection limit.
*Fe2O3 and FeO are calculated from charge balance.
Corundum and hematite
Corundum and hematite are the main constituents of most opaque intergrowths (Fig. 3). Corundum essentially varies in Fe2O3 (4.2–11.8 wt.%); minor components are Cr2O3 (up to 0.6 wt.%) and TiO2 (up to 0.4 wt.%). The content of other minor oxides is negligible (<0.1 wt.%). Variations in Fe2O3 content for the Hatrurim corundum are given in Fig. 7. In general, some corundum grains show mosaic (spotty) zonation, especially in direct contact with hematite (Fig. 3, Fig. S5). The compositions richer in Fe are common near the corundum-hematite boundary. Solid inclusions in corundum are represented by anorthite, hematite and calcite. No oriented exsolution structure is found in this mineral.

Fig. 7. Variations of Al2O3 vs. Fe2O3 contents (in wt.%) in corundum from opaque segregation in plagioclase-clinopyroxene rock, Hatrurim Basin, Israel.
As noted above, orthorhombic FeAlO3 was identified in one of the opaque assemblages in vesicles in the rock investigated (Sharygin and Sokol, Reference Sharygin and Sokol2017). Therefore hematite from the corundum-bearing segregation was investigated for other polymorphic modifications of Fe2O3 using Raman spectroscopy, Fig. S6. Five crystalline polymorphs of Fe2O3 are known: hematite (α-Fe2O3, trigonal); maghemite (γ-Fe2O3, cubic with tetragonal superstructure); luogufengite (ε-Fe2O3, orthorhombic) (Sakurai et al., Reference Sakurai, Namai, Hashimoto and Ohkoshi2009; Xu et al., Reference Xu, Lee and Xu2017); β-Fe2O3 (synthetic, cubic) (Zboril et al., Reference Zboril, Mashlan, Krausova and Pikal1999; Sakurai et al., Reference Sakurai, Namai, Hashimoto and Ohkoshi2009), and ξ-Fe2O3 (synthetic, monoclinic) (Tucek et al., Reference Tucek, Machala, Ono, Namai, Yoshikiyo, Imoto, Tokoro, Ohkoshi and Zboril2015). In addition, orthorhombic ε-FeAlO3, perovskite and post-perovskite FeAlO3 and AlFeO3 have been synthesised (Polli et al., Reference Polli, Lange and Levi1996; Majzlan et al., Reference Majzlan, Navrotsky and Evans2002; Feenstra et al., Reference Feenstra, Samann and Wunder2005; Pissas et al., Reference Pissas, Stamopoulos, Sanakis and Simopoulos2008; Caracas, Reference Caracas2010). The hematite studied from the Hatrurim Basin differs drastically from FeAlO3 with lower Al2O3. Raman spectra indicate a suite of bands characteristic of hematite (Hanesch, Reference Hanesch2009; Jubb and Allen, Reference Jubb and Allen2010) and it differs significantly from maghemite and, possibly, from other polymorphs (Fig. S6).
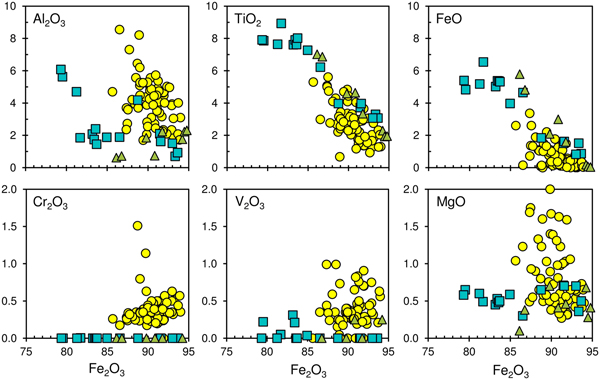
Hematite also varies notably in composition (in wt.%): TiO2 0.7–5.6; Al2O3 0.7–8.6; Cr2O3 0.2–1.5; V2O3 0.1–1.0; MgO 0.3–2.0; and up to 0.4 MnO, NiO and CaO. Compositional variations of hematite from the opaque segregation in comparison with those from the leucocratic zone and host rock are shown in Fig. 8. In general, hematite from the opaque segregation is richer in Al2O3, Cr2O3, V2O3 and MgO and poorer in TiO2 and FeO than the same mineral from other associations (Fig. 8). In addition to these peculiarities, hematite from the opaque segregation is inhomogeneous and sporadically contains oriented exsolution structures of corundum (Fig. 3, Fig. S5). Rare μm-sized inclusions of unidentified Ti–Fe- and Ca–V–Fe-oxide phases were also found in this hematite (Fig. S5). Within intergrowths the Ti–Fe phase (ilmenite ?) occurs both in hematite and at the boundary with other oxide minerals. The average EDS compositions for Ca–V–Fe oxide is (in wt.%, excitation of host hematite is possible): TiO2 3.19; Cr2O3 0.14; V2O3 20.39; Al2O3 4.45; Fe2O3 47.58; and MgO 0.61.

Fig. 8. Variations of Fe2O3 vs. major oxides (in wt.%) for hematite from the opaque segregation (circles), leucocratic zone (squares) and host plagioclase-clinopyroxene rock (triangles), Hatrurim Basin, Israel.
The compositions of the coexisting corundum and hematite within intergrowths for Hatrurim are given in Table 3 (WDS spot analyses, 1–2 μm) and Table S5 (EDS rastered analyses, >3 μm ×3 μm).
Table 3. Representative composition (WDS, wt.%) of coexisting corundum (Crn) and hematite (Hem) from opaque segregation in plagioclase-clinopyroxene rock, Hatrurim Basin, Israel.

n.a. = not analysed; b.d.l. = below detection limit; *Fe2O3 and FeO are calculated from charge balance; other data (EDS) are given in Table S4 (Supplementary material).
Discussion
Anorthite-clinopyroxene rock: hornfels or paralava?
As noted above the plagioclase-clinopyroxene rock investigated might be interpreted as paralava (the product of a local melting event), which formed after hornfels. This conclusion is supported by petrographic observations, in particular, by the high abundance of vesicles, which are now partly or completely filled with secondary minerals, such as calcite, zeolite-supergroup minerals, quartz and Ca-sulfates (Fig. S2-S3, Table S2). The classic term ‘hornfels’ refers to a dense fine-grained metamorphic rock, the product of solid-state transformations. The appearance of vesicles in the sample investigated is evidence of at least partial (or precursor) melting of parent plagioclase-clinopyroxene hornfels possibly near 1240°C (temperature of the anorthite–diopside eutectic) by the influence of superheated oxidised gases. An investigation of different plagioclase-clinopyroxene paralavas in the ‘olive’ unit at Hatrurim Basin indicated minimal melting temperatures as high as 1100°C (Vapnik et al., Reference Vapnik, Sharygin, Sokol, Shagam and Stracher2007), whereas the formation of the same hornfels (solid phase transformation) remains enigmatic in the temperature context. Such a two-stage model of sedimentary transformation (initial marly sediment → hornfel/clinker → paralava) was suggested for some Hatrurim plagioclase-clinopyroxene and melilite-rich paralavas (Sharygin et al., Reference Sharygin, Vapnik, Sokol, Kamenetsky, Shagam, Ni and Li2006; Vapnik et al., Reference Vapnik, Sharygin, Sokol, Shagam and Stracher2007; Sokol et al., Reference Sokol, Novikov, Zateeva, Sharygin and Vapnik2008; Reference Sokol, Novikov, Zateeva, Vapnik, Shagam and Kozmenko2010; Reference Sokol, Kozmenko, Kokh and Vapnik2012; Reference Sokol, Seryotkin, Kokh, Vapnik, Nigmatulina, Goryainov, Belogub and Sharygin2015; Seryotkin et al., Reference Seryotkin, Sokol and Kokh2012; Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Wlodyka, Dzierżanowski and Murashko2015c) and for melted combustion metamorphism rocks from natural coal fires and coal-mine burned dumps (Sokol et al., Reference Sokol, Maksimova, Nigmatulina, Sharygin and Kalugin2005; Grapes, Reference Grapes2011; Grapes et al., Reference Grapes, Korzhova, Sokol and Seryotkin2011 and references therein).
Genesis and temperature estimation for the opaque segregation with corundum
The origin of the corundum-bearing opaque segregation (as well as other opaque assemblages) in the Hatrurim Basin plagioclase-clinopyroxene rock remains enigmatic. In principle, they might result from high-temperature transformation of segregations with Al- and Fe-rich oxy/hydroxides (gibbsite, diaspore, böhmite and goethite) originally present in sediments. Note however that all opaque assemblages are localised inside or near large vesicles. In this respect, another possibility might be that hot oxidised gases provoked local melting of hornfels and subsequent recrystallization with Fe–Al-oxide components accumulating into, or near, gas vesicles (Sharygin et al., Reference Sharygin, Vapnik and Sokol2008b). The participation of hot gas jets in a melting event is supported by the presence of fluid inclusions in the Hatrurim corundum (Fig. 4). Possibly, the gas effect was a one-time event, which led to formation of both the paralava and Fe–Al-oxide opaque assemblages. Nevertheless, all the Fe–Al-oxide associations are quite different in mineralogy, possibly due to variable rate of temperature decrease (quenching) near individual vesicles. The maximum temperature for crystallisation of the Hatrurim assemblage with orthorhombic FeAlO3 may be estimated as T ≥1318°C (Sharygin and Sokol, Reference Sharygin and Sokol2017) according to experimental data for the system Al2O3–Fe2O3 (Muan and Gee, Reference Muan and Gee1956; Popović et al., Reference Popović, Ristić and Musić1995; Polli et al., Reference Polli, Lange and Levi1996; Majzlan et al., Reference Majzlan, Navrotsky and Evans2002). It should be noted that natural orthorhombic ε-Fe2O3 (luogufengite) was also noted in vesicles, in late Pleistocene basaltic scoria from the Menan Volcanic Complex, Idaho, USA (Xu et al., Reference Xu, Lee and Xu2017). This mineral is considered to be an oxidation product of Fe-bearing basaltic glass at high temperature and is associated with maghemite and hematite.
The Hatrurim corundum-bearing assemblage (Figs 1,2) apparently crystallised at a lower temperature. In general, the presence of hibonite, spinel, Fe-rich corundum and Al-rich hematite indicates high-temperature crystallization (>900–1000°С). In addition, the absence of orthorhombic FeAlO3 implies a temperature lower than 1318°C, as according to the Al2O3–Fe2O3 diagram the phase FeAlO3 should inevitably decay with gradual temperature decrease into Fe-rich corundum and Al-rich hematite (Muan and Gee, Reference Muan and Gee1956; Polli et al., Reference Polli, Lange and Levi1996; Majzlan et al., Reference Majzlan, Navrotsky and Evans2002; Feenstra et al., Reference Feenstra, Samann and Wunder2005). More exact constraints on the crystallization temperature of the corundum-bearing assemblage were obtained using the corundum–hematite pair. The limitations in application of this pair as a geothermometer were discussed by Feenstra et al. (Reference Feenstra, Samann and Wunder2005). Primarily, the available experimental data for the behaviour of hematite at high temperatures are controversial (Majzlan et al., Reference Majzlan, Navrotsky and Evans2002, Feenstra et al., Reference Feenstra, Samann and Wunder2005), possibly, because of different quenching conditions, though the data for corundum are more consistent. Moreover, hematite from the Hatrurim corundum-bearing assemblage obviously contains oriented exsolution structures of corundum (Fig. 3, Fig. S6) indicating further solid phase decay after the corundum-hematite co-crystallisation. Thus, the concentration of Fe2O3 in corundum in the case of the corundum-hematite coexistence could be used for a better temperature estimation. In general, the Fe-rich corundum + Al-rich hematite + spinel + hibonite assemblage in the plagioclase-clinopyroxene rock at Hatrurim Basin allows the temperature to be estimated as 1000–1200°C (Fig. 9).

Fig. 9. Compositions of Fe-rich corundum and Al-rich hematite from the opaque segregation in plagioclase-clinopyroxene rock (Hatrurim Basin) on the Al2O3–Fe2O3 diagram after Muan and Gee (Reference Muan and Gee1956), Popović et al. (Reference Popović, Ristić and Musić1995), Polli et al. (Reference Polli, Lange and Levi1996), Majzlan et al. (Reference Majzlan, Navrotsky and Evans2002) and Feenstra et al. (Reference Feenstra, Samann and Wunder2005). Dotted curve = data from Majzlan et al. (Reference Majzlan, Navrotsky and Evans2002); dashed curve = data from Feenstra et al. (Reference Feenstra, Samann and Wunder2005). Our data: circle is WDS spot analysis (1–2 μm); square is EDS rastered analysis (>3 × 3 μm). Initial data see Tables 3 and S5. Values for hematite were assumed as intermediate between two experimental lines.
It should be noted that all multi-stage transformations in the plagioclase-clinopyroxene rock investigated (initial siliceous marly sediment → hornfels → paralava → segregation of Fe–Al–Mg-oxides) are related to crystallisation at high oxygen fugacity (possibly higher or near the HM buffer). In the case of the corundum-bearing assemblage (as the culmination of the combustion metamorphic process) it is indicated by: (1) the presence of hematite; (2) high concentrations of Fe2O3 in corundum, hibonite and spinel; and (3) gradual increasing of the esseneite component in clinopyroxene.
Final remarks
The presence of abundant hematite incrustations around cavities in rocks is a common phenomenon for the Hatrurim Basin, especially for the ‘olive unit’ (Vapnik et al., Reference Vapnik, Sharygin, Sokol, Shagam and Stracher2007). However, its formation (at active participation of a gas phase) does not always suggest a very high-temperature process. Crystallisation of the specific hibonite-spinel-corundum-hematite association in the Hatrurim Basin plagioclase-clinopyroxene rock can be assumed at 1000–1200°C using the corundum–hematite pair (Fig. 9). Feenstra et al. (Reference Feenstra, Samann and Wunder2005) noted that “the corundum–hematite solvus has limited potential as a geothermometer because of the scarcity of suitable rock types for application and the restricted Fe3+–Al exchange in the solution at T ≤ 1000°C that introduces large inaccuracy in T”. Meanwhile, the Hatrurim Basin is an example of realisitic temperature evaluations for both the host rock and this specific mineral pair.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2018.138
Acknowledgements
The author thanks N.S. Karmanov, M.V. Khlestov, L.N. Pospelova and E.N. Nigmatulina for help with scanning microscope and microprobe studies at the V.S. Sobolev Institute of Geology and Mineralogy, Novosibirsk, Russia. E.V. Sokol (IGM, Novosibirsk, Russia) and Ye. Vapnik (Beer-Sheva, Israel) are thanked for helpful comments on an earlier version of the manuscript. The last version of the manuscript was improved through comments and suggestions by T. Perepelova, a translator of an Elsevier journal (IGM, Novosibirsk), L.A. Hartmann and an anonymous reviewer. This work was supported by the Russian Science Foundation (grant 17-17-01056).