Introduction
Schizophrenia is a complex disorder and deficits relative to controls have been seen across a broad range of behaviours. In the present study we were interested in examining the impact of social stimuli on learning and decision-making processes. Although patients with schizophrenia have deficits in both decision making and emotion recognition, these two avenues of research have proceeded independently. By investigating decision making within a social context, we aimed to explore how deficits in these two domains interact with one another. Our task also allowed us to examine the implicit impact of emotional stimuli when a decision had to be made about those stimuli as opposed to the explicit responses, which are often used in emotion naming tasks.
Altered decision making in patients with schizophrenia has been demonstrated using several different behavioural paradigms. For example, patients have been shown to have reversal learning deficits in associative learning tasks (Waltz & Gold, Reference Waltz and Gold2007; Murray et al. Reference Murray, Cheng, Clark, Barnett, Blackwell, Fletcher, Robbins, Bullmore and Jones2008) in addition to deficits in learning from negative feedback (Waltz et al. Reference Waltz, Frank, Robinson and Gold2007), learning categories in the Wisconsin Card Sorting Task (Prentice et al. Reference Prentice, Gold and Buchanan2008) and learning to select from the advantageous deck in the Iowa Gambling Task (Premkumar et al. Reference Premkumar, Fannon, Kuipers, Simmons, Frangou and Kumari2008b).
Another, perhaps more striking, feature of schizophrenia is that of poor social performance. The DSM-IV (APA, 1994) stipulates that some level of social or occupational dysfunction be present for a formal diagnosis of schizophrenia and social impairment in childhood and adolescence is associated with subsequent psychosis onset (Done et al. Reference Done, Crow, Johnstone and Sacker1994). Patients with schizophrenia are found to be impaired on various aspects of social cognition (Penn et al. Reference Penn, Sanna and Roberts2008), including a reliable deficit in explicit emotion perception (Feinberg et al. Reference Feinberg, Rifkin, Schaffer and Walker1986; Archer et al. Reference Archer, Hay and Young1994; Mueser et al. Reference Mueser, Doonan, Penn, Blanchard, Bellack, Nishith and DeLeon1996; Salem et al. Reference Salem, Kring and Kerr1996; Premkumar et al. Reference Premkumar, Cooke, Fannon, Peters, Michel, Aasen, Murray, Kuipers and Kumari2008a; Chen et al. Reference Chen, Norton, McBain, Ongur and Heckers2009; Meyer & Kurtz, Reference Meyer and Kurtz2009; Norton et al. Reference Norton, McBain, Holt, Ongur and Chen2009; Laroi et al. Reference Laroi, Fonteneau, Mourad and Raballo2010; Linden et al. Reference Linden, Jackson, Subramanian, Wolf, Green, Healy and Linden2010). Although several studies have reported some form of deficit in emotion perception, methodological shortcomings and task variability have led to inconsistencies (Edwards et al. Reference Edwards, Jackson and Pattison2002). Some authors have found impairments in the recognition of all emotions, including happiness and surprise (Archer et al. Reference Archer, Hay and Young1994; Schneider et al. Reference Schneider, Gur, Gur and Shtasel1995), whereas others have reported only difficulties with negative emotions, particularly fear (Mandal, Reference Mandal1987; Gaebel & Wolwer, Reference Gaebel and Wolwer1992), although this could simply reflect the fact that positive emotions are easier to recognize (Gosselin et al. Reference Gosselin, Kirouac and Dore1995).
The study reported here focused on how facial affect impacts on decision making. We have developed a task in which participants are asked to determine, through trial and error, which of two faces (one happy, one angry) is associated with a higher probability of reward. In healthy participants, the expressions biased decision making even when the emotional valence of the face stimuli was irrelevant to the task (Averbeck & Duchaine, Reference Averbeck and Duchaine2009). Given that previous work has shown that patients with schizophrenia have difficulty in some inference and associative learning tasks, and also in evaluating emotional expressions, we sought to clarify how social stimuli biased decision making in this population. Our hypotheses, not all of which were borne out by the data, were that patients with schizophrenia would (a) recognize clear explicit expressions of happiness and anger; (b) learn successfully the expectancies associated with the more rewarding faces, responding to implicit expressions; (c) demonstrate a differential response to negative feedback, associated with poorer learning; and (d) demonstrate a differential response to happy versus angry faces, associated with increased aversion to negative emotion.
Method
Participants
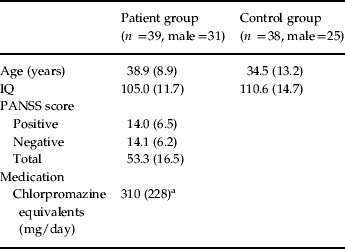
We recruited 39 individuals (31 males) who met the DSM-IV criteria for schizophrenia from the out-patient department of the South London and Maudsley National Health Service (NHS) Trust. Patients were stable on treatment with antipsychotic medication (Table 1); those with dual diagnoses and drug and alcohol problems were excluded from the study. A control group (n=38, 25 males) matched on age (t 75=1.8, p =0.088) and IQ (t 75=1.7, p =0.070) was also recruited. Patients underwent a Positive and Negative Syndrome Scale (PANSS) diagnostic interview on the day of testing (Table 1).
Table 1. Participant demographic information
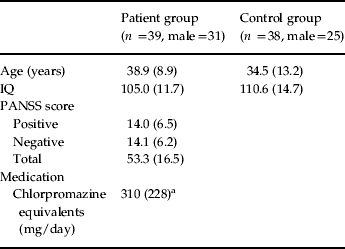
PANSS, Positive and Negative Syndrome Scale.
Values given as mean (standard deviation).
a These data were not available for five participants.
Task
We used a stochastically rewarded learning task using emotional faces as stimuli. The aim of the task was for the participant to ‘win’ on as many trials as possible, that is to determine through trial and error which face had a higher chance of being rewarded and select that face as many times as possible. On each trial, two faces were presented on a screen (Fig. 1). These faces had the same identity but differed in terms of facial expression: one was angry, the other happy. Each expression appeared pseudo-randomly on either the left or the right of the screen in each trial. The participants selected one of the faces using the keyboard and they were then told whether they had won or lost on that trial. Feedback followed participant choice and was accompanied by the face that was chosen. A ‘win’ corresponded to 10p being added to the total winnings, a ‘loss’ meant that nothing was added, although they were explicitly given the feedback ‘You lose’ (Fig. 1). Participants were told that the amount they were paid depended in part on the amount of money they accrued. However, in reality they were all paid the same amount. The financial incentive helped to ensure that participants engaged with the task.

Fig. 1. On each trial, participants were asked to choose between two faces: one angry, one happy. A subsequent feedback screen informed them whether they had won or lost on that trial. The faces are rewarded differentially. The aim was to determine which face had a higher probability of leading to a ‘win’.
Each face was rewarded in each block according to a predetermined probability. The participant was told that one face in each block would be rewarded more often than the other but not given any further information (in fact, one face was rewarded at 40%, the other 60%). The probabilities assigned to each face remained constant throughout each block, which contained 26 trials. At the end of each block the probabilities were reassigned and the participant was informed when this happened and given a short break.
Two different identities were also used and these alternated across blocks: identity 1, identity 2, identity 1, identity 2. The reward associations were counterbalanced across blocks such that the identity 1 happy face was most often rewarded in one block and the identity 1 angry face was most often rewarded in one block and the same for identity 2. The order of happy versus angry faces being rewarded was balanced as much as possible across participants, such that happy faces were more often rewarded following angry faces as often as angry faces were more often rewarded following happy faces.
Before performing the task, a subset of participants was tested on a control task, in which they were asked to determine whether a face shown to them was happy or angry. The faces used in the main task were included in this series. This was to ensure that participants could perceive and differentiate the stimuli. All faces used in the study were male, and drawn from the Ekman set (Ekman & Friesen, Reference Ekman and Friesen1971).
Data analysis
At the beginning of each block of trials the participants were told that the probabilities had been reassigned and they should try to work out by trial and error which face was best. Thus, at the beginning of the block the participants had no evidence about which face was best and they had to begin selecting one or the other face, registering the feedback, and trying to work out which face was best. Although one face was rewarded more often than the other, the probabilities used were 0.6 and 0.4 and as such the task was challenging. It was even possible that, over short intervals, the face that had a lower probability of being rewarded would be rewarded more often than the other. Therefore, we referenced the participants' behaviour to an ideal observer model that estimated, based on all of the feedback received up to the current trial in the block, which face was best. By comparing each participant's choices to the ideal observer trial by trial, the fraction correct could be derived, as a baseline estimate of performance.
As the outcome in each trial was either win or lose, the ideal observer was based upon a binomial model. The likelihood that the rewards were being generated probabilistically by an underlying probability θi is given by:
where D is the observed series of outcomes, θi is the probability that face i (angry or happy) is rewarded, r i is the number of times face i was rewarded, and N i is the number of times face i was selected. This equation provided us with the distributions over reward probabilities for each face. Specifically, as the participants did not know the underlying probabilities, they would infer a distribution of possible probabilities, given the reward outcomes. For example, if seven heads are observed in 10 coin tosses, it would be possible that the coin was fair (i.e. p=0.5 of heads versus tails), but it would also be possible, in fact more likely given the outcomes, that the coin was unfair and had a probability of heads equal to 0.7. Equation (1) would give the complete distribution over the probabilities for some set of outcomes.
To make a decision, the participants had to assess which face was better. We operationalized this decision step by assuming that participants would compute the probability that face i was more often rewarded than face j. This was given by:
The integral is over the posterior. For the ideal observer the prior was flat, and as such, the posterior is just the normalized likelihood. As a decision rule, this probability was thresholded at chance. This gives the ‘ideal choice’ (![]() ).
).
For the analyses that compared the decision of the participant to the ‘choice’ of the ideal observer, the case of p(θi>θj)=0.5 was handled by incrementing both the happy and the angry choices of the ideal observer by 0.5.
For details of the parameterized Bayesian learning model, see Supplementary online material.
Results
We carried out a control task in a subset of patients (n=20) and controls (n=20) and found that they all showed 100% accuracy in being able to discriminate between the angry and happy faces we used in this study. In the first analyses of the decision-making task, behaviour was referenced to an ‘ideal observer’ model (see Method section). It was not possible to ensure that participants were rewarded a fixed number of times for each face in each block because we could not control which face they picked. The ideal observer, however, allowed us to indicate what the participants should do, while allowing the participants unconstrained choice behaviour, which is more natural. Thus, the ideal observer allowed us (a) to check whether participants were able to do the task, as the ideal observer provided an estimate of whether the participants were learning from the feedback, and (b) to see whether participant choices showed a preference for the happy or angry expression. Percent correct was given by the percentage of responses that agreed with that of the ideal observer.
In general, this is a difficult learning task as the reward probabilities we used in each block were 60% and 40% for the most and least often rewarded face respectively. Analysed group-wise, patients and controls performed significantly above chance (50%) on the task (patients: t 38=3.89, p<0.001; controls: t 37=6.35, p<0.001). Patient choices concurred with the ideal observer 57% of the time and control choices concurred 62% of the time (Fig. 2a). The difference in performance between patients and controls approached but did not achieve significance (t 75=1.84, p=0.070). Analysed individually, 44% of patients (p<0.05, χ2 on choices of individual participant) and 53% of controls (p<0.05, χ2) were above chance. These proportions were, however, not significantly different (z=0.549, p =0.583). Furthermore, both patients and controls showed a significant preference for the happy face. The average difference between choosing the happy face when they should have chosen the angry face [p(participant=happy|model=angry)] and choosing the angry face when they should have chosen the happy face [p(participant=angry|model=happy)] was 0.17 for both groups (patients: t 38=4.79, p<0.001; controls: t 37=4.49, p<0.001). That is, when responses differed from the predictions of the ideal observer, there was a significantly higher probability of participants choosing happy when they should have chosen angry, compared to choosing angry when they should have chosen happy. There was, however, no significant difference in the overall preference between patients and controls (t 75=1.31, p=0.195), when averaged across conditions in this way. Thus, both groups learned from the feedback, although this was not the case for all individual participants, and both groups showed a preference for the happy face.

Fig. 2. Between-group differences in learning and face preferences. (a) Fraction correct, referenced to the ideal observer when choosing between happy and angry faces in drug and placebo conditions. Error bars are ±1 standard error of the mean (s.e.m.). (b) Difference in learning from positive and negative feedback. Error bars are ±1 s.e.m. Pos (positive) is parameter α from equation (4) in the Supplementary material. Neg (negative) is parameter β from equation (5) in the Supplementary material. (c) Evidence versus choice probability curves. The fraction of times the participants picked the happy face gives the evidence for the happy face, under drug and placebo conditions. The evidence comes from the ideal observer model, and is given by p(θhappy >θangry) from equation (2).
In the next analysis we split the total learning in the task into the amount learned from positive and negative feedback. This is a more detailed analysis, and previous studies have suggested that striatal dopamine levels may affect the relative amount learned from positive and negative feedback (Frank et al. Reference Frank, Seeberger and O'Reilly2004; Pessiglione et al. Reference Pessiglione, Seymour, Flandin, Dolan and Frith2006; Waltz et al. Reference Waltz, Frank, Robinson and Gold2007; Cools et al. Reference Cools, Frank, Gibbs, Miyakawa, Jagust and D'Esposito2009). Thus, given the dopamine hypothesis in schizophrenia (Weinberger, Reference Weinberger1987; Davis et al. Reference Davis, Kahn, Ko and Davidson1991; Laruelle et al. Reference Laruelle, Abi-Dargham, van Dyck, Gil, D'Souza, Erdos, McCance, Rosenblatt, Fingado, Zoghbi, Baldwin, Seibyl, Krystal, Charney and Innis1996; Abi-Dargham et al. Reference Abi-Dargham, Gil, Krystal, Baldwin, Seibyl, Bowers, van Dyck, Charney, Innis and Laruelle1998), we wanted to examine group differences in these parameters. We used a Bayesian reinforcement learning model (see Supplementary material) to estimate the amount that individual participants learned from positive and negative feedback (Fig. 2 b). When we compared this across groups using a mixed-effects ANOVA, however, we found that there was no main effect of group [F(1, 75)=0.01, p=0.941] and no interaction of group and valence [i.e. positive (α) versus negative (β); F(1, 75)=1.35, p=0.249]. There was a main effect of valence, across groups [F(1, 75)=7.88, p=0.006], attributable to consistent task strategies between groups.
Next, we examined the evidence versus choice probability curves, which characterize how often participants picked each of the faces, as a function of the strength of the evidence supporting each face. For example, if a participant had picked the happy face four times and won three times and picked the angry face four times and won three times, the evidence would be equivocal (feedback evidence for happy=0.5). However, if the participant had received proportionally more positive feedback for the angry face than the happy face, the feedback evidence would favour the angry face, and vice versa. We found that both groups showed the preference towards the happy face revealed by the analysis above (Fig. 2 c), indicated by the shift of the evidence versus choice probability curves up and to the left. However, the choice aversion to the angry face was stronger in the patients than the controls, evidenced by the separation of the curves in the left half of the plot, when the evidence strongly favoured the angry face. Bin-by-bin t tests confirmed this difference (Fig. 2 c: horizontal line at y=0.15). These t tests are subject to the multiple comparisons criticism, but the results are robust when examined using a regression-based approach, which avoids this criticism. To evaluate this effect while avoiding the problem of multiple comparisons, we fit the following regression to the evidence–choice probability data, participant by participant in both groups:
This analysis gives us four parameters (a 0, a 1, a 2, a 3) for each participant. Across participants within a group we can then carry out t tests on each individual parameter distribution to see if it is significant within that group. In other words, we get 39 values of parameter a 0 for the patient group, and a t test on that distribution tells us if that parameter is significantly positive or negative within that group. We found that the second- and third-order terms were not significant in either group so we refit the equations with only the intercept (a 0) and linear (a 1) terms. Both of these terms were significant in both groups (patients: a 0: t 38=11.8, p<0.001; a 1: t 38=3.1, p=0.003; controls: a 0: t 37=8.1, p<0.001; a 1: t 37=6.9, p<0.001). We then carried out t tests on the parameter distributions between groups to assess significance. This is equivalent to a random effects model (Holmes & Friston, Reference Holmes and Friston1998) and thus we only need to carry out two t tests to determine significance. We found that both the intercept and linear terms were significantly different between groups (a 0: t 75=2.81, p=0.006; a 1: t 75=2.46, p=0.016). Both of these would remain significant if corrected for the two comparisons using Bonferroni correction. Importantly, the significant intercept effect shows that, when the evidence strongly supports the angry face (feedback evidence happy=0), control participants selected the angry face significantly more often. Incidentally, both the intercept and linear terms were also significantly different between groups when we included the second- and third-order terms (data not shown). The change in the slope reflects the overall decrease in performance of the participants (Fig. 2 a). When assessed as the slope of the evidence versus choice probability curve, there is a significant difference between groups.
To further characterize this effect, we examined whether or not patients and controls were able to identify the best face at above-chance levels when the evidence either strongly supported the happy face (feedback evidence for happy=1) or strongly supported the angry face (feedback evidence for happy=0). When the evidence strongly supported the happy face, both groups were above chance (patients: t 38=5.3, p<0.001; controls: t 37=7.8, p<0.001). However, when the evidence strongly supported the angry face, only the controls were significantly above chance (patients: t 38=1.0, p=0.329; controls: t 37=5, p<0.001). Thus, patients were able to pick the happy face as better when the evidence supported it, but not the angry face.
Patients with schizophrenia have been shown to have reversal learning deficits (Waltz & Gold, Reference Waltz and Gold2007). However, we were not specifically interested in studying those deficits, so the task was designed to minimize those factors, using explicit breaks between blocks, and onscreen instructions to the participants during the break between blocks that probabilities were being reassigned randomly. Furthermore, two identities were used and they were always presented in interleaved blocks I1, I2, I1, I2. There were no effects of carryover within either group (see Supplementary material online).
Next, we carried out a series of control analyses to assess correlations between PANSS scores, medication levels and our dependent behavioural variables, specifically the regression parameters for the face preference effect (a 0 or a 1) and the parameters assessing learning from positive and negative feedback (α and β from equations (4) and (5), shown in the Supplementary material online, plotted as positive and negative in Fig. 2 b). There were no significant correlations between any of the three PANSS scores (positive, negative and general) and the two parameters from the regression (a 0 or a 1), with all p>0.05. We also examined correlations between a combined delusion score (delusions, hallucinations, suspiciousness and hostility) and the regression parameters, but there were no significant correlations (p>0.05). There was a significant correlation between the general symptom score and learning from positive feedback (α, r 38=0.36, p=0.04) but the rest of the correlations were non-significant. There was a significant correlation between medication levels measured in chlorpromazine equivalent units and both parameters from the regression (a 0: r 38=0.36, p=0.047 and a 1: r 38=−0.40, p=0.025), but no correlation between medication levels and learning from positive and negative feedback.
As we found a correlation between medication levels and the regression parameters, we included medication level as a covariate, along with overall percent correct and IQ. Even when these variables were partialled out of the regression, there was still a group difference in the intercept term (a 0, t 75=11.7, p<0.001). Finally, we ran an analysis to control for the effect of gender, as the proportions were not identical across groups. Gender was not a significant predictor of the intercept (a 0) across groups [F(1, 73)=0.39, p=0.534], nor was there a significant interaction between group and sex [F(1, 73)=0, p=0.979], and the effect of groups was still significant [F(1, 73)=5.51, p=0.022]. Thus, this effect survives correction for possible confounding factors.
Discussion
We tested patients with schizophrenia and healthy controls on a stochastically rewarded decision-making task using emotional faces as stimuli. Both patients and controls were able to recognize faces explicitly and showed a significant preference for selecting the happy face, a preference that has been documented previously in an independent group of control participants (Averbeck & Duchaine, Reference Averbeck and Duchaine2009). Assessed as overall percent correct, we found that patients could determine which face was being most often rewarded as well as control participants, with a trend towards a deficit. The slope of the regression line from the evidence versus choice probability curves was, however, significantly different between groups. Of importance in the present study, the choice behaviour of the patients was significantly more averse to the angry faces than that of the healthy controls, such that when the feedback evidence strongly supported the angry face as the best choice, patients chose it significantly less often, and in fact patients performed at chance in this condition. This difference did not exist for happy faces, however, as patients were able to perform at above-chance levels when the happy face was more often rewarded. Thus, patients and controls learn to associate rewards to happy and angry faces differently.
Although excessive striatal dopamine has been implicated consistently in schizophrenia (Laruelle et al. Reference Laruelle, Abi-Dargham, van Dyck, Gil, D'Souza, Erdos, McCance, Rosenblatt, Fingado, Zoghbi, Baldwin, Seibyl, Krystal, Charney and Innis1996; Breier et al. Reference Breier, Su, Saunders, Carson, Kolachana, de Bartolomeis, Weinberger, Weisenfeld, Malhotra, Eckelman and Pickar1997; Abi-Dargham et al. Reference Abi-Dargham, Gil, Krystal, Baldwin, Seibyl, Bowers, van Dyck, Charney, Innis and Laruelle1998; Bortolozzi et al. Reference Bortolozzi, Díaz-Mataix, Artigas, Buschmann, Diaz, Holenz, Parraga, Torrens and Vela2007) and modelling and experimental work has suggested that striatal dopamine levels are related to differences in learning from positive and negative feedback (Frank et al. Reference Frank, Seeberger and O'Reilly2004, Reference Frank, Moustafa, Haughey, Curran and Hutchison2007; Cools et al. Reference Cools, Altamirano and D'Esposito2006, Reference Cools, Frank, Gibbs, Miyakawa, Jagust and D'Esposito2009; Pessiglione et al. Reference Pessiglione, Seymour, Flandin, Dolan and Frith2006; Waltz et al. Reference Waltz, Frank, Robinson and Gold2007; Rutledge et al. Reference Rutledge, Lazzaro, Lau, Myers, Gluck and Glimcher2009; Djamshidian et al. Reference Djamshidian, Jha, O'Sullivan, Silveira-Moriyama, Jacobson, Brown, Lees and Averbeck2010; Voon et al. Reference Voon, Reynolds, Brezing, Gallea, Skaljic, Ekanayake, Fernandez, Potenza, Dolan and Hallett2010), we did not find strong differences between patients and controls in this aspect of the task. In the study of Waltz et al. (Reference Waltz, Frank, Robinson and Gold2007), patients slightly outperformed controls in initial acquisition of the most difficult 60/40 split, which used reward contingencies equivalent to ours. Consistent with this, it has been reported that patients have intact initial acquisition of preference for rewarded stimuli (Herbener, Reference Herbener2009). Some authors have reported that patients with schizophrenia show deficits specifically when reward contingencies are reversed (Waltz & Gold, Reference Waltz and Gold2007), and other authors have shown deficits in first-episode patients specifically during initial acquisition but not in all forms of reversal learning (Murray et al. Reference Murray, Cheng, Clark, Barnett, Blackwell, Fletcher, Robbins, Bullmore and Jones2008). Others have suggested that deficits, at least in the Wisconsin Card Sorting Task, which uses deterministic and not stochastic feedback, are in initial acquisition and not reversal (Prentice et al. Reference Prentice, Gold and Buchanan2008). Our results show relatively intact acquisition of associations in the patient group, with most of the deficit in learning being driven by an increased aversion to the angry faces. Finally, with respect to this finding it is worth noting that the reward prediction error hypothesis of dopamine (Schultz, Reference Schultz2002) does not predict differences between initial acquisition and reversals. Such differences are often seen, however, which suggests that there is much about this process that is still not understood.
Although studies have documented deficits in processing emotional expressions in patients (Feinberg et al. Reference Feinberg, Rifkin, Schaffer and Walker1986; Archer et al. Reference Archer, Hay and Young1994; Mueser et al. Reference Mueser, Doonan, Penn, Blanchard, Bellack, Nishith and DeLeon1996; Salem et al. Reference Salem, Kring and Kerr1996; Chen et al. Reference Chen, Norton, McBain, Ongur and Heckers2009; Meyer & Kurtz, Reference Meyer and Kurtz2009; Norton et al. Reference Norton, McBain, Holt, Ongur and Chen2009; Laroi et al. Reference Laroi, Fonteneau, Mourad and Raballo2010; Linden et al. Reference Linden, Jackson, Subramanian, Wolf, Green, Healy and Linden2010), including a specific misattribution of fearful faces as angry (Premkumar et al. Reference Premkumar, Cooke, Fannon, Peters, Michel, Aasen, Murray, Kuipers and Kumari2008a), our study shows that, under some conditions, patients are more sensitive to expressions than controls. Specifically, patients and controls both showed a statistically significant preference for the happy face. Patients, however, were significantly more averse to the angry face and thus patients seemed to be relatively more sensitive to the angry face. Other studies have reported emotion recognition effects that were specific to angry. This is consistent with work showing that positively valenced emotional material does not improve long-term memory in patients as it does in controls, but negatively valenced emotion material does (Herbener et al. Reference Herbener, Rosen, Khine and Sweeney2007). This is in contrast to work examining labelling of emotions, which has shown that there are deficits in identifying happy or angry faces (Archer et al. Reference Archer, Hay and Young1994; Schneider et al. Reference Schneider, Gur, Gur and Shtasel1995; Laroi et al. Reference Laroi, Fonteneau, Mourad and Raballo2010; Linden et al. Reference Linden, Jackson, Subramanian, Wolf, Green, Healy and Linden2010). Although it might be argued that the behavioural differences between patients and controls seen for angry faces in our task might be due to an underlying perceptual deficit, it should be noted that the same perceptual process is engaged independent of the evidence favouring happy or angry faces, and our control task showed that given a two-alternative forced choice decision, patients can reliably determine whether a face is happy or angry. The behavioural difference we see, however, is specific to cases in which participants have received reward evidence that the angry face is the choice that will most often win.
There are limitations to our study. First, we did not use an explicit punishment condition. Our loss condition used negative text, ‘You lose’, but in fact participants simply did not earn anything on those trials. It is possible that an explicit loss condition would have affected learning behaviour differentially, perhaps enhancing the negative feedback more specifically. However, our approach is consistent with previous work that used lack of reward as a negative outcome (Waltz et al. Reference Waltz, Frank, Robinson and Gold2007). Furthermore, care must be taken when losses are used explicitly, as they are not treated the same as gains (Kahneman et al. Reference Kahneman, Slovic and Tversky1982), and adding a condition to study losses would have extended the task considerably. In addition, as discussed earlier, patients were at chance performance when the evidence strongly supported the angry face. The choice behaviour in this case should have been driven by both a lack of reward for the happy face and reward assigned to the angry face. However, if patients simply could not assign reward to the angry face, the two faces may have seemed equally rewarding. This seems unlikely, however, as the patients do pick the angry face more often when it is being more often rewarded, as shown by the significant slope in the evidence versus choice probability curve, which suggests that they do incorporate reward information about the angry face.
Conclusions
We used a task that allowed us to examine the effects of financial feedback and emotional expressions simultaneously on choice behaviour and found that patients with schizophrenia showed a larger aversion to angry faces than control participants. As our task is not a simple perceptual task, but rather requires participants to engage in choice behaviour, it may engage brain processes that are closer to social behavioural deficits seen in patients. Additional studies linking this task with parallel social cognition tasks will be necessary to examine this hypothesis.
Note
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/psm).
Acknowledgements
This work was supported by a Welcome Trust Project Grant to B.B.A., a Higher Education Funding Council for England (HEFCE) Clinical Senior Lecturer Award to S.S.S. and Medical Research Council (MRC) funding within the UCL 3-year PhD in Neuroscience to S.E. This work was also supported in part by the Intramural Research Program of the National Institute of Mental Health (NIMH), National Institutes of Health (NIH).
Declaration of Interest
None.