Introduction
Geophysical data (i.e. seismic and multibeam) collected on the Antarctic continental shelves show that large-scale glacial troughs were eroded by ice streams when grounded ice advanced to the outer shelf during the Last Glacial Maximum (LGM) (e.g. Jakobsson et al. Reference Jakobsson, Anderson, Nitsche, Dowdeswell, Gyllencreutz, Kirchner, Mohammad, O’Regan, Alley, Anandakrishnan, Eriksson, Kirshner, Fernandez, Stolldorf, Minzoni and Majewski2011, O’Brien et al. Reference O’Brien, Smith, Stark, Johnston, Riddle and Franklin2015). Since the LGM, grounded ice has retreated to the inner continental shelf. During the retreat, some palaeo-ice-stream troughs were partially filled with a backstepping succession of grounding zone wedges (GZWs), i.e. subaqueous terminal moraines (e.g. Graham et al. Reference Graham, Larter, Gohl, Dowdeswell, Hillenbrand, Smith, Evans, Kuhn and Deen2010). The GZWs are of interest because they provide unequivocal evidence showing the locations at which the retreat of grounded ice was temporarily interrupted by a pause and slight re-advance.
Piston cores collected on the outer continental shelf always show that ice sheet retreat is recorded by an overall upcore lithologic transition from diamict that is tens of metres thick to a condensed section of diatom ooze (e.g. Domack et al. Reference Domack, Jacobsen, Shipp and Anderson1999). The diamict was deposited by or in proximity to grounded ice whereas ooze has accumulated in an ice-distal open-marine setting since ice shelf retreat. The challenge has been to find in situ material to radiocarbon date the retreat history with all its discrete pauses. The task is complicated because ice-contact and ice-proximal sediments contain much recycled material.
During recent years, denser seismic grids and large-area multibeam surveys (e.g. Graham et al. Reference Graham, Larter, Gohl, Dowdeswell, Hillenbrand, Smith, Evans, Kuhn and Deen2010) reveal that the 3D morphology of GZWs includes eroded topset morphology, which is a surface over which grounded ice streamed. The eroded sediment is transported, in this case northward, to a depositional foreset surface, formed beyond the limit of grounded ice. The GZW foreset received sediment delivered by ice streaming over the topset. The topset and foreset zones are distinct and are clearly separated by an ice sheet grounding line. Megascale glacial lineations (MSGLs, i.e. subglacial bedforms) are not found on the GZW foreset surface. Furthermore, given that the GZW foreset probably was a site of rapid sedimentation, long cores through the foreset lessen the possibility that benthic organisms have created a post-depositional bioturbation disturbance possibly reworking younger post-glacial material downward into the underlying marine diamict.
In a recent study of a GZW on the middle continental shelf of the Glomar Challenger Basin palaeo-ice stream trough in eastern Ross Sea, Bart & Cone (Reference Bart and Cone2012) emphasized the existence of a GZW topset with MSGLs that can be distinguished from a GZW foreset surface. The foreset has tens of metres of relief and is a few kilometres wide (Fig. 1). The existence of an undisturbed GZW foreset opens the possibility that in situ foraminifera and other life assemblages inhabited this setting and that the stratigraphic record has not experienced post-depositional disturbance. Video images acquired by the Whillans Ice Stream Subglacial Access Research Drilling (WISSARD) project show that an ecosystem, including fish and invertebrates, exists at the modern marine termination of a GZW. This discovery of surprisingly rich life at this site is remarkable because this unique setting is below a 1000 metre thick floating ice shelf, and is more than 500 km from the nearest open-water environment. The existence of fauna in this environment is of special interest to palaeoclimatologists who use the backstepping succession of ice-proximal deposits and submarine landforms on the Antarctic continental shelf to reconstruct the ice sheet’s post-glacial retreat (e.g. Post et al. Reference Post, Galton-Fenzi, Riddle, Herraiz-Borreguero, O’Brien, Hemer, McMinn, Rasch and Craven2014). An in situ fossil assemblage in ice-proximal marine sediments could be used to i) reconstruct the changing environmental conditions at the grounding line environment (e.g. Mackensen Reference Mackensen2012), and ii) radiocarbon date the various positions occupied by grounded ice during the post-glacial retreat of ice from the continental shelf (e.g. Bart & Cone Reference Bart and Cone2012).

Fig. 1a Global Challenger Basin (GCB), eastern Ross Sea, Antarctica. Dark yellow indicates the drainage basin for the palaeo Whillans Ice Stream when the West Antarctic Ice Sheet was grounded at the middle continental shelf of GCB. Light yellow indicates the modern drainage, where the WISSARD project sampled the grounding zone wedge (GZW) environment. b. Multibeam bathymetry of the middle continental shelf showing core locations on the GZW foreset. c. Cross section of the GZW at PC12 and PC5 in grey. White dashed lines show the inferred time-transgressive GZW progradation. M=McMurdo Sound, MSGLs=megascale glacial lineations, WA=West Antarctic.
Here, we show that ice-proximal diamict sediment deposited on the foreset of a GZW in the Glomar Challenger Basin palaeo-ice-stream trough of the eastern Ross Sea contains 18 species of well-preserved extant benthic foraminifera. In combination with the ecosystem discovered by the WISSARD team, our results open the possibility that an in situ ice-proximal sub-ice shelf assemblage lived on the GZW foreset prior to decoupling retreat of the West Antarctic Ice Sheet (WAIS) and is preserved in marine diamict deposited on the middle continental shelf.
Methods
Our study focused on isolating foraminifera from ice-proximal marine diamict that was deposited at a GZW when the WAIS was grounded on the middle continental shelf within the axis of the Glomar Challenger Basin palaeo-ice-stream trough in eastern Ross Sea. Two cores collected during an RVIB Nathaniel B. Palmer 2008 cruise (NBP0802) were selected based on the morphology of the GZW as interpreted from a seismic grid and large-area multibeam survey of the trough. Both cores penetrated olive-green diatom-rich ooze overlying homogenous grey diamict. PC12 was selected because it penetrates the GZW foreset at a location corresponding to a zone where the last interval of marine diamict deposition occurred prior to the onset of lift-off and retreat of grounded ice (Fig. 1b). PC5 penetrated GZW diamict at an interior location on the eroded scarp of the GZW topset (Fig. 1c).
Fifty-five 15 ml samples of marine diamict and two 15 ml core-top samples of post-glacial diatom ooze were processed individually following the techniques described by Bart & Cone (Reference Bart and Cone2012), i.e. before picking, foraminifera were concentrated by floating air-dried>45 μm residue. This procedure removed potentially reworked specimens with sediment filled tests. Distinction between in situ and reworked foraminifera was made on the basis of preservation state (e.g. Majewski & Anderson Reference Majewski and Anderson2009). Foraminifera tests (i.e. the hard shells) that appeared to be whole and devoid of physical/chemical damage in visible light were picked and analysed by scanning electron microscope (SEM) to confirm whether or not the tests were indeed damage free. The SEM images were also used for taxonomic identification. The core-top samples of open-marine diatom ooze sediment from PC12 and PC5 were processed for comparison with the foraminiferal assemblages isolated from glacial-age diamict. Foraminiferal abundances from the marine diamict and ooze sediment were calculated.
Results
Foraminiferal preservation state
A total of 302 whole foraminifera were picked from 55 samples of diamict from PC12 and PC5. In visible light, 98% of tests (296 specimens) are white and translucent to opaque, whereas the remaining 2% are discoloured yellow-brown. All tests are small and thin, i.e. there are no heavily calcified or sediment filled tests. Among the 40 tests selected for SEM analysis, 32 (80%) show no surface damage (Fig. 2). A detailed visual examination of the SEM images showed that the majority of tests possess well-defined pores and sharp edges. Two tests show signs of minor chemical dissolution such as etching and/or slightly enlarged pores (e.g. Fig. 2a). Five show physical damage, which appears as fractures or holes on the test walls (e.g. Fig. 2l & m) and one (a planktonic) shows an eroded surface (Fig. 2t). Aside from the damage to the planktonic foraminifera, no significant differences in the degree of physical or chemical damage of the tests were observed between the two core sites.

Fig. 2 Foraminifera recovered from NBP0802-PC12 and NBP0803-PC5 cores. Scale bar indicates 80 μm. a. Astrononion echolsi Kennett, b. Bulimina aculeata d’Orbigny, c. Cassidulina teretis Tappan, d. Cibicides sp., e. Ehrenbergina glabra Heron-Allen & Earland, f. Epistominella spp., g. Fissurina sp., h. Stainforthia concava (Höglund), i. Globocassidulina subglobosa (Brady), j. Homalohedra acuticosta (Reuss), k. Lagena sp., l. Melonis sp. (broken), m. unidentified Miliolid, n. Nonionella sp., o. Oolina spp., p. Oolina spp., q. Trifarina earlandi (Parr), r. Textularia sp. (agglutinated), s. Trochammina sp. (agglutinated), t. Neogloboquadrina pachyderma (Ehrenberg) (planktonic).
Assemblage diversity: the grounding zone wedge marine diamict
We isolated 302 foraminifera, averaging less than six minute specimens per 15 ml sample (i.e. 0.3 specimens cm-3). No extinct taxa were found in the processed picked samples. Nineteen taxa (18 benthics and the planktonic Neogloboquadrina pachyderma (Ehrenberg)) were identified from the diamict samples from PC12 and PC5 (Fig. 3). The assemblage is dominated by Globocassidulina subglobosa (Brady) and Epistominella spp. All other species generally constitute<5% of the assemblages. We found only two agglutinated genera, Textularia and Trochammina, in our samples at 2.3% and 8.8% in PC12 and PC5, respectively (e.g. Fig. 2r & s).

Fig. 3 Distribution of foraminiferal species abundances (i.e. number of specimens) in a. NBP0802-PC12, and b. NBP0803-PC5 on the middle shelf grounding zone wedge diamict of Global Challenger Basin, eastern Ross Sea. Names highlighted are those of agglutinated species.
Assemblage diversity: the interglacial-age ooze
Foraminiferal abundance was 20 times higher in the core-top diatom ooze samples with an average of 120 tests per 15 ml sample. The assemblage of the core-top ooze samples includes mainly agglutinated forms (Table I). Among the nine genera found in the surface samples, Trochammina is the most common (> 70% in both PC5 and PC12) and is associated mainly with species of Pseudobolivina, Miliolinella and Miliammina. Six of the nine genera are the same in the two core-top diatom ooze samples (Table I). Four of the nine genera in the core-top sample appear in the GZW diamict assemblage.
Table I Distribution of foraminiferal species in NBP0802-PC12 and NBP0803-PC5 from core-top open-marine ooze overlying the middle shelf grounding zone wedge of Global Challenger Basin, eastern Ross Sea.

* Agglutinated species.
Stable isotopic data
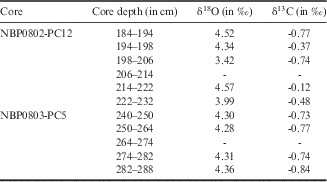
Small size and low abundances of foraminifera precluded obtaining geochemical data for individual specimens. For this reason, isotopic results were obtained on bulk foraminiferal samples. The low abundance of calcareous species in the core-top ooze precluded obtaining any stable isotopic data on core-top foraminifera. Results of the oxygen and carbon isotope analyses are given in Table II. The δ18O values were highly positive ranging from 3.42‰ to 4.57‰, with mean δ18O values of 4.17±0.47‰ and 4.31±0.04‰ for PC12 and PC5, respectively. Values of δ13C measured on foraminiferal tests range from -0.84‰ to -0.12‰.
Table II δ13C and δ18O data from benthic foraminifera.
Discussion
Preservation of minute benthic foraminifera in grounding zone wedge diamict
Discolouration and other preservation issues may be the way to distinguish between in situ and reworked foraminiferal tests. Foraminiferal biostratigraphical analyses of Cape Roberts Project core data (e.g. Webb & Strong Reference Webb and Strong2006) show that a low diversity and low abundance assemblage similar to that found in our piston core diamict samples has existed, more or less continuously in the Ross Sea region since at least the early Oligocene. In other words, the diamict assemblage that was isolated in piston cores might be recycled from pre-LGM or even much older strata.
A good example of reworked calcareous benthic foraminifera from Antarctic Holocene sediment was illustrated in Majewski & Anderson (Reference Majewski and Anderson2009). They showed three populations of thickly testate Globocassidulina biora (Crespin) from a single sample. Some of the worst preserved specimens were clearly compacted with poorly preserved walls and no signs of pores. The less altered were light-to-dark orange in colour with a rough surface and partly or completely obscured pores, and finally the best preserved were white specimens with smooth walls and well-preserved pores. This example shows what kind of alterations could be expected for reworked foraminiferal tests.
The SEM images confirm that the majority (80%) of foraminiferal tests that were isolated from diamict are devoid of significant damage (Fig. 2). A majority of our specimens from GZW diamict show smooth test walls with well-preserved pores. They are all small and hollow, with thin walls that would seemingly make them susceptible to breakage during compaction or transport. When damage is present, it is mostly found on either the last chamber or along test margins. The slight physical breakage observed on some foraminifera may be due to in situ predation or sample processing.
Highly eroded and discoloured surfaces are observed on only the three planktonic foraminifera (e.g. Fig. 2t), which suggests that those three specimens may be reworked from older sediment. Conversely, these specimens may have been exposed to a shallow Carbon Compensation Depth (CCD). All other tests are benthic foraminifera and these exhibit at most only minor effects of dissolution manifested as microscopic surface etching or slightly enlarged pores (Fig. 2). This relatively slight chemical damage might have been due to less prolonged exposure to corrosive waters in the Ross Sea where the average depth of the CCD is c. 550 m (Osterman & Kellogg Reference Osterman and Kellogg1979).
The assemblage also includes a few agglutinated forms. Most of them tend to disintegrate after death because of the fragile nature of their tests (Corliss Reference Corliss1985). Such paucity of agglutinated forms in sediments older than a few thousands of years is not uncommon (e.g. Majewski & Anderson Reference Majewski and Anderson2009) and it may obscure reconstructions of fossil assemblages. In this case, it seems to provide no arguments for either a reworked or in situ character of the GZW assemblage, as the original ratio of calcareous forms is unknown.
In summary, the overall excellent preservation state of the foraminifera is consistent with an assemblage that is in situ. However, this physical criterion cannot be considered definitive proof of the in situ assemblage because it cannot be proved that recycled foraminifera cannot survive physical and chemical damage intact.
Difference between grounding zone wedge diamict and open-marine benthic foraminiferal assemblages
We assumed that the modern ooze assemblage can be taken as an approximation to the foraminiferal assemblage that inhabited the open-marine assemblage during pre-LGM interglacials. In this line of reasoning, if the foraminifera isolated from GZW diamict were reworked from an ooze deposited during a previous interglacial, then the diamict assemblage should be composed of a subset of the ooze assemblage.
The high abundance of foraminifera in the core-top oozes analysed is due at least in part to the higher productivity and extremely low influx of terrigenous sediment to the open water continental shelf. The dominance of agglutinated foraminifera in our core-top assemblage (Table I) is typical for the modern eastern Ross Sea open-marine conditions (McKnight Reference McKnight1962, Pflum Reference Pflum1966, Osterman & Kellogg Reference Osterman and Kellogg1979). If this modern open-marine assemblage is a potential proxy for pre-LGM open-marine assemblages in the eastern Ross Sea, then it can be expected that the reworked fraction would be dominated by the most abundant robust tests from the modern-like ooze that, following Schröder (Reference Schröder1988), should be the agglutinated Trochammina. Its occurrence in the diamict permits the possibility that it was reworked from pre-LGM interglacial ooze, but alternately, its very low abundance may indicate the opposite, i.e. that its occurrences are primarily in situ and it belongs to a very different assemblage dominated by calcareous forms.
The diamict assemblage is dominated by two calcareous taxa, i.e. G. subglobosa and Epistominella spp. (Fig. 3), both of which are also known from the Ross Sea (Osterman & Kellogg Reference Osterman and Kellogg1979, Ward et al. Reference Ward, Barrett and Vella1987, Melis & Salvi Reference Melis and Salvi2009) and other modern Antarctic continental shelf areas (Pudsey et al. Reference Pudsey, Murray, Appleby and Evans2006, Majewski Reference Majewski2013). Although neither of these species is found in the modern diatom ooze from our two core-top samples (Table I), it cannot be ruled out that pre-LGM open-marine conditions in the eastern Ross Sea were different than Recent, facilitating much different foraminiferal assemblages.
The environmental affinities of the well-preserved benthic foraminifera
The in situ assemblage should include only those species that could tolerate the expected conditions for an ice-proximal environment. Benthic foraminiferal assemblages are controlled by many factors including food availability and its seasonal delivery, sediment input, substrate type, water mass properties and bathymetry, temperature, salinity and oxygen content (e.g. Osterman & Kellogg Reference Osterman and Kellogg1979, Majewski Reference Majewski2005, Melis & Salvi Reference Melis and Salvi2009, Gooday et al. Reference Gooday, Rothe, Bowser and Pawlowski2014). The dominant foraminifera in the diamict assemblage (G. subglobosa, Epistominella spp. and Bulimina aculeata d’Orbigny) are typical of cold-water benthic assemblages (Nelson et al. Reference Nelson, Cook, Hendy and Cuthbertson1993, Polyak & Solheim Reference Polyak and Solheim1994, Ishman & Foley Reference Ishman and Foley1996). Figure 4 summarizes the ranges for three environmental parameters for the benthic species that were isolated in the diamict based on data from McKnight (Reference McKnight1962), Pflum (Reference Pflum1966), Ward et al. (Reference Ward, Barrett and Vella1987), Mackensen & Hald (Reference Mackensen and Hald1988), Asioli (Reference Asioli1995), Harloff & Mackensen (Reference Harloff and Mackensen1997), Majewski (Reference Majewski2005) and other summary data (Encyclopedia of Life 2009, http://www.eol.org). The grey-shaded area shows that there is a common range of environmental conditions that all species in the diamict share (Fig. 4). This overlap is a minimum requirement for the GZW assemblage to be considered in situ.

Fig. 4 Environmental tolerances for foraminiferal species found in PC12 and PC5 of the grounding zone wedge diamict. Grey shading indicates a common range of environmental conditions that all species in the diamict share.
Reports on foraminiferal communities from under ice sheets around Antarctica are not uncommon. The best studied are fossil assemblages from the area of the Larsen Ice Shelf collapse aimed at developing foraminiferal proxies for tracking past ice shelf retreats (Murray & Pudsey Reference Murray and Pudsey2004). Abundant planktonic foraminifera were described from beneath Amery Ice Shelf reflecting sub-ice shelf circulation (Hemer et al. Reference Hemer, Post, O’Brien, Craven, Truswell, Roberts and Harris2007). Foraminifera were also encountered from below the Ross Ice Shelf. Lipps et al. (Reference Lipps, Ronan and Delaca1979) reported empty foraminiferal tests from under 420 m of ice as far south as 430 km from open sea. More recently, Pawlowski et al. (Reference Pawlowski, Fahrni, Guiard, Conlan, Hardecker, Habura and Bowser2005) described allogromiid foraminifera and gromiids along with empty tests from the ANDRILL site HWD-2, 12 km east of the ice edge. They interpret the presence of the rather diverse living protist community as the result of advected phytodetritus that was apparently lacking at the site investigated by Lipps et al. (Reference Lipps, Ronan and Delaca1979).
The question to be answered is whether our GZW diamict assemblage is similar to what could be hypothetically anticipated in habitats located many tens of kilometres from open ocean as its potential food source. Korsun et al. (Reference Korsun, Pogodina, Forman and Lubinski1995) showed that ice-proximal Arctic fjord settings lack large tests but have abundant juveniles, which they argued is an opportunistic response to environmental stress of glacier proximity. These glacier-proximal settings were also dominated by abundant monothalamous allogromiid species that lack calcified tests (Korsun & Hald Reference Korsun and Hald2000, Sabbatini et al. Reference Sabbatini, Morigi, Negri and Gooday2007). The allogromiid foraminifera are also prominent in marginal settings of the South Shetland Islands (Majewski et al. Reference Majewski, Lecroq, Sinniger and Pawlowski2007) and in McMurdo Sound (Gooday et al. Reference Gooday, Bowser and Bernhard1996), and were reported from below the Ross Ice Shelf (Pawlowski et al. Reference Pawlowski, Fahrni, Guiard, Conlan, Hardecker, Habura and Bowser2005). Because they do not possess robust tests, they are not present in the fossil record and are also commonly overlooked in studies of Recent assemblages, if sediment samples are washed and dried prior to picking. The minute size and scarcity of the predominantly calcareous benthic foraminifera found in GZW diamict seems to coincide with an assemblage originally dominated by allogromiid foraminifera with only a minor share of calcareous forms. Their small size and thin walls suggest that this could have been an element of an opportunistic assemblage strongly affected by scarce and/or seasonal delivery of phytodetritus to the sea floor (Gooday Reference Gooday1993).
It is true, however, that similar suites of minute calcareous benthic foraminifera were commonly described also from open-sea environments where food is limited and/or strongly seasonal, for example, the Epistominella trough assemblage found off the Larsen A Ice Shelf (Ishman & Szymcek Reference Ishman and Szymcek2003) or the Epistominella spp. dominated assemblage from central Pine Island Bay (Majewski Reference Majewski2013), where persistent sea ice often hinders primary production. A widespread assemblage dominated by G. subglobosa was also described from surface sediments of the western Ross Sea (Assemblage 3 from Osterman & Kellogg Reference Osterman and Kellogg1979). Thus, one could argue that the major components of our GZW assemblage are actually more characteristic of open-water conditions.
On the other hand, marginal, especially sub- or near-glacier environments, are definitely under-investigated. There are simply hardly any modern analogue data. Sub-ice shelf facies with abundant calcareous foraminifera were reported from Marguerite Trough (Kilfeather et al. Reference Kilfeather, Cofaigh, Lloyd, Dowdeswell, Xu and Moreton2011). The most glacier-proximal assemblage of the Holocene record from Firth of Tay (Majewski & Anderson Reference Majewski and Anderson2009) corresponds in terms of species composition and minute size of tests with the GZW assemblage. Sediments interpreted to represent sub-ice shelf environments from Pine Island Bay contain benthic foraminifera that represent roughly similar assemblages dominated by sparse and minute species (unpublished data).
Following these arguments, species composition of the GZW fossil assemblage seems to be coherent and to represent opportunistic, food-limited communities. This assemblage seems to correspond well with conditions of scarce phytodetritus only occasionally delivered by subglacial currents, but on the other hand could also be associated with an open-sea environment with primary production restricted by persistent sea ice and/or oligotrophic waters.
Evaluation of stable isotopic data
Both δ18O and δ13C values may differ significantly between different benthic foraminiferal species from a single site (e.g. Majewski et al. Reference Majewski, Wellner, Szczuciński and Anderson2012). Because, due to the minute size of the specimens, our measurements were performed on bulk samples grouping together specimens belonging to different species, it is difficult to unequivocally evaluate these data. At face value, the oxygen and carbon stable isotopic values are reasonable and consistent between different samples (Table II). They do not have values that would suggest possible diverse provenances of analysed foraminifera, in turn supporting the hypothesis that at least a majority of analysed specimens are of similar origin.
Implications for dating of ice sheet grounding events
Throughout the earlier discussion, we showed that diamict deposited on a GZW foreset contains a low abundance of minute, well-preserved, extant foraminifera. Our analyses of the preservation states, assemblage compositions, as well as δ18O and δ13C values, failed to provide conclusive evidence that the benthic foraminifera are in situ. Nonetheless, nothing in our data requires that the in situ assumption be abandoned as a working hypothesis. This finding may have a pronounced significance for radiocarbon dating deglaciation events after the LGM.
So far, two basic strategies have been used to directly date the marine record of Antarctic ice sheet retreat. The most widely used approach is to constrain the time corresponding to the resumption of marine sedimentation that followed ice sheet retreat. In this approach, dateable materials, either foraminifera and/or acid insoluble organic matter (AIOM), are isolated from the basal-most section of post-glacial diatom ooze that overlies the glacial-age diamict. This approach has its weaknesses, however. It assumes that ooze sedimentation began immediately after ice sheet retreat and has since been continuous and undisturbed. If this assumption is not correct, it may lead to significant underestimating of deglaciation ages.
The second strategy is to date the grounding event itself. This relies on isolating dateable material from glacial-age diamict. Licht & Andrews (Reference Licht and Andrews2002) reasoned that the advance of grounded ice eroded and recycled proglacial sediment that was originally deposited on the previously open-water continental shelf. Given that proglacial sediments (and the dateable material it contains) become recycled and incorporated into the diamict, the youngest radiocarbon dates of either AIOM or foraminifera in diamict provide a maximum age of the grounding event.
In a departure from the strategy employed by Licht & Andrews (Reference Licht and Andrews2002), we suggest that well-preserved foraminifera from the marine diamict deposited on the middle continental shelf GZW foreset are in situ. This assumption has already been employed by Bart & Cone (Reference Bart and Cone2012). Their radiocarbon dates from two widely separated core sites are tightly clustered and stratigraphically ordered, providing support for the in situ nature of the well-preserved foraminiferal assemblages. Still, those dates could also be considered suspect because i) many specimens had to be combined to produce a single radiocarbon date, and ii) the dates suggest that the WAIS retreated significantly earlier than is deduced from other marine and terrestrial locations.
Our present study does not show unequivocally that the in situ assumption of Bart & Cone (Reference Bart and Cone2012) is correct, but nothing in the nature of the well-preserved minute calcareous foraminifera assemblages found in GZWs suggests that they are reworked. The possibility that the best preserved fraction is in situ is important because radiocarbon dates of carefully isolated in situ foraminifera can be used to date the timing and durations of grounding events in the many places where GZWs are found on the Antarctic continental shelves (e.g. Graham et al. Reference Graham, Larter, Gohl, Dowdeswell, Hillenbrand, Smith, Evans, Kuhn and Deen2010) more precisely than by dating post-glacial diatom ooze. This new approach can significantly improve our knowledge of Antarctic deglaciation history and environmental conditions existing where grounded ice streams meet the marine settings, providing better data for climatic modelling.
Conclusions
To our knowledge, foraminifera have not yet been observed from samples and observations made by the WISSARD team, thus this is the first analysis of GZW foreset ecology. As for our analyses of the GZW preserved on the eastern Ross Sea middle continental shelf, we propose that further work is needed before the in situ hypothesis is confirmed because the data do not convincingly require that the foraminifera that were isolated from the diamict are in situ. Conversely, nothing in our new data requires that the in situ assumption be abandoned as a working hypothesis.
Acknowledgements
The research was supported by NSF OPP grant #1246357 to Bart. Thanks are extended to the ECO Captain, ship crew and marine/electronic support crews of the RVIB Nathaniel B. Palmer for assistance acquiring core and geophysical data during difficult field conditions during the NBP0802 and 0803 cruises to Glomar Challenger Basin. Thanks are also extended to the curatorial staff of the Antarctic Marine Geology Research Facilities in Tallahassee for providing the core samples requested from the archive. We thank Patrick Quilty and the journal editor for suggestions that improved the manuscript.
Author contribution
Coquereau sampled, processed and analysed the samples for foraminifera under the supervision of Bart and Warny. Foraminifera identifications were confirmed by Majewski and Barun Sen Gupta. All authors participated in the data interpretation and writing of the manuscript.









