Introduction
Cryptococcal meningitis is an infection of the cerebral meninges caused by Cryptococcus neoformans. C neoformans is typically found in the soil and pigeon faeces.Reference Hughes, Green, Alvarez and Reimer1 Infections occur via the inhalational route,Reference Chayakulkeeree and Perfect2 and may then spread haematogenously to the central nervous system and other extra-pulmonary sites.Reference Hughes, Green, Alvarez and Reimer1, Reference Lewis and Rabinovich3 Infections most commonly occur in association with underlying immunosuppression.Reference Day, Hoang, Duong, Hong, Diep and Campbell4 In an era with an increasing population of patients with immunosuppression associated with human immunodeficiency virus (HIV) infections, cytotoxic drugs and corticosteroids, cryptococcosis and other opportunistic infections are of greater significance.Reference Pukkila-Worely and Mylonakis5
Sensorineural hearing loss (SNHL) is a known complication of cryptococcal meningitis, occurring in between 27 and 30.8 per cent of affected individuals.Reference Hughes, Green, Alvarez and Reimer1, Reference Wang, Chang, Lui, Peng, Huang and Chang6 In this case report, we examined a patient who presented with bilateral, profound SNHL and right vestibular dysfunction as a result of cryptococcal meningitis, who subsequently displayed a marked recovery in hearing prior to the planned cochlear implant surgery.
Case report
A 52-year-old Chinese man presented with non-vertiginous giddiness and bilateral hearing loss. He had been suffering from headaches over the occipital region and double vision for two months. He had been admitted twice to another institution because of his non-specific symptoms, where he was diagnosed to have partial left abducens palsy; however, further investigations had failed to elucidate the cause of his symptoms. His past medical history included a pre-existing, right-sided, moderate to severe SNHL of unknown aetiology (Figure 1), hyperlipidaemia and hypertension.

Fig. 1 Audiogram conducted prior to presentation, demonstrating mild sensorineural hearing loss (SNHL) in the left ear and moderate to severe SNHL in the right ear. × = Air conduction (unmasked) left ear; > = bone conduction (unmasked) left ear; ○ = air conduction (unmasked) right ear; [=bone conduction (masked) right ear; △ = air conduction (masked) right ear
On examination, he was afebrile and non-toxic looking, with bilateral impaired abduction of his eyes. No other focal neurological deficits or signs of meningism were present. Further investigations revealed a mildly elevated white cell count (10.4 × 109/l), with 78.1 per cent neutrophils and 11.3 per cent lymphocytes. The erythrocyte sedimentation rate was raised, at 15 mm/hour, and serum procalcitonin levels were normal (0.03 ug/l). Both magnetic resonance imaging (MRI) and computed tomography (CT) scans revealed mild hyper-intensity in the bilateral temporal and parieto-occipital sulci, suggesting meningitis. No other abnormalities were noted. In addition, the tests for HIV, syphilis and tuberculosis were all negative. A lumbar puncture revealed a raised protein count (1 g/l), low glucose (1.4 mmol/l) and 119 nucleated cells/ul, with 57 per cent lymphocytes. Cryptococcus antigen was positive, with a titre of 1:512. An Indian ink stain showed C neoformans.
The patient completed a 16-day course of intravenous amphotericin B and a 15-day course of flucytosine, with good clinical response. This was subsequently switched to a four-week course of fluconazole.
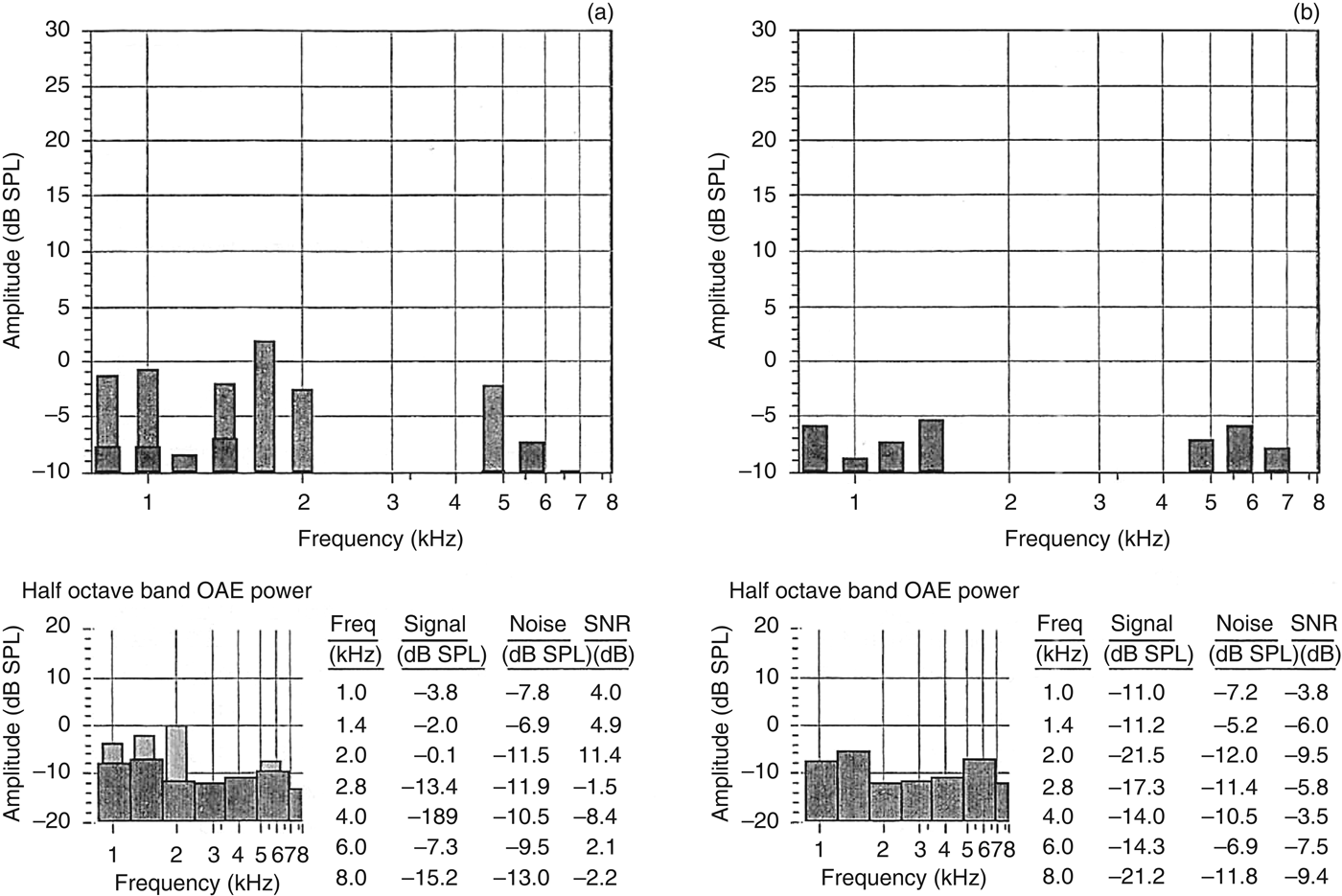
Given his acute medical issues, such as raised intracranial pressure and malignant hypertension, audiological assessment could only be conducted one month after admission. The initial pure tone audiometry showed severe SNHL in the right ear and profound SNHL in the left ear (Figure 2). His unaided and aided speech discrimination scores, performed with monosyllabic Mandarin words, were 0 per cent bilaterally. Both distortion product otoacoustic emissions (Figure 3) and transient evoked otoacoustic emissions (Figure 4) were present, with reduced amplitudes up to 2–4 kHz in the left ear and absent responses in the right ear. Click-evoked auditory brainstem response testing showed absent responses in both ears at 80 dB nHL presented at a rate of 11.1 pulses per second. The patient was scheduled for a cochlear implant candidacy evaluation because of the poor benefit with a conventional hearing aid.

Fig. 2 Initial pure tone audiometry results, showing profound sensorineural hearing loss (SNHL) in the left ear and moderate to severe SNHL in the right ear. ↘ = No response in left ear; ↙ = no response in right ear; ○ = air conduction (unmasked) right ear; × = air conduction (unmasked) left ear

Fig. 3 Distortion product otoacoustic emission (DPOAE) results for: (a) left ear, showing reduced amplitudes up to 2 kHz, and (b) right ear, for which DPOAEs are absent. OAE = otoacoustic emission; Freq = frequency; SNR = signal-to-noise ratio

Fig. 4 Transient evoked otoacoustic emission (TEOAE) results for: (a) left ear, showing reduced amplitudes up to 2 kHz, with absent TEOAEs for higher frequencies, and (b) right ear, for which TEOAEs are absent. OAE = otoacoustic emission; Freq = frequency; SNR = signal-to-noise ratio
Subsequent pure tone audiometry, conducted during the second appointment, revealed an average of 10–20 dB hearing improvement in the right ear, and an average of 15–35 dB, with inconsistent responses, in the left ear. Furthermore, testing of the left ear showed SNHL with a reverse sloping pattern to a profound degree up to 1 kHz, and moderately severe to severe hearing loss for the high frequencies (Figure 5). Aided word recognition scores for Mandarin monosyllabic words improved significantly for the right ear (Table I). However, performance for the left ear remained poor (these scores are not included in this report).

Fig. 5 Pure tone audiometry results at one month, showing a reverse sloping pattern with severe hearing loss for low frequencies and moderate to severe hearing loss for higher frequencies in the left ear, and moderate to severe hearing loss in the right ear. ○ = Air conduction (unmasked) right ear; < = bone conduction (unmasked) right ear; ] = bone conduction (masked) left ear; ↘ = No response in left ear; × = air conduction (unmasked) left ear; > = bone conduction (unmasked) left ear
Table I Right ear aided speech discrimination scores at one month

SNR = signal-to-noise ratio
Of note, an MRI scan with intravenous contrast (performed two months after the onset of hearing impairment) revealed that both vestibulocochlear nerves were of normal configuration. There was no abnormal enhancement of the labyrinth, and the T2-weighted signal within the cochlear and labyrinth was preserved bilaterally. Hence, there was no evidence of intracochlear fibrosis.
In view of the patient's balance problems, videonystagmography was performed to evaluate vestibular function. The results revealed significant spontaneous left-beating nystagmus (slow phase velocity was 7 degrees per second) without optic fixation, and with weak caloric responses in the right ear, suggesting right vestibular hypofunction.
As the patient showed significant fluctuations in hearing in the left ear and good aided benefits in the right ear (Table I), we decided to postpone cochlear implantation and to closely monitor hearing thresholds instead.
At 11 months after the first presentation, the patient showed moderate SNHL in the right ear (Figure 6), similar to his baseline hearing thresholds. However, his left ear hearing thresholds had improved significantly to between 15 and 35 dB, with a dip to 65 dB at 2 kHz. The patient was coping well and even declined the use of a hearing aid.

Fig. 6 Pure tone audiometry results at 11 months after the first presentation, showing moderate hearing loss in the left ear and hearing that had returned to baseline in the right ear. × = Air conduction (unmasked) left ear; > = bone conduction (unmasked) left ear; ○ = air conduction (unmasked) right ear; [ = bone conduction (masked) right ear; △ = air conduction (masked) right ear
Discussion
Various mechanisms, such as invasion of the temporal bone, destruction of the spiral ganglion cells and cochlear nerve fibres, and cryptococcal meningeal infiltration with arachnoiditis, have been shown to cause SNHL in cryptococcal meningitis.Reference Wang, Chang, Lui, Peng, Huang and Chang6, Reference Moberly, Naumann and Cordes7 The pattern of hearing loss may vary from mild to profound in severity, and may present unilaterally or bilaterally.Reference Hughes, Green, Alvarez and Reimer1, Reference Wang, Chang, Lui, Peng, Huang and Chang6–Reference Maslan, Graham and Flood9 In the case reported, the damage was more severe in the left ear compared to the right ear. In addition, the presence of otoacoustic emissions with reduced amplitudes and an absent auditory brainstem response suggests that the lesion was more pronounced in the inner hair cells, spiral ganglion cells or the auditory nerve itself, rather than in the outer hair cells. Although the damage was more severe in the left ear, it showed a significant improvement, with hearing levels even recovering to baseline levels of below 1.5 kHz. This suggests that the presence of otoacoustic emissions may be a good predictor of hearing recovery,Reference Mori, Suzuki, Hiraki, Hashida, Ohbuchi and Katoh10 and highlights the need for their inclusion in hearing assessments, especially in patients with SNHL following cryptococcal meningitis.
However, no conclusive evidence may be used to predict the clinical course of the hearing loss, as patients have shown varying results according to the literature. Indeed, several authors have described varying degrees of recovery in patients with hearing loss post-cryptococcal meningitis.Reference Hughes, Green, Alvarez and Reimer1, Reference Wang, Chang, Lui, Peng, Huang and Chang6, Reference Mayer, Chevalier, Albert, Casteran, Boutin and Ponsot11 In a case series reported by Wang et al., three of seven survivors had shown improvement in hearing after a three-year follow-up period.Reference Wang, Chang, Lui, Peng, Huang and Chang6 The authors suggested that risk factors for SNHL include a high cryptococcal antigen titre (more than 1:1024), concomitant visual impairment and the presence of meningeal enhancement on MRI studies. However, none of these studies have reported on the degree of improvement in hearing amongst their patients.
Temporal bone studies have shown varying patterns of vestibulocochlear nerve involvement and end organ damage.Reference Low8, Reference Igarishi, Weber, Alford, Coats and Jerger12 However, many histopathological studies have implicated the cochlear nerve, with varying involvement of the vestibular component.Reference Igarishi, Weber, Alford, Coats and Jerger12–Reference Harada, Sando and Myers14 Consequently, most would agree that this retrocochlear damage may limit the efficacy of a cochlear implant.Reference Low8 This is in contrast to patients with bacterial meningitis in whom retrocochlear involvement is limited.Reference Wang, Chang, Lui, Peng, Huang and Chang6
• Hearing loss is a recognised complication of cryptococcal meningitis
• Hearing loss in cryptococcal meningitis is variable, with potential reversibility and greater retrocochlear involvement than in bacterial meningitis
• Cryptococcal meningitis patients have not been shown to develop labyrinthitis ossificans
• Hence, cochlear implantation is less urgent, and, perhaps, a more conservative approach (close monitoring of hearing thresholds) may be adopted
• Otoacoustic emissions may be a good predictor of hearing recovery and should be included in audiometric assessment
The literature regarding cochlear implantation post-cryptococcal meningitis remains limited. In bacterial meningitis, there is typically some urgency for cochlear implantation because of the risk of labyrinthitis ossificans, which may be present as early as two months post-meningitis.Reference Caye-Thomson, Dam, Omland and Mantoni15 However, this case report illustrates that, unlike in bacterial meningitis, perhaps there is room for a more conservative approach initially, such as close monitoring of hearing thresholds in the immediate and short-term post-meningitis period. Furthermore, cryptococcal meningitis predominantly occurs in immune-compromised hosts,Reference Day, Hoang, Duong, Hong, Diep and Campbell4, Reference Pukkila-Worely and Mylonakis5 though it may occasionally affect immune-competent hosts.Reference Day, Hoang, Duong, Hong, Diep and Campbell4 Co-existing co-morbidities may limit the value of cochlear implantation.
Conclusion
Sensorineural hearing loss is a known complication of cryptococcal meningitis. However, because of its unique features of potential reversibility (i.e. hearing recovery following resolution of infection) and retrocochlear involvement, hearing loss associated with cryptococcal meningitis should be managed more conservatively than hearing loss associated with bacterial meningitis. In addition, otoacoustic emissions testing may be used as a predictor of hearing recovery.