INTRODUCTION
The asexual multiplication of protozoa of the genus Plasmodium in human erythrocytes causes malaria (Fig. 1). This is still the most prevalent parasitic disease on earth with ca. 225 million cases and 0·8 million deaths annually (WHO, 2010b). The number of deaths, mostly children under 5 years infected by Plasmodium falciparum in sub-Saharan Africa, has steadily declined in many endemic countries since the introduction of artemisinin combination therapies (ACT) as first line treatment for uncomplicated malaria (WHO, 2010b; O'Brien et al. Reference O'Brien, Henrich, Passi and Fidock2011). However, the emergence of resistance against artemisinins in South East Asia has raised general concern because of the lack of effective alternative treatments in case of spread to other geographical areas (WHO, 2010a; O'Brien et al. Reference O'Brien, Henrich, Passi and Fidock2011).

Fig. 1. Plasmodium spp. cycle. (A) Anopheles spp. female mosquitoes inoculate sporozoites, present in their salivary glands, in the host. Upon invasion of hepatocytes, sporozoites differentiate and replicate to produce liver schizonts. These cells contain specialized cells (merozoites) capable of infecting erythrocytes once released into bloodstream. The liver infection is asymptomatic. In a few species, including P. vivax and P. ovale, a fraction of parasites enters a quiescent stage (hypnozoite). Reactivated hypnozoites are thought to be the cause of the characteristic cyclic vivax and ovale malaria relapses that happen months after infection; (B) Released liver merozoites invade erythrocytes, mature and undergo endomitotic divisions to produce blood schizonts, which contain merozoites infective to new erythrocytes upon schizont rupture. The erythrocyte stage causes the clinical symptoms and is the target of chemotherapy of malaria. The different Plasmodium spp. show marked differences in preference to infect reticulocytes or mature erythrocytes and on the erythrocytic cycle duration. For example, P. vivax selectively invades reticulocytes whereas P. falciparum is not selective. The erythrocytic cycle is of about 24 h in rodent malarial species and P. knowlesi, 48 h for P. falciparum, P. vivax and P. ovale whereas P. malariae shows a cycle of 72 h; (C) A low percentage of parasites per erythrocytic cycle differentiate into male and female gametocytes. These gametocytes are specialized sexual cells taken up in mosquitoes’ blood meals. Upon gametocyte activation and fertilization in the mid-gut of mosquitoes, new infective sporozoites that reach their salivary glands are produced.
Since the call by The Bill and Melinda Gates Foundation in 2007, malaria eradication has gained momentum as a public health objective. Nowadays, eradication is perceived as an attainable objective based on the lessons learned from previous control, elimination and eradication programmes, particularly from the Global Malaria Eradication Programme between 1955 and 1969 (Nájera et al. Reference Nájera, González-Silva and Alonso2011). As drugs are the mainstay for malaria eradication (The malERA Consultative Group on Drugs, 2011), several target product profiles (TPP) have been proposed for drugs aimed at specific indications (Burrows et al. Reference Burrows, Leroy, Lotharius and Waterson2011; The malERA Consultative Group on Drugs, 2011). The specifications of each TPP define the characteristics of a new medicine for a therapeutic indication. Currently, the most ambitious TPPs look for radical cure and transmission blocking of P. falciparum and P. vivax infections (Burrows et al. Reference Burrows, Leroy, Lotharius and Waterson2011; The malERA Consultative Group on Drugs, 2011). These requirements call for drugs that kill liver stages, gametocytes and hypnozoites, which are the dormant liver stage responsible for the characteristic relapses of vivax malaria (see Fig. 1) (Galinski and Barnwell, Reference Galinski and Barnwell2008).
Animal models of malaria are key tools for drug discovery (Fidock et al. Reference Fidock, Rosenthal, Croft, Brun and Nwaka2004). These tools provide integrated systems in which the efficacy of drugs is assessed in a physiological context. Efficacy essentially depends on drug disposition (e.g. absorption, distribution, metabolism and excretion, abbreviated as ADME), the toxic effects elicited in the host and the intrinsic anti-parasitic activity of drugs. Both disposition and anti-parasitic activity may be substantially different in animal models and humans because of dissimilarity in body size, physiology and susceptibility to different Plasmodium spp. However, many pharmacological interactions between drugs and pathogens are independent of the host involved. As such, animal models allow gaining insight into the in vivo pharmacological properties of drugs and their combinations.
In this review, we analyse the use of animal models of malaria in current drug discovery programmes whose objective is to develop drugs against the erythrocyte stage of Plasmodium spp. This paradigm of animal model use could be applied to drug discovery programmes aiming at different TPPs or other infectious diseases.
EFFICACY MODELS IN MALARIA DRUG DISCOVERY
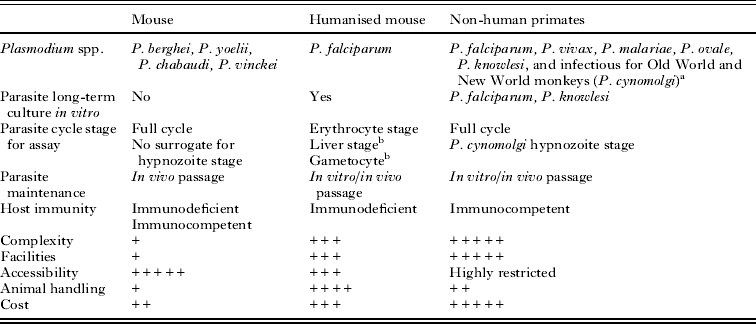
Malaria is caused by pathogens that infect humans and some non-human primates (White, Reference White2008; Prugnolle et al. Reference Prugnolle, Ollomo, Durand, Yalcindag, Arnathau, Elguero, Berry, Pourrut, Gonzalez, Nkoghe, Akiana, Verrier, Leroy, Ayala and Renaud2011). This host selectivity has limited the number and type of animal models available for drug evaluation (Table 1).
Table 1. Models for malaria drug discovery

a Strains of P. falciparum, P. vivax, P. malariae, P. ovale adapted to grow in monkeys.
b No reports on use for chemotherapy studies in drug discovery programmes.
Adapted from Angulo-Barturen and Ferrer (Reference Angulo-Barturen and Ferrer2012).
Models using human plasmodial pathogens
The Plasmodium species that cause malaria in humans are P. falciparum, P. vivax, P. malariae, P. ovale and P. knowlesi (White, Reference White2008). At pre-clinical stages of drug discovery, the efficacy of drugs against human malaria can only be tested using susceptible non-human primates or humanised mice (huMouse) as hosts.
Several species of New World Monkeys belonging to the genus Aotus and Saimiri are susceptible to infection by the five species mentioned above (Collins, Reference Collins2002b). P. falciparum, P. vivax and P. malariae require a process of adaptation in vivo to grow reproducibly in New World monkeys. In addition, splenectomy is often necessary to obtain significant parasitaemias and production of infective gametocytes (Collins, Reference Collins2002a, Reference Collinsb). Conversely, P. knowlesi, a natural parasite of monkeys, readily infects Aotus, Saimiri and Rhesus monkeys (Macaca mulatta). As a major advantage of non-human primates, all stages of the biological cycle of the human parasites can be reproduced for drug evaluation by choosing appropriate host-parasite pairs (Collins, Reference Collins2002a; Stewart, Reference Stewart2003). Nonetheless, the use of monkey models is quite limited due to ethical concerns and experimental complexity because highly specialized facilities are required.
The humanised mouse (huMouse) model is the only pre-clinical in vivo system in which parasites grow in human cells. Humanised mice are generated by engraftment of human tissues into immunodeficient mice (Shultz et al. Reference Shultz, Brehm, García-Martínez and Greiner2012). These mice lack T and B lymphocytes thereby enabling reproducible engraftment of human erythrocytes by intraperitoneal (Angulo-Barturen et al. Reference Angulo-Barturen, Jiménez-Díaz, Mulet, Rullas, Herreros, Ferrer, Jiménez, Mendoza, Regadera, Rosenthal, Bathurst, Pompliano, Gómez de las Heras and Gargallo-Viola2008) or intravenous injections (Arnold et al. Reference Arnold, Tyagi, Meija, Swetman, Gleeson, Perignon and Druilhe2011). The erythrocyte stages of P. falciparum can grow reproducibly inside engrafted human erythrocytes (Angulo-Barturen et al. Reference Angulo-Barturen, Jiménez-Díaz, Mulet, Rullas, Herreros, Ferrer, Jiménez, Mendoza, Regadera, Rosenthal, Bathurst, Pompliano, Gómez de las Heras and Gargallo-Viola2008; Arnold et al. Reference Arnold, Tyagi, Meija, Swetman, Gleeson, Perignon and Druilhe2011) and produce infective gametocytes (Moore et al. Reference Moore, Kumar, Shultz and Rajan1995). Using the P. falciparum huMouse (PfhuMouse) a number of standardized assays are available to evaluate the efficacy of antimalarial drugs targeting erythrocytic stages (Moreno et al. Reference Moreno, Badell, Van Rooijen and Druilhe2001; Angulo-Barturen et al. Reference Angulo-Barturen, Jiménez-Díaz, Mulet, Rullas, Herreros, Ferrer, Jiménez, Mendoza, Regadera, Rosenthal, Bathurst, Pompliano, Gómez de las Heras and Gargallo-Viola2008; Jiménez-Díaz et al. Reference Jiménez-Díaz, Mulet, Viera, Gómez, Garuti, Ibañez, Alvarez-Doval, Shultz, Martínez, Gargallo-Viola and Angulo-Barturen2009b).
Although their use in new drug discovery programmes has not been reported, the development of liver stages of P. falciparum is also supported in different strains of immunodeficient mice engrafted with human liver cells in ectopic (Morosan et al. Reference Morosan, Hez-Deroubaix, Lunel, Renia, Giannini, Van Rooijen, Battaglia, Blanc, Eling, Sauerwein, Hannoun, Belghiti, Brechot, Kremsdorf and Druilhe2006; Sacci et al. Reference Sacci, Alam, Douglas, Lewis, Tyrrell, Azad and Kneteman2006) or orthotopic (Vaughan et al. Reference Vaughan, Mikolajczak, Wilson, Grompe, Kaushansky, Camargo, Bial, Ploss and Kappe2012) locations. Of note, Fah–/–, Rag2–/–, Il2rg–/– (FRG) immunodeficient mice can be used to serially expand functional human liver cells in vivo from primary donors (Azuma et al. Reference Azuma, Paulk, Ranade, Dorrell, Al-Dhalimy, Ellis, Strom, Kay, Finegold and Grompe2007). The development of FRG mice is a breakthrough that has been used to demonstrate for the first time the production of viable liver merozoites on P. falciparum sporozoite infection in huMouse (Vaughan et al. Reference Vaughan, Mikolajczak, Wilson, Grompe, Kaushansky, Camargo, Bial, Ploss and Kappe2012). This study is particularly relevant because the quality of the P. falciparum liver stages found in FRG mice suggests its suitability to support P. vivax as well.
Models using surrogate plasmodial species
The mouse is the most widespread used host in malaria drug discovery owing to its versatility and accessibility. There are four major Plasmodium species adapted to grow in mice: P. berghei, P. yoelii, P. chabaudi and P. vinckei (Landau and Gautret, Reference Landau, Gautret and Sherman1998). Of these, P. berghei is the most widely used rodent species whereas P. yoelii, P. vinckei and P. chabaudi are used to a lesser extent. The severity of the disease in mice, which ranges between lethal infections and self-limited disease, depends on the strain of parasite species and the murine genetic background (Landau and Gautret, Reference Landau, Gautret and Sherman1998). The choice of plasmodial species is often based on empirical basis because not all rodent Plasmodium spp. are equally susceptible to drugs (Landau and Gautret, Reference Landau, Gautret and Sherman1998). The rodent Plasmodium spp. have biological cycles of about 24 h, which is a major difference from the classical human pathogens (Landau and Gautret, Reference Landau, Gautret and Sherman1998; Sanni et al. Reference Sanni, Fonseca, Langhorne and Doolan2002). In spite of these differences, the rodent-adapted parasites can reproduce the full cycle of malaria in mammals (Scheller et al. Reference Scheller, Wirtz and Azad1994). As a downside, the evaluation of drugs is performed with parasites that show significant evolutionary divergence from P. falciparum and P. vivax (Prugnolle et al. Reference Prugnolle, McGee, Keebler and Awadalla2008).
Macaques are the natural host of P. cynomolgi, a malaria parasite that has a biological cycle similar to P. vivax and can infect humans (Coatney et al. Reference Coatney, Elder, Contacos, Getz, Greenland, Rossan and Schmidt1961, Reference Coatney, Collins, McWarren and Contacos1971). P. cynomolgi has a cycle of maturation in blood of 48 h with reticulocytes being the preferred cell target of infection. The blood-stage infection is self-limited but presents characteristic relapses (Kocken et al. Reference Kocken, Remarque, Dubbeld, Wein, Van Der Wel, Verburgh, Vial and Thomas2009). P. cynomolgi is also noteworthy as it can also form hypnozoites upon liver infection. All these characteristics make P. cynomolgi an interesting surrogate model for P. vivax infection (DiTusa et al. Reference DiTusa, Gettayacamin, Kozar, Lin, Fracisco, Ohrt and Magill2010).
ROLE OF EFFICACY MODELS IN CURRENT DRUG DISCOVERY
The drug discovery process
Modern drug discovery is structured as a series of sequential iterative steps in which the properties of drugs as potential medicines are investigated (Payne et al. Reference Payne, Gwynn, Holmes and Pompliano2007). The drug discovery paradigm is typically divided in four stages: hit to lead, lead optimization, preclinical development and clinical development. At each step, test compounds are evaluated in different assays to understand risk and assess efficacy, toxicity, DMPK and physicochemical properties as indicators of their potential as future drug molecules. Ultimately, progression from one stage of discovery to the next indicates that drugs have no overt issues or the risks identified can be managed at a later stage in the development process (see Fig. 2).

Fig. 2. Comparison of efficacy evaluation in critical pathways. The Fig. summarizes two strategies of evaluation in vivo in hit-to-lead, lead optimization and candidate selection. (A) Efficacy evaluation is performed at the end of each progression step. This strategy of evaluation is followed by most current drug discovery programmes. In general, efficacy evaluation relies on relatively large experiments (dose–response, abbreviated as D/R, >10 mice per compound) designed to estimate the potency of compounds (e.g. ED90, etc.). The compounds tested are generally selected after extensive filtering using in silico/in vitro filtering; (B) Efficacy is performed at the beginning of each evaluation step. Efficacy evaluation relies on screening format assays involving small number of mice per compound (⩽3 per compound) aiming at detecting improvement or not over a reference compound of the series. In this strategy all in silico, in vitro and in vivo data are integrated in decision-making on compounds at early stage.
Hit to lead
Hits are drugs that have shown specific activity in vitro against an essential molecule of Plasmodium or the whole parasite itself. The sources of hits are usually high-throughput molecular or phenotypic screenings, depending on whether the objective is to inhibit a specific molecular target or the intact parasite/host cell, respectively. The availability of the full genome sequence for P. falciparum and, more recently, P. vivax, had raised hopes for the identification of hundreds of new targets (Gardner et al. Reference Gardner, Hall, Fung, White, Berriman, Hyman, Carlton, Pain, Nelson, Bowman, Paulsen, James, Eisen, Rutherford, Salzberg, Craig, Kyes, Chan, Nene, Shallom, Suh, Peterson, Angiuoli, Pertea, Allen, Selengut, Haft, Mather, Vaidya, Martin, Fairlamb, Fraunholz, Roos, Ralph, McFadden, Cummings, Subramanian, Mungall, Venter, Carucci, Hoffman, Newbold, Davis, Fraser and Barrell2002; Carlton et al. Reference Carlton, Adams, Silva, Bidwell, Lorenzi, Caler, Crabtree, Angiuoli, Merino, Amedeo, Cheng, Coulson, Crabb, del Portillo, Essien, Feldblyum, Fernandez-Becerra, Gilson, Gueye, Guo, Kang/'a, Kooij, Korsinczky, Meyer, Nene, Paulsen, White, Ralph, Ren, Sargeant, Salzberg, Stoeckert, Sullivan, Yamamoto, Hoffman, Wortman, Gardner, Galinski, Barnwell and Fraser-Liggett2008). However, no new class of antimalarials has been discovered in the last twenty years using target-based approaches (Chatterjee and Yeung, Reference Chatterjee and Yeung2012; Guiguemde et al. Reference Guiguemde, Shelat, García-Bustos, Diagana, Gamo and Guy2012). These negative results are in close agreement with the experience accumulated in anti-bacterial drug discovery (Payne et al. Reference Payne, Gwynn, Holmes and Pompliano2007) and highlight the importance of testing compounds in systems in which the target is in its native functional context.
Recently, a series of phenotypic screens have delivered more than twenty thousand compounds that have activity in vitro against the asexual erythrocyte stage of P. falciparum (Chong et al. Reference Chong, Chen, Shi, Liu and Sullivan2006; Weisman et al. Reference Weisman, Liou, Shelat, Cohen, Guy and DeRisi2006; Baniecki et al. Reference Baniecki, Wirth and Clardy2007; Plouffe et al. Reference Plouffe, Brinker, McNamara, Henson, Kato, Kuhen, Nagle, Adrian, Matzen, Anderson, Nam, Gray, Chatterjee, Janes, Yan, Trager, Caldwell, Schultz, Zhou and Winzeler2008; Gamo et al. Reference Gamo, Sanz, Vidal, de Cózar, Alvarez, Lavandera, Vanderwall, Green, Kumar, Hasan, Brown, Peishoff, Cardon and García-Bustos2010; Guiguemde et al. Reference Guiguemde, Shelat, Bouck, Duffy, Crowther, Davis, Smithson, Connelly, Clark, Zhu, Jiménez-Díaz, Martínez, Wilson, Tripathi, Gut, Sharlow, Bathurst, El Mazouni, Fowble, Forquer, McGinley, Castro, Angulo-Barturen, Ferrer, Rosenthal, Derisi, Sullivan, Lazo, Roos, Riscoe, Phillips, Rathod, Van Voorhis, Avery and Guy2010). This work has identified an unprecedented number of potential starting points for drug discovery. The prioritization of hits according to their physicochemical and ADMET (absorption, distribution, metabolism, excretion and toxicity) properties in in silico and in vitro assays is employed to select the most promising new scaffolds to start drug discovery programmes (Keldenich, Reference Keldenich2009; Gleeson et al. Reference Gleeson, Hersey and Hannongbua2011). Selected scaffolds are then modified to improve their physicochemical and DMPK properties, identify early toxicological flags and increase their antimalarial potency.
The demonstration of efficacy in vivo for a new scaffold is a major goal of the hit-to-lead stage. Murine models are the most widely used to achieve this proof of concept because they require low amounts of compound (tens of mg of solid) and are accessible to many laboratories. While P. berghei is still the most widely used model, other rodent-infecting species have also been employed across a number of drug discovery programmes (Jain et al. Reference Jain, Vangapandu, Sachdeva, Singh, Singh, Jena, Tikoo, Ramarao, Kaul and Jain2004; Chong et al. Reference Chong, Chen, Shi, Liu and Sullivan2006; Bhattacharjee et al. Reference Bhattacharjee, Nichols, Gerena, Roncal and Gutteridge2007; Coslédan et al. Reference Coslédan, Fraisse, Pellet, Guillou, Mordmuller, Kremsner, Moreno, Mazier, Maffrand and Meunier2008; Kelly et al. Reference Kelly, Smilkstein, Brun, Wittlin, Cooper, Lane, Janowsky, Johnson, Dodean, Winter, Hinrichs and Riscoe2009).
The choice of model may depend on the genetic similarity at the level of the molecular target. For example, P. yoelii was chosen to test 4-(1H) pyridones because of the high sequence homology with P. falciparum for the target cytochrome bc1, which is a key protein in the mitochondrial respiratory chain (Yeates et al. Reference Yeates, Batchelor, Capon, Cheesman, Fry, Hudson, Pudney, Trimming, Woolven, Bueno, Chicharro, Fernandez, Fiandor, Gargallo-Viola, Gomez de las Heras, Herreros and Leon2008). In other cases, the choice is guided by empirical considerations based on the relative sensitivity of each model to the drugs tested. Thus, diamidines have been tested in the P. vinckei model because P. berghei is almost insensitive to these drugs (Angulo-Barturen et al. Reference Angulo-Barturen, Jiménez-Díaz, Mulet, Rullas, Herreros, Ferrer, Jiménez, Mendoza, Regadera, Rosenthal, Bathurst, Pompliano, Gómez de las Heras and Gargallo-Viola2008).
To address the efficacy proof of concept two different assays, or variants of them, are typically employed: the Thompson test and the Peters’ suppressive 4-day test (Thompson and Werbel, Reference Thompson and Werbel1972; Peters and Robinson, Reference Peters, Robinson, Zak and Sande1999). In the Thompson test, mice having patent infections with P. berghei are treated with a drug for three days and the survival time of treated mice is compared to vehicle-treated controls. In contrast, in the Peters’ 4-day test, the administration of compounds starts one to three hours after infection with parasitized-erythrocytes and the resulting parasitaemia is measured on the fifth day, that is 24 h after the last dose administration, and compared to vehicle-treated controls. Both assays are robust and reproducible, as demonstrated by the numerous projects that rely on them (Fidock et al. Reference Fidock, Rosenthal, Croft, Brun and Nwaka2004).
Assessing the pharmacokinetics of compounds tested in vivo before evaluation enhances the power of discrimination of the efficacy study. Prior knowledge of exposure enables researchers to select those compounds that have the greatest potential of showing in vivo efficacy (Lowes et al. Reference Lowes, Pradhan, Iyer, Parman, Gow, Zhu, Furimsky, Lemoff, Guiguemde, Sigal, Clark, Wilson, Tang, Connelly, DeRisi, Kyle, Mirsalis and Guy2012; Nagle et al. Reference Nagle, Wu, Kuhen, Gagaring, Borboa, Francek, Chen, Plouffe, Lin, Caldwell, Ek, Skolnik, Liu, Wang, Chang, Li, Liu, Hollenbeck, Tuntland, Isbell, Chuan, Alper, Fischli, Brun, Lakshminarayana, Rottmann, Diagana, Winzeler, Glynne, Tully and Chatterjee2012; Zhang et al. Reference Zhang, Clark, Connelly, Zhu, Min, Guiguemde, Pradhan, Iyer, Furimsky, Gow, Parman, El Mazouni, Phillips, Kyle, Mirsalis and Guy2012). This strategy is particularly important in cases in which drugs fail to inhibit rodent Plasmodium spp. even though sufficient drug exposure is achieved in the blood. A differential susceptibility to the drug between the human pathogen in vitro and the rodent surrogate in vivo is inferred in these situations. In these cases, evaluating the efficacy of drugs either in non-human primates or humanized mouse models is the only realistic alternative.
Lead optimization
In lead optimization, efficacy studies seek to detect small differences in potency between compounds that show small structural differences. As in hit-to-lead, the Thompson test and the Peters’ suppressive 4-day test are the most widely used assays. The parameters of efficacy are essentially measurements of potency, that is, the mg per kg of body weight necessary to achieve a specific biological endpoint (Fidock et al. Reference Fidock, Rosenthal, Croft, Brun and Nwaka2004). The usual biological endpoints are the number of days until recrudescence (Saenz et al. Reference Saenz, Mutka, Udenze, Oduola and Kyle2012; Anderson et al. Reference Anderson, Sarantakis, Terpinski, Kumar, Tsai, Kuo, Ager, Jacobs, Schiehser, Ekins, Sacchettini, Jacobus, Fidock and Freundlich2013) and the reduction in parasitaemia with respect to vehicle-treated mice (e.g. ED50 and ED90, defined as the effective dose levels that reduce parasitaemia by 50 or 90%, respectively) (Coslédan et al. Reference Coslédan, Fraisse, Pellet, Guillou, Mordmuller, Kremsner, Moreno, Mazier, Maffrand and Meunier2008; Khan et al. Reference Khan, Levi, Tekwani, Khan, Kimura and Borne2009; Booker et al. Reference Booker, Bastos, Kramer, Barker, Skerlj, Sidhu, Deng, Celatka, Cortese, Guerrero Bravo, Crespo Llado, Serrano, Angulo-Barturen, Jiménez-Díaz, Viera, Garuti, Wittlin, Papastogiannidis, Lin, Janse, Khan, Duraisingh, Coleman, Goldsmith, Phillips, Muñoz, Wirth, Klinger, Wiegand and Sybertza2010; Barker et al. Reference Barker, Urgaonkar, Mazitschek, Celatka, Skerlj, Cortese, Tyndall, Liu, Cromwell, Sidhu, Guerrero-Bravo, Crespo-Llado, Serrano, Lin, Janse, Khan, Duraisingh, Coleman, Angulo-Barturen, Jiménez-Díaz, Magán, Gómez, Ferrer, Martínez, Wittlin, Papastogiannidis, O'Shea, Klinger, Bree, Lee, Levine, Wiegand, Muñoz, Wirth, Clardy, Bathurst and Sybertz2011; Biagini et al. Reference Biagini, Fisher, Shone, Mubaraki, Srivastava, Hill, Antoine, Warman, Davies, Pidathala, Amewu, Leung, Sharma, Gibbons, Hong, Pacorel, Lawrenson, Charoensutthivarakul, Taylor, Berger, Mbekeani, Stocks, Nixon, Chadwick, Hemingway, Delves, Sinden, Zeeman, Kocken, Berry, O'Neill and Ward2012; Brunner et al. Reference Brunner, Aissaoui, Boss, Bozdech, Brun, Corminboeuf, Delahaye, Fischli, Heidmann, Kaiser, Kamber, Meyer, Papastogiannidis, Siegrist, Voss, Welford, Wittlin and Binkert2012; Younis et al. Reference Younis, Douelle, Feng, Gonzalez Cabrera, Le Manach, Nchinda, Duffy, White, Shackleford, Morizzi, Mannila, Katneni, Bhamidipati, Zabiulla, Joseph, Bashyam, Waterson, Witty, Hardick, Wittlin, Avery, Charman and Chibale2012). Although it should be noted that assessments of the minimum number of animals necessary to estimate the parameters of efficacy at a predetermined power and confidence level are scarce and typically not calculated.
The drugs that selectively inhibit human pathogens represent a challenge during the lead optimization. Although non-human primates have been utilized for profiling of efficacy of choline analogues (Salom-Roig et al. Reference Salom-Roig, Hamze, Calas and Vial2005), these hosts are mostly used in projects seeking drugs for radical cure (Lin et al. Reference Lin, Kozar, O'Neil, Melendez, Saunders and Magill2009). In contrast, the PfhuMouse model offers a practical alternative that has been exploited by several recent drug discovery projects. For example, the poor activity of some triazolopyrimidines against P. berghei dihydroorotate dehydrogenase (DHODH) compared to the P. falciparum enzyme prompted researchers to use the PfhuMouse Model to measure efficacy in vivo (Coteron et al. Reference Coteron, Marco, Esquivias, Deng, White, White, Koltun, El Mazouni, Kokkonda, Katneni, Bhamidipati, Shackleford, Angulo-Barturen, Ferrer, Jiménez-Díaz, Gamo, Goldsmith, Charman, Bathurst, Floyd, Matthews, Burrows, Rathod, Charman and Phillips2011). Noteworthy, an increasing number of projects employ the PfhuMouse model at some point during the lead optimization process irrespective of the difference in susceptibility between P. falciparum and P. berghei (Coslédan et al. Reference Coslédan, Fraisse, Pellet, Guillou, Mordmuller, Kremsner, Moreno, Mazier, Maffrand and Meunier2008; Jiménez-Díaz et al. Reference Jiménez-Díaz, Mulet, Viera, Gómez, Garuti, Ibañez, Alvarez-Doval, Shultz, Martínez, Gargallo-Viola and Angulo-Barturen2009b; Booker et al. Reference Booker, Bastos, Kramer, Barker, Skerlj, Sidhu, Deng, Celatka, Cortese, Guerrero Bravo, Crespo Llado, Serrano, Angulo-Barturen, Jiménez-Díaz, Viera, Garuti, Wittlin, Papastogiannidis, Lin, Janse, Khan, Duraisingh, Coleman, Goldsmith, Phillips, Muñoz, Wirth, Klinger, Wiegand and Sybertza2010; Barker et al. Reference Barker, Urgaonkar, Mazitschek, Celatka, Skerlj, Cortese, Tyndall, Liu, Cromwell, Sidhu, Guerrero-Bravo, Crespo-Llado, Serrano, Lin, Janse, Khan, Duraisingh, Coleman, Angulo-Barturen, Jiménez-Díaz, Magán, Gómez, Ferrer, Martínez, Wittlin, Papastogiannidis, O'Shea, Klinger, Bree, Lee, Levine, Wiegand, Muñoz, Wirth, Clardy, Bathurst and Sybertz2011; Sanz et al. Reference Sanz, Jiménez-Díaz, Crespo, De Cózar, Almela, Angulo-Barturen, Castañeda, Ibañez, Fernandez, Ferrer, Herreros, Lozano, Martínez, Rueda, Burrows, García-Bustos and Gamo2011; Brunner et al. Reference Brunner, Aissaoui, Boss, Bozdech, Brun, Corminboeuf, Delahaye, Fischli, Heidmann, Kaiser, Kamber, Meyer, Papastogiannidis, Siegrist, Voss, Welford, Wittlin and Binkert2012; Nilsen et al. Reference Nilsen, LaCrue, White, Forquer, Cross, Marfurt, Mather, Delves, Shackleford, Saenz, Morrisey, Steuten, Mutka, Li, Wirjanata, Ryan, Duffy, Kelly, Sebayang, Zeeman, Noviyanti, Sinden, Kocken, Price, Avery, Angulo-Barturen, Jiménez-Díaz, Ferrer, Herreros, Sanz, Gamo, Bathurst, Burrows, Siegl, Guy, Winter, Vaidya, Charman, Kyle, Manetsch and Riscoe2013).
Candidate selection
Candidate selection is a milestone in the progression of a project and represents a commitment to clinical development of a specific asset. At this stage, the candidate molecule is thoroughly evaluated for DMPK, toxicity, efficacy and physicochemical properties. The difference between the levels of compound in blood that are efficacious and those at which toxicity is observed in animals is known as the therapeutic index (TI) or therapeutic window. The TI is an important parameter in order to decide whether a drug should be progressed to clinical development.
Efficacy animal models should provide estimates of the efficacious levels to support TI calculations. Ideally, this should be addressed through detailed pharmacokinetic/pharmacodynamic (PK/PD) studies linking drug exposure and parasitological inhibition or cure. Even though detailed PK/PD studies are hardly addressed in recent literature describing new candidates, some valuable dose-fractionation studies assessing cure of mice have been performed. For example, the candidate drugs MK-4815, NITD609 or OZ439 were shown to cure Balb/c mice infected with P. berghei (Rottmann et al. Reference Rottmann, McNamara, Yeung, Lee, Zou, Russell, Seitz, Plouffe, Dharia, Tan, Cohen, Spencer, González-Páez, Lakshminarayana, Goh, Suwanarusk, Jegla, Schmitt, Beck, Brun, Nosten, Renia, Dartois, Keller, Fidock, Winzeler and Diagana2010; Charman et al. Reference Charman, Arbe-Barnes, Bathurst, Brund, Campbell, Charman, Chiu, Chollet, Craft, Creek, Don, Matile, Maurer, Morizzi, Nguyen, Papastogiannidis, Scheurer, Shackleford, Sriraghavan, Stingelin, Tang, Urwyler, Wang, White, Wittlin, Zhou and Vennerstrom2011; Powles et al. Reference Powles, Allocco, Yeung, Nare, Liberator and Schmatz2012).
A proof of concept in an animal model of infection with human plasmodial pathogens is also often included in the candidate evaluation package. For example, efficacy against P. falciparum and P. cynomolgi in non-human primates has been tested in choline analogues that inhibit phosphatidylcholine biosynthesis (Wengelnik et al. Reference Wengelnik, Vidal, Ancelin, Cathiard, Morgat, Kocken, Calas, Herrera, Thomas and Vial2002). However, as described above, the PfhuMouse model is an alternative test system that has been successfully exploited to estimate drug efficacy in an increasing number of projects (Coslédan et al. Reference Coslédan, Fraisse, Pellet, Guillou, Mordmuller, Kremsner, Moreno, Mazier, Maffrand and Meunier2008; Jiménez-Díaz et al. Reference Jiménez-Díaz, Mulet, Viera, Gómez, Garuti, Ibañez, Alvarez-Doval, Shultz, Martínez, Gargallo-Viola and Angulo-Barturen2009b; Booker et al. Reference Booker, Bastos, Kramer, Barker, Skerlj, Sidhu, Deng, Celatka, Cortese, Guerrero Bravo, Crespo Llado, Serrano, Angulo-Barturen, Jiménez-Díaz, Viera, Garuti, Wittlin, Papastogiannidis, Lin, Janse, Khan, Duraisingh, Coleman, Goldsmith, Phillips, Muñoz, Wirth, Klinger, Wiegand and Sybertza2010; Barker et al. Reference Barker, Urgaonkar, Mazitschek, Celatka, Skerlj, Cortese, Tyndall, Liu, Cromwell, Sidhu, Guerrero-Bravo, Crespo-Llado, Serrano, Lin, Janse, Khan, Duraisingh, Coleman, Angulo-Barturen, Jiménez-Díaz, Magán, Gómez, Ferrer, Martínez, Wittlin, Papastogiannidis, O'Shea, Klinger, Bree, Lee, Levine, Wiegand, Muñoz, Wirth, Clardy, Bathurst and Sybertz2011; Coteron et al. Reference Coteron, Marco, Esquivias, Deng, White, White, Koltun, El Mazouni, Kokkonda, Katneni, Bhamidipati, Shackleford, Angulo-Barturen, Ferrer, Jiménez-Díaz, Gamo, Goldsmith, Charman, Bathurst, Floyd, Matthews, Burrows, Rathod, Charman and Phillips2011; Skerlj et al. Reference Skerlj, Bastos, Booker, Kramer, Barker, Celatka, O'Shea, Munoz, Sidhu, Cortese, Wittlin, Papastogiannidis, Angulo-Barturen, Jiménez-Díaz and Sybertz2011; Nilsen et al. Reference Nilsen, LaCrue, White, Forquer, Cross, Marfurt, Mather, Delves, Shackleford, Saenz, Morrisey, Steuten, Mutka, Li, Wirjanata, Ryan, Duffy, Kelly, Sebayang, Zeeman, Noviyanti, Sinden, Kocken, Price, Avery, Angulo-Barturen, Jiménez-Díaz, Ferrer, Herreros, Sanz, Gamo, Bathurst, Burrows, Siegl, Guy, Winter, Vaidya, Charman, Kyle, Manetsch and Riscoe2013).
Pre-clinical and clinical development
Drug development starts after candidate selection. At this stage, pre-clinical studies aim at preparing Phase I (first time in human) and Phase II (proof of concept in humans). Efficacy experiments in animals are rarely performed during the pre-clinical or clinical development phases before the drug has demonstrated efficacy in humans. Once this proof of concept in humans has been achieved, efficacy models can be used to find new therapeutic indications for known drugs as exemplified by the repositioning of azithromycin (Andersen et al. Reference Andersen, Ager, McGreevy, Schuster, Wesche, Kuschner, Ohrt, Ellis, Rossan and Berman1995) and iron chelators (Ferrer et al. Reference Ferrer, Tripathi, Clark, Hand, Rienhoff and Sullivan2012) as antimalarials. Particularly important is the use of animal models to study the optimal partnering of marketed drugs to create new antimalarial combinations. Examples of this type of study include characterization of new combinations of artemisinin derivatives in P. berghei (Guo et al. Reference Guo, Guiguemde, Bentura-Marciano, Clark, Haynes, Chan, Wong, Hunt, Guy and Golenser2012), the evaluation of artemisone and mefloquine in Aotus monkeys infected with P. falciparum (Obaldia et al. Reference Obaldia, Kotecka, Edstein, Haynes, Fugmann, Kyle and Rieckmann2009) and the evaluation of chloroquine and azithromycin in combination with R-amlodipine to reverse chloroquine resistance in P. yoelii (Pereira et al. Reference Pereira, Henrich, Sidhu, Johnson, Hardink, Van Deusen, Lin, Gore, O'Brien, Wele, Djimde, Chandra and Fidock2011).
Summary
The general pattern of the current use of efficacy models in drug discovery indicates that in vivo evaluation is performed with a relatively low number of compounds after extensive testing and selection by in silico and in vitro assays. The evaluation in vivo aims to measure the potency of compounds by estimating ED90 or analogous parameters of efficacy utilizing experimental designs widely employed in the scientific community. These experiments are executed using relatively large numbers of animals per compound tested (>10 mice/compound/assay).
NEW DIRECTIONS USING EFFICACY MODELS
The animal models of malaria are unique tools to analyse the properties of drugs as future medicines. Because of their nature, best practices in animal experimentation demand a periodic critical review of the current procedures in the field. As a final objective, only optimally designed experiments necessary to obtain crucial information for drug development should be addressed. Here we propose several lines of improvement that we have started to implement in our strategy of evaluation.
A translational approach
The drug discovery process would benefit from efficacy models able to inform the design of clinical studies. This entails that the parasitological response of infected individuals to therapy is studied using comparable methods and parameters of efficacy in both drug discovery and clinical development.
Efficacy models that employ human pathogens may have a critical role in translational medicine for malaria (Burrows et al. Reference Burrows, Leroy, Lotharius and Waterson2011; Angulo-Barturen and Ferrer, Reference Angulo-Barturen and Ferrer2012). Not surprisingly, humanised murine models are being increasingly used in drug discovery programmes (Coslédan et al. Reference Coslédan, Fraisse, Pellet, Guillou, Mordmuller, Kremsner, Moreno, Mazier, Maffrand and Meunier2008; Booker et al. Reference Booker, Bastos, Kramer, Barker, Skerlj, Sidhu, Deng, Celatka, Cortese, Guerrero Bravo, Crespo Llado, Serrano, Angulo-Barturen, Jiménez-Díaz, Viera, Garuti, Wittlin, Papastogiannidis, Lin, Janse, Khan, Duraisingh, Coleman, Goldsmith, Phillips, Muñoz, Wirth, Klinger, Wiegand and Sybertza2010; Barker et al. Reference Barker, Urgaonkar, Mazitschek, Celatka, Skerlj, Cortese, Tyndall, Liu, Cromwell, Sidhu, Guerrero-Bravo, Crespo-Llado, Serrano, Lin, Janse, Khan, Duraisingh, Coleman, Angulo-Barturen, Jiménez-Díaz, Magán, Gómez, Ferrer, Martínez, Wittlin, Papastogiannidis, O'Shea, Klinger, Bree, Lee, Levine, Wiegand, Muñoz, Wirth, Clardy, Bathurst and Sybertz2011; Coteron et al. Reference Coteron, Marco, Esquivias, Deng, White, White, Koltun, El Mazouni, Kokkonda, Katneni, Bhamidipati, Shackleford, Angulo-Barturen, Ferrer, Jiménez-Díaz, Gamo, Goldsmith, Charman, Bathurst, Floyd, Matthews, Burrows, Rathod, Charman and Phillips2011; Nilsen et al. Reference Nilsen, LaCrue, White, Forquer, Cross, Marfurt, Mather, Delves, Shackleford, Saenz, Morrisey, Steuten, Mutka, Li, Wirjanata, Ryan, Duffy, Kelly, Sebayang, Zeeman, Noviyanti, Sinden, Kocken, Price, Avery, Angulo-Barturen, Jiménez-Díaz, Ferrer, Herreros, Sanz, Gamo, Bathurst, Burrows, Siegl, Guy, Winter, Vaidya, Charman, Kyle, Manetsch and Riscoe2013). One practical advantage of these models is their simplicity compared to non-human primates (Collins, Reference Collins2002a, Reference Collinsb). Moreover, latest advances in huMouse models suggest that mice engrafted with human bone marrow and liver cells will be commercially available shortly. Nonetheless, surrogate plasmodial species may also have a valuable role in predicting human clinical doses. As an example, a PK/PD analysis using the Peters’ 4-day test in P. yoelii- infected mice has been employed to estimate the effective daily dose of R-amlodipine necessary to overcome chloroquine resistance in humans treated with a combination of chloroquine and azithromycin (Pereira et al. Reference Pereira, Henrich, Sidhu, Johnson, Hardink, Van Deusen, Lin, Gore, O'Brien, Wele, Djimde, Chandra and Fidock2011).
The efficacy of a drug in humans is measured by the rate at which the parasites are cleared from peripheral blood and the rate of cure after treatment (White, Reference White2011). In experimental models, the parasite clearance rate is not a common measurement of efficacy, although it has been used to assess the onset of action upon treatment (Charman et al. Reference Charman, Arbe-Barnes, Bathurst, Brund, Campbell, Charman, Chiu, Chollet, Craft, Creek, Don, Matile, Maurer, Morizzi, Nguyen, Papastogiannidis, Scheurer, Shackleford, Sriraghavan, Stingelin, Tang, Urwyler, Wang, White, Wittlin, Zhou and Vennerstrom2011). Differences in parasitaemia at a given time of the assay with respect to vehicle-treated controls are the most used parameters of efficacy (Thompson and Werbel, Reference Thompson and Werbel1972; Peters and Robinson, Reference Peters, Robinson, Zak and Sande1999; Fidock et al. Reference Fidock, Rosenthal, Croft, Brun and Nwaka2004). However, a new type of assay based on modelling the treatment of patients in mice and measuring the parasite clearance rate has been recently developed in a P. berghei murine model (Jiménez-Díaz et al. Reference Jiménez-Díaz, Viera, Ibáñez, Mulet, Magán-Marchal, Garuti, Gómez, Cortés-Gil, Martínez, Ferrer, Fraile, Calderón, Fernández, Shultz, Leroy, Wilson, García-Bustos, Gamo and Angulo-Barturen2013). This type of assay allows estimates of treatment duration with new drugs in early drug discovery to be assessed because the elimination of the parasite is a first order process (White, Reference White2011). As a downside, it is still pending the implementation of suitable metrics for the parameters that allow comparisons of parasite clearance rates in animals and humans. On the contrary, the comparison of the rate of cure in mice and humans treated with antimalarials is straightforward. This interspecies comparison is possible by using logistic analysis, which relates a dichotomous response variable (cured/not cured) with continuous explanatory variables (dose level, exposure, time of treatment, etc…). Thus, logistic analysis offers a powerful tool to validate murine systems for human dose prediction (Angulo-Barturen and Ferrer, Reference Angulo-Barturen and Ferrer2012).
Animal models can inform the design of clinical studies by estimating the efficacious exposure of drugs in vivo. These estimations have been indirectly addressed by extrapolating data from separated PK and efficacy studies (Barker et al. Reference Barker, Urgaonkar, Mazitschek, Celatka, Skerlj, Cortese, Tyndall, Liu, Cromwell, Sidhu, Guerrero-Bravo, Crespo-Llado, Serrano, Lin, Janse, Khan, Duraisingh, Coleman, Angulo-Barturen, Jiménez-Díaz, Magán, Gómez, Ferrer, Martínez, Wittlin, Papastogiannidis, O'Shea, Klinger, Bree, Lee, Levine, Wiegand, Muñoz, Wirth, Clardy, Bathurst and Sybertz2011). By blood microsampling of animals employed in efficacy studies, the PK/PD relationships governing the efficacy of compounds can be studied at the desired level of precision (Pereira et al. Reference Pereira, Henrich, Sidhu, Johnson, Hardink, Van Deusen, Lin, Gore, O'Brien, Wele, Djimde, Chandra and Fidock2011; Nilsen et al. Reference Nilsen, LaCrue, White, Forquer, Cross, Marfurt, Mather, Delves, Shackleford, Saenz, Morrisey, Steuten, Mutka, Li, Wirjanata, Ryan, Duffy, Kelly, Sebayang, Zeeman, Noviyanti, Sinden, Kocken, Price, Avery, Angulo-Barturen, Jiménez-Díaz, Ferrer, Herreros, Sanz, Gamo, Bathurst, Burrows, Siegl, Guy, Winter, Vaidya, Charman, Kyle, Manetsch and Riscoe2013). The efficacy-coupled microsampling approach is particularly powerful, because it allows the discrimination between the intrinsic antimalarial potency of compounds and their DMPK liabilities in the host species chosen.
Methodological improvement
The improvement of the experimental designs employed in efficacy studies can reduce the number of animals required while increasing the translational value of the parameters measured. It is surprising that the statistical techniques used in clinical trials to minimize the number of patients are not widely implemented in experimental efficacy (van der Worp et al. Reference van der Worp, Howells, Sena, Porritt, Rewell, O'Collins and Macleod2010). For example, a substantial reduction in the number of animals has been achieved in estimating the in vivo potency of ELQ-300 against P. falciparum by using individuals as experimental units instead of groups of individuals (Nilsen et al. Reference Nilsen, LaCrue, White, Forquer, Cross, Marfurt, Mather, Delves, Shackleford, Saenz, Morrisey, Steuten, Mutka, Li, Wirjanata, Ryan, Duffy, Kelly, Sebayang, Zeeman, Noviyanti, Sinden, Kocken, Price, Avery, Angulo-Barturen, Jiménez-Díaz, Ferrer, Herreros, Sanz, Gamo, Bathurst, Burrows, Siegl, Guy, Winter, Vaidya, Charman, Kyle, Manetsch and Riscoe2013). This approach can also take advantage of advanced statistical methods, for example, non-linear mixed-effect (NLME) models (Paterson and Lello, Reference Paterson and Lello2003). A NLME model has been successfully utilized to measure the efficacy of G25, an inhibitor of phosphatidylcholine biosynthesis, in P. cynomolgi-infected macaques (Kocken et al. Reference Kocken, Remarque, Dubbeld, Wein, Van Der Wel, Verburgh, Vial and Thomas2009). The NLME model controlled the influence of pseudo-replication and auto-correlation on multiple samples taken from the same individual over time and allowed accounting for the individual variability of the course of parasitaemia on the overall effect of drug treatment.
The properties of drugs as antimalarial medicines are established at early stages of drug discovery when the chemical scaffold is chosen. By comparison with drugs of known mechanisms of action, it would be possible to classify the new drugs according to their phenotypic effects on parasites. Among the many techniques available, multiparametric flow cytometry is particularly useful for compound classification (Jiménez-Díaz et al. Reference Jiménez-Díaz, Mulet, Gómez, Viera, Alvarez, Garuti, Vázquez, Fernández, Ibañez, Jiménez, Gargallo-Viola and Angulo-Barturen2009a; Apte et al. Reference Apte, Groves, Roddick, V and Doolan2011; Malleret et al. Reference Malleret, Claser, Ong, Suwanarusk, Sriprawat, Howland, Russell, Nosten and Renia2011). Flow cytometry requires very small blood samples (units of μl) that do not interfere with the evaluation of efficacy in vivo. Therefore, incorporating high-content experimental designs might increase the decision-making and translational value of in vivo models.
Critical pathway
Animal models of efficacy are usually go/no go decision points in critical paths. This means that the sooner the level of in vivo efficacy is established for a given compound the quicker it can be eliminated for further progression thus saving resources. Therefore, it seems logical to perform efficacy studies at the earliest possible point in the critical path.
In vivo screening for efficacious compounds can be addressed immediately after the identification of hits (Fig. 2). In support of this contention, all antimalarial families on the market belong to chemical scaffolds identified through large screening campaigns performed during the 20th century in avian or murine malaria models (Kinnamon and Rothe, Reference Kinnamon and Rothe1975; Ockenhouse et al. Reference Ockenhouse, Magill, Smith and Milhous2005; Slater, Reference Slater2005). Nowadays, the identification of more than twenty thousand compounds that are active in vitro against the erythrocyte stage of P. falciparum raises the question of how to exploit this knowledge (Guiguemde et al. Reference Guiguemde, Shelat, García-Bustos, Diagana, Gamo and Guy2012). A detailed consideration of the in vivo primary screening addressed in the past comes after the evaluation of ca. 800 compounds from the Tres Cantos Antimalarial Collection (TCAMS) against P. berghei (Jiménez-Díaz et al. Reference Jiménez-Díaz, Viera, Ibáñez, Mulet, Magán-Marchal, Garuti, Gómez, Cortés-Gil, Martínez, Ferrer, Fraile, Calderón, Fernández, Shultz, Leroy, Wilson, García-Bustos, Gamo and Angulo-Barturen2013). This study indicates that performing in vivo screens on a large number of hit compounds is a feasible task whose main bottleneck is compound synthesis. Moreover, about 10% of the compounds tested had some efficacy and about 3% were as efficacious as marketed antimalarials. The main advantage of the in vivo screening approach is that the starting points for drug discovery programmes are compounds with sufficient exposure to be efficacious and without overt toxicities. The downside is the risk of losing compounds with bad PK properties that might be improved through rational chemical optimization (Fig. 2).
Full PK/PD analysis of the pharmacological properties of compounds could be addressed as the starting point of lead optimization. The importance of PK/PD studies to understand the efficacy of compounds is widely recognized (Gabrielsson et al. Reference Gabrielsson, Dolgos, Gillberg, Bredberg, Benthem and Duker2009). A series of PK/PD studies in P. berghei-infected mice on the efficacy of doxycycline alone or in combination with dihydroartemisinin (Batty et al. Reference Batty, Law, Stirling and Moore2007), piperaquine (Moore et al. Reference Moore, Batty, Andrzejewski, Jago, Page-Sharp and Ilett2008, Reference Moore, Ilett, Page-Sharp, Jago and Batty2009) and chloroquine (Moore et al. Reference Moore, Page-Sharp, Stoney, Ilett, Jago and Batty2011) have been published recently. The methodology described in these papers, which address the parasitological response to treatment (e.g. parasite clearance and relapse during and after treatment), might be useful for efficacy studies in late hit-to-lead or early lead optimization. This early PK/PD analysis would also allow a judgment on whether the pharmacological characteristics of the compound series meet the desired efficacy end-points.
CONCLUDING REMARKS
Animal models of efficacy play a crucial role in malaria drug discovery. As tools that integrate efficacy, drug disposition and toxicology, efficacy models can provide new insights into the PK/PD properties of antimalarial drugs. No doubt, these studies could improve decision-making and likely inform the design of clinical trials. All these improvements are compatible with a more effective use of the variety of efficacy models available, which offer alternatives to design different critical pathways for drug progression.
There is compelling evidence indicating that the use of animal models in drug discovery can be optimized. Here we propose that performing more informative efficacy studies at an earlier point in discovery screening cascades might dramatically accelerate the development of new antimalarial medicines while reducing the number of animals employed.
ACKNOWLEDGEMENTS
The authors are indebted to Drs Santiago Ferrer (GSK) and Didier Leroy for their scientific input on the subject. The authors are indebted to Dr David M. Wilson (GSK) for critical reading and discussion of the manuscript.
FINANCIAL SUPPORT
This work has been supported in part by the Miniportfolio Agreement between GlaxoSmithKline and Medicines for Malaria Venture (Geneva, Switzerland).