Published online by Cambridge University Press: 13 May 2004
Among the variety of virulence factors of Entamoeba histolytica, an adherence lectin (Gal/GalNAc, 260 kDa) is known to mediate colonization and subsequent host responses. Gal/GalNAc lectin is universally recognized by the immune sera of patients with amoebic liver abscess. It plays a crucial role in cytolysis and phagocytosis of human and rat colonic mucin glycoproteins. The objective of the present study was to elucidate the role of antioxidants in E. histolytica Gal/GalNAc lectin-induced signals in the target epithelial cells. We have attempted to define a pathway in target cells, Henle-407 cells (human intestinal epithelial cell line), that could link this immunodominant antigen to a known biological pathway for target cell activation and triggering of subsequent disease pathology/parasite survival. Since several workers have demonstrated that cAMP and cGMP may act as important cellular signals for altering ion transport, so in the present study, cAMP and cGMP levels were measured in Henle-407 cells which showed significant increase at 15 min after stimulation. Elevated cAMP and cGMP levels are implicated in altered electrolyte transport and conductance. Results showed that there were increased levels of ROS and RNI which led to reduced activities of antioxidant enzymes – catalase, superoxide dismutase and glutathione peroxidase. Despite the increased glutathione (reduced) levels, the enzymes were not able to combat the damage caused by ROS and RNI. Thus, there was an increased local concentration of the free radicals and reduced activities of all the three enzymes which could damage the target cell in terms of cytoskeleton and permeability changes.
Entamoeba histolytica infection in humans is one of the leading causes of parasitic diseases among the developing countries, including India (Prakash et al. 2002). Most of the amoebic infections remain restricted to the colon and the majority of individuals (approximately 90%) with colonic amoebic infection are carriers (Walsh, 1986).
Adherence of the parasite to intestinal epithelial cells is a prerequisite for the pathogenesis of disease (Ravdin, 1986). While different adhesion molecules have been identified on the surface of E. histolytica, only 260 kDa Gal/GalNAc lectin is considered to play a crucial role in the interaction between the parasite and an epithelial cell (Carrero & Laclette, 1996). This Gal/GalNAc lectin is a novel multifunctional virulence factor and the native protein is a heterodimeric glycoprotein composed of 170 and 35 kDa subunits (Petri et al. 1989). Further, the role of this Gal/GalNAc lectin in cytolysis and phagocytosis of human and rat colonic mucin glycoproteins has also been established by Chadee et al. (1987).
Intracellular mediators proposed to regulate intestinal electrolyte transport directly are cAMP, cGMP and intracellular cytosol free Ca2+ (Fondacaro, 1986). These intracellular messengers are thought to activate protein kinases which phosphorylate membrane proteins, thereby altering transport carriers or conductance channel (Asaoka et al. 1992). Reactive oxygen species (ROS) have been implicated in a wide spectrum of human diseases including diseases of the gastrointestinal tract (Naya et al. 1997) as they play an important physiological function and can also cause extensive damage. The balance between physiological function and damage is determined by the formation of and the protection against reactive oxygen species (ROS). When, however, the rate of production of ROS exceeds the capacity of antioxidant defences, substantial tissue damage can occur. Generation of increased amounts of antioxidant enzymes (like SOD and catalase) help in combating the generation of ROS by spontaneous/catalytic dismutation (Yamamoto & Droffner, 1985). E. histolytica has been shown to recruit thiol-specific antioxidant (TSA) by Gal/GalNAc lectin for its own defence against reactive oxygen intermediates generated by the host cells (Hughes et al. 2003). Several studies have suggested that NO or NO-derived species are capable of causing oxidative injury (Nguyen et al. 1992) as they inactivate the antioxidant enzymes. 170 kDa subunit of Gal/GalNAc lectin has been reported to activate macrophages to produce TNF-α which enhances nitric oxide dependent cytotoxicity (Seguin et al. 1995).
We have designed experiments to measure cAMP and cGMP levels in Gal/GalNAc lectin induced epithelial cells and investigated the potential role of Gal/GalNAc lectin in induction of oxidative stress in the epithelial cells. Further, the activation/suppression of antioxidant defences, which are known to mediate intestinal damage in inflammatory disease of the bowel, were studied.
The axenic strain of E. histolytica trophozoites (HM-1-IMSS) was cultivated in TYI-S-33 medium. The trophozoites were solubilized and the supernatant fraction (10000 g, 10 min) was applied at 4 °C to an affinity column. The bound amoebic Gal/GalNAc lectin was eluted with 0·2 M acetic acid (pH 2·5) into test tubes containing 1·5 M Tris, pH 8·3 to neutralize the acid rapidly. The protein content of the Gal/GalNAc lectin was determined and the purity of the isolated Gal/GalNAc lectin was assessed by SDS–PAGE (10%, 10 μg/lane) along with the standard mixture of molecular weight markers (Sigma, Cat. no. 6539). The purity of Gal/GalNAc lectin was also confirmed by native PAGE (7·5%) (Davies, 1964). A haemagglutination assay with purified Gal/GalNAc lectin was performed, using heparinized human erythrocytes to confirm Gal/GalNAc lectin activity in the preparation (Petri & Schnaar, 1995).
Henle-407 (human intestinal epithelial cell line), obtained from the National Centre for Cell Sciences (NCCS), Pune (India), was grown in 25 cm2 plastic tissue-culture flasks (Costar Corporations, USA) in RPMI 1640 with the usual supplements (Freshney, 1987). After 48–72 h confluent cells were trypsinized and then suspended in RPMI-1640 without foetal calf serum (FCS). After 1 wash with the medium, the cell number was adjusted to 1×106 cells/ml and dispensed in multi-well tissue-culture plates (Nunc, Germany) for adherence (2 h). Subsequently, purified Gal/GalNAc lectin (10 μl, 20 μg/ml) was gently layered on to the monolayer of epithelial cells for specific time-intervals, after which the Gal/GalNAc lectin was gently washed off. The epithelial cells were then harvested (with slight tapping) and labelled as Gal/GalNAc lectin-stimulated cells. Simultaneously, unstimulated cells were taken as control.
Both stimulated and unstimulated cells were sonicated (30 sec bursts for 2 min) in an MSE (Measuring and Scientific Equipments, UK) ultrasonic disintegrator at 4 °C and 2 ml of absolute alcohol were added drop-wise. The cell suspension was kept at room temperature to precipitate protein and then centrifuged at 5000 g for 10 min. The supernatant obtained was dried with a gentle stream of nitrogen and stored at −70 °C. The sample was reconstituted with 700 μl of absolute alcohol (Merck Chemicals) and analysed for cAMP and cGMP by HPLC (Waters India Ltd, USA) (Harding, Carretero & Lapointe, 1995). Sodium salts of cAMP and cGMP (Sigma, USA) were run as standards on HPLC with a stock standard concentration of 10 mM and working standard concentration of 0·1–0·5 nM. Data were analysed using Millennium 32 software. The levels were expressed as nM per million cells.
The total free oxygen radicals released by the cells were measured by estimating the Luminol-dependent chemiluminescence response (LDCL) as described by Cheung, Archibald & Robinson (1984). Briefly, after stimulation, Luminol (B) and latex (C) were added to the cells. Background counts (A) were recorded for 1 min. Counts were recorded in a Micro β plus counter (1450 Wallace Inc, USA) according to the manufacturer's operation manual.
The Chemiluminescence Index (CI) was calculated as C−A/B−A.
Nitrite levels were measured by the method of Green et al. (1982). Griess reagent containing naphthylene diamine and sulfanilamide was added to the cells and purple azo dye complex formation was measured by recording OD at 546 nm. Results were expressed as nM of nitrite formed/million cells/ml.
For enzymatic assays, the cells were sonicated as above and centrifuged at 105000 g for 1 h. Enzymes (catalase, glutathione peroxidase (GPx), superoxide dismutase (SOD) and reduced glutathione levels were assayed in the cytosolic fraction of Henle-407 cells after determining the protein content.
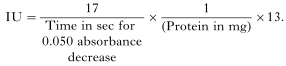
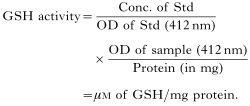
Results were expressed as mean±S.D. of at least 3 sets of experiments. Student's unpaired t-test was applied where applicable. A value of P<0·05 was considered significant.
Gal/GalNAc lectin was purified by galactose affinity column and SDS–PAGE analysis of the preparation (Fig. 1A) depicts a heterodimer of purified Gal/GalNAc lectin showing heavy and light subunits corresponding to 170 kDa and 35 kDa respectively. Isolated Gal/GalNAc lectin showed a single band on native PAGE confirming its purity (Fig. 1B). The haemagglutination assay was done to confirm the Gal/GalNAc lectin activity in the isolated and purified fraction which was indicated up to 1[ratio ]32 dilution of Gal/GalNAc lectin preparation (results not shown). The protein content of control and Gal/GalNAc lectin stimulated cell lysates (5, 15 and 25 min) was equalized for calculating enzymatic activity.

Fig. 1. (A) Lane-A shows SDS–PAGE (10%) of purified Gal/GalNAc lectin subunits of 170 kDa and 35 kDa and Lane-B shows standard mixture of wide range molecular weight markers. (B) Purified Gal/GalNAc lectin of 260 kDa showing a single band on silver staining.
After 5 min of Gal/GalNAc lectin stimulation, there was an increase in cAMP level corresponding to 0·784±0·02 nM which was highly significant in comparison to the control (P<0·001). At 25 min, cAMP levels decreased to 0·781±0·03 nM which was still significantly high. Maximum cAMP levels were observed at 15 min (P<0·001) (Table 1).

The level of cGMP was estimated at all 3 time-periods but was detectable only at 15 min of stimulation corresponding to 1·28±0·11 nM and it was highly significant (P<0·01) (Table 1) as compared to the control.
ROS production in Henle-407 cells was estimated by Luminol-dependent chemiluminescence (LDCL). There was a significant increase in ROS production upon stimulation with Gal/GalNAc lectin (P<0·05) as compared to the control. Maximum production of ROS was observed at 5 min of incubation with Gal/GalNAc lectin showing Chemiluminescence Index (CI) of 4·5±0·22. Thereafter, ROS levels decreased with increase in time of Gal/GalNAc lectin stimulation.
RNI production in Henle-407 cells in response to Gal/GalNAc lectin stimulation was studied by estimating nitrite levels. Significantly high nitrite levels were found at all time-periods in response to Gal/GalNAc lectin (P<0·05) as compared to the control. Maximum levels were observed at 5 min (3·1±0·08 nM/ml). Even though with increasing incubation with Gal/GalNAc lectin, there was a decrease in nitrite levels, they were still significant in comparison with the control (P<0·05) (Table 2).

Catalase activity was assayed in Henle-407 cells and the results showed that the activity was significantly increased (P<0·05) at 5 min of stimulation as compared to the control. This increase was later followed by a significant decrease in activity at 15 min and 25 min (P<0·05) as compared to the control and 5 min of stimulation with Gal/GalNAc lectin (Table 3).

Table 3 also shows the SOD activity of control and test samples stimulated with Gal/GalNAc lectin. There was a significant (P<0·05) decrease in the SOD activity of the cells stimulated with Gal/GalNAc lectin at 5 min (2·2±0·10 units) as compared to the control. This decrease was also significant at later time-intervals after stimulation (P<0·05).
GPx activity was estimated in Henle-407 cells as shown in Table 3. A significant (P<0·05) decrease in the activity of GPx in the cells stimulated with Gal/GalNAc lectin at 5 min (34·20±1·65 nM) was observed as compared to the control. This pattern of action in enzyme activity was followed at later time-intervals also and was found to be highly significant as compared to the control (P<0·05).
GPx levels were determined in the Henle-407 cells stimulated with Gal/GalNAc lectin. There was a significant increase (P<0·05) in reduced GSH levels in 5 min-stimulated cells (27·35±1·8 nM). This increase was significant up to 15 min (24·26±0·62 nM) of incubation with Gal/GalNAc lectin but later, at 25 min (17·63±3·49 nM), the reduced GSH levels decreased significantly (P<0·05) as compared to the control unstimulated cells (Table 3).
The invasion of the colon by Entameoba histolytica may lead to metastasis to vital organs of the body. The most common site of metastasis is the liver, resulting in the development of amoebic liver abscesses (Ravdin, 1995). The exact pathway by which the amoeba can kill the target cells is unknown, although one of the earlier studies had shown transfer of parasitic Gal/GalNAc lectin to the lateral surface of enterocytes which preceeds their killing (Leroy et al. 1995). Mammalian cells without N-terminal galactose or N-acetyl-galactosamine residues are resistant to adherence by trophozoites and killing by E. histolytica. Cytolysis is finally undertaken by amoebapores, a family of at least 3 small peptides which form pores in the lipid bi-layer (Leippe, 1997). During passage of amoeba trophozoites to liver, the 170 kDa subunit of Gal/GalNAc lectin protects trophozoites from complement-mediated lysis as it has homology with CD59 (Braga, Ninomiya & McCoy, 1992). The Gal/GalNAc lectin has a role in adherence and cytotoxicity through its cytoplasmic domain which contains a β2-integrin motif. Reduced virulence and decreased ability to form liver abscesses in a gerbil model was observed when the cytoplasmic domain of the Gal/GalNAc lectin was modified (Vines et al. 1998). This domain recruits thiol-specific antioxidant, in vivo, at the host–parasite interface and thus allows E. histolytica trophozoites to protect themselves from the oxidative attack from activated phagocytic and epithelial cells. Clearly, while some functions of this Gal/GalNAc lectin have become known, others remain obscure in the absence of availability of a suitable experimental model for the disease. Several past studies have employed cell lines like CaCO-2, HT-29, Henle-407 and even CHO cells (Chadee et al. 1987; Rigothier et al. 1991; Burchard, Prange & Mirelman, 1992) to study host–parasite interaction.
It has been reported that in many cellular responses, Ca2+ and cAMP act as synorchic messengers in regulating cell function (Rasmussen et al. 1992). We measured the cAMP levels upon stimulation with Gal/GalNAc lectin and results showed a significant increase in the level of cAMP. Several workers have demonstrated that cAMP may act as an important cellular signal for altering ion transport (Field, 1981). In context with the present study, increased cAMP levels in Gal/GalNAc lectin- stimulated cells might be mediated via calcium mobilization through intracellular stores (Rasmussen et al. 1992) and thus induce cytoskeleton and permeability changes. Increased cAMP is known to activate protein kinases which phosphorylate membrane transport carriers or conductance of electrolytes. Our results also showed increased cGMP levels, which is another second messenger known to regulate intestinal electrolyte transport. In mammalian intestinal cells, increase in cGMP levels causes changes in ion transport similar to the transport changes that result from increases in cAMP and Ca2+ (Rao, 1985).
Because gastrointestinal epithelial cells are likely to be exposed to free radicals generated in the intestinal mucosa and the lumen (Parks, 1989), in the present study, an attempt was first made to find out whether ROS and RNI sources are exacerbated in response to Gal/GalNAc lectin stimulation.
Both ROS and RNI levels were increased early upon Gal/GalNAc lectin stimulation. Increase in ROS may be due to increased generation of superoxide anions and the preferential formation of peroxynitrite which can be formed by a direct reaction of NO with the superoxide radical (Klebanoff, 1993). We observed a significant increase in LDCL response, suggesting increased ROS and NO generation by Henle-407 cells upon Gal/GalNAc lectin stimulation. An increased capacity to generate ROS has also been seen in the macrophages isolated from the patients with Crohn's disease (Kitahora et al. 1988). Further, the increased production of NO in the Gal/GalNAc lectin-stimulated cells was confirmed. Nitrites are the end-products of the oxidative metabolism of the labile NO and their quantification is regarded as an indicator of NO generation (Stuehr & Marletta, 1985). An increased nitric oxide production by the activated macrophages during an inflammatory response has been seen by Carreras et al. (1994).
The simultaneous production of ROS and NO, observed in the present study, indicates generation of these two mediators in the same cell at the same time. This might be due to the presence of cells at different stages of activation. Reactions between ROS and NO are, therefore, possible and will result in the generation of free radicals like peroxynitrite anion, which has powerful cytotoxic properties. Peroxynitrite anion induces tissue injury through mechanisms entailing lipid peroxidation and sulfhydryl oxidation (Beckman et al. 1990). The decomposition products of peroxynitrite OH− and NO2− can also cause tissue injury and further can contribute to damage (Radomski et al. 1992).
Since the colonic mucosa is subjected to significant oxidant stress during times of acute and chronic inflammation, knowledge of oxidant defence mechanisms in the colon is of biological and potential clinical importance. Therefore, our objective was to quantify the specific activities of antioxidant enzymes including catalase, SOD, GPx and reduced glutathione levels in Gal/GalNAc lectin-stimulated and unstimulated Henle-407 cells. The gastrointestinal tract is a rich source of antioxidant enzymes, GSH and α-tocopherol, all of which detoxify the ROS. However, when the rate of production of ROS exceeds the capacity of the antioxidant defences, substantial tissue damage can occur.
In the last phase of the study, the changes in the antioxidant levels in Henle-407 cells on stimulation with Gal/GalNAc lectin were estimated. There was a significant decrease in the activity of all the antioxidant enzymes i.e. SOD, GPx and catalase in the Henle-407 cells as compared to the control. Probably the decrease in the level of antioxidant defences sensitized to an increased flux of ROS and RNI led to membrane damage and permeability changes.
The enzyme catalase converts H2O2 to H2O. The catalase-mediated epithelial cell protection has been seen in a number of studies (Baker & Campbell, 1991). In the present study, there was an initial increase in catalase levels but, later, there was a significant decrease. The decrease in catalase activity might be correlated with an increase in the level of lipid peroxidation. NO has been shown to inhibit catalase at low concentrations (Brown, 1995). The significant decrease in the catalase activity might be due to its inhibition by NO.
SOD functions in the dismutation of the O2− to H2O2. The relevance of SOD-mediated tissue protection against injury related to ROS has been shown in an experimental colitis model, where inflammation was appreciably reduced by the treatment with SOD. The significant decrease in the SOD activity we observed might be caused by increased inactivation through a local concentration of the superoxide radical or by reduced local concentration of the enzymes. A similar decrease in the SOD activity of intestinal mucosa has been reported during the ischaemia reperfusion injury (Grisham, Ryan & von Ritler, 1987) and inflammatory bowel disease (Mulder et al. 1991). This decrease in the SOD activity might result in the hampered dismutation of the superoxide anion.
Reduced glutathione is a specific substrate for GPx (Stadman, 1980). Although in the present study, there was an increase in reduced GSH levels, but there was a significant decrease in GPx activity which can be due to its inactivation by NO. With a significant decrease in the activities of GPx and catalase, H2O2 is not efficiently catabolized. It would then accumulate to a point where its reaction with iron initiates the peroxidation of lipids faster or to a greater extent than can be controlled by the cells. Although, there was an increase in GSH levels in response to Gal/GalNAc lectin stimulation as compared to control, cells showed decreased GPx levels which indicate that cells were not able to scavenge the oxygen radicals such that high concentration of free radicals reduced the concentration of enzyme. Thus, the increased GSH levels were not able to combat the damage (Iantomasi et al. 1997).
Taken together, Gal/GalNAc lectin induced a significant increase in ROS and RNI and a significant decrease in the level of antioxidant enzymes. Probably, this decrease in the level of antioxidant defence enzymes sensitized the cells to an increased flux of ROS and RNI which led to an increase in the extent of membrane damage and loss of cell adherence in the presence of Gal/GalNAc lectin.
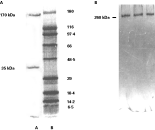
Fig. 1. (A) Lane-A shows SDS–PAGE (10%) of purified Gal/GalNAc lectin subunits of 170 kDa and 35 kDa and Lane-B shows standard mixture of wide range molecular weight markers. (B) Purified Gal/GalNAc lectin of 260 kDa showing a single band on silver staining.

Table 1. Levels of cAMP and cGMP in epithelial cells (nM/106 cells)

Table 2. ROS and RNI production in Henle-407 cells

Table 3. Estimation of enzymatic activity