Introduction
Successful cryopreservation of mammalian oocytes is quite difficult because of the high volume to surface ratio, chilling injury, alterations of the zona pellucida and cumulus cell interactions (Vajta, Reference Vajta1997). In addition to the large size of the oocyte, water content and high degree of cytoplasmic specialization are considered as obstacles in the way of safe oocyte vitrification (Nottola et al., Reference Nottola, Coticchio and Sciajno2009).
Different freezing methods, cryoprotectant concentrations, oocyte maturity and the specific biological structure of mammalian oocytes make their cryopreservation difficult (Arav et al., Reference Arav, Shehu and Mattioli1993; Asada et al., 2000). Moreover, the cell cycle stage during meiosis may influence both survival and cryopreservation results due to a varying sensitivity to the cooling procedure. Accordingly, oocyte cryopreservation could be performed at two meiotic stages: germinal vesicle (GV) and metaphase II (MII). As the genetic material in GV oocytes remains confined within the nucleus and protected by a membrane, it is probably less sensitive to cooling when compared with MII (Tucker et al., Reference Tucker, Morton, Wright and Massey1998; Ghelter et al., Reference Ghelter, Skutelsky and Nun2006; Bogliolo et al., Reference Bogliolo, Ariu and Fois2007). Unfortunately the broad difference in size between the oocyte and its associated cumulus cells in COCs yields distinct responses to stress during cryopreservation (Ruppert-Lingham et al., Reference Ruppert-Lingham, Paynter and Godfrey2003). However, the survivability of immature oocytes, as well as their latter maturation and development are significantly impaired after cryopreservation in comparison with fresh ones (Bogliolo et al., Reference Bogliolo, Ariu and Fois2007).
Different lines of evidence have demonstrated that GV-stage oocytes that have been stripped of cumulus cells show a lack of coordination between nuclear and cytoplasmic maturation (Goud et al., Reference Goud, Goud and Qian2000; Combelles et al., Reference Combelles, Cekleniak, Racowsky and Albertini2002) and undergo a lower development compared with cumulus-enclosed oocytes (Wongsrikeao et al., Reference Wongsrikeao, Kanashige and Ooki2005), thus gap functional coupling between oocytes and cumulus cells plays an important role in the maturation process (Gilchrist et al., Reference Gilchrist, Ritter and Armstrong2004; Li et al., Reference Li, Bunch and White2006). Accordingly, the success of immature oocyte cryopreservation could depend on the ability to preserve the structural and functional integrity of a COC as a whole.
It is obvious that oocyte quality determination by phase contrast microscopy is not an adequate measure of oocyte fertilization potential and developmental competence (Swain & Pool, Reference Swaine and Pool2008), so evaluation of the cryopreservation effects on oocyte quality by using transmission electron microscopy (TEM) seems essential and could be effective. TEM allows observation of the fine details of cell microanatomy and suprisingly it has not been used in the cryopreservation field very much. This inattention exists even though structural damage is one of the most likely adverse events associated with cryopreservation and its concomitant osmotic stress (Coticchio et al., Reference Coticchio, Borini and Distratis2010).
For the ultrastructural aspect, a constant association between mitochondria and smooth endoplasmic reticulum (SER) is observed at all stages of follicular development in most mammalian oocytes (Cran et al., Reference Cran, Hay and Hay1980) and this aggregation serves different functions such as: providing a rich pool of energy reserves, the production/secretion of substances (nutrients, growth factors) useful for fertilization, and rapid neogenesis of plasma and nuclear membranes during the first embryo cleavages (Motta et al., Reference Motta, Nottola and Pereda1995, Reference Motta, Nottola, Makabe and Heyn2000, Reference Motta, Nottola and Familiari2003). Also, mitochondria–SER aggregates may regulate local concentrations of free calcium and ATP production, thus acting on different cellular calcium including mediation of calcium-dependent signal transduction pathways at fertilization (Makabe et al., Reference Makabe, Van Blerkom and Nottola2006). In sheep, hooded mitochondria appear during late estrous (Russe, Reference Russe1975) and clearly increase their surface area. They may also provide a specific microenvironment to facilitate the exchange of metabolic intermediates with the endoplasmic reticulum (Fleming & Saake, Reference Fleming and Saake1972; Cran et al., Reference Cran, Hay and Hay1980). It has been demonstrated that cryopreservation affects the pericortical distribution of mitochondria and smooth endoplasmic reticulum. These organelles are associated with the cytoskeleton and cryopreservation might cause their displacement, by directly affecting the associated cytoskeleton elements or merely through physiological forces that break their links with the cytoskeleton (Gualtieri et al., Reference Gualtieri, Iaccarino and Mollo2009). Swelling of the smooth endoplasmic reticulum elements might also be observed after cryopreservation. Such damage is markedly higher if mitochondria are also injured (Kline & Kline, Reference Kline and Kline1992; Whitaker, Reference Whitaker2006).
Another ultrastructural characteristic of ovine oocytes is the presence of numerous large vesicles. In sheep, the number of vesicles increases during the early stage of follicular development. Information regarding their chemical nature or their derivation is lacking. Small vesicular elements of endoplasmic reticulum are often observed among them and profiles suggestive of fusion or budding of the large vesicles are also noted. However, no clear intermediates between endoplasmic reticulum and the vesicles have been observed (Cran et al., Reference Cran, Hay and Hay1980). The presence of vacuoles after vitrification can be considered as a non-specific feature commonly found in cells that are responding to an injury (Ghadially, Reference Ghadially1982) and is probably the most apparent characteristic in human oocytes when routinely examined by microscopy (Ebner et al., Reference Ebner, Moser and Sommergruber2005). Oocyte vacuolization causes fertilization failure (both IVF and ICSI) and rare cleavage (Ebner et al., Reference Ebner, Moser and Sommergruber2005).
Generally, limited numbers of studies have focused on the effects of low temperatures on the oocyte ultrastructure, and little is known about ultrastructural modifications of sheep oocytes following different methods of vitrification. As a result, the object of this study was to evaluate the effects of three different vitrification methods on the ultrastructural changes of sheep COCs.
Materials and methods
Collection of immature oocytes
Sheep ovaries were transported in phosphate-buffered saline (PBS) supplemented with gentamycin (0.5%), streptomycin (0.05 mg/ml) and penicillin (0.06 mg/ml) at 35–37 °C within 2–4 h from the slaughter house. COCs were aspirated from antral follicles (2–6 mm in diameter) with a 19-gauge needle fitted to a 5 ml syringe. After aspiration, COCs were washed three times in HEPES-buffered TCM199 (Tissue Culture Medium 199, Sigma) supplemented with 10% fetal bovine serum (FBS, HyClone). The mean diameter of good isolated COCs (fine homogenous granular ooplasm surrounded with compact layers of cumulus cells) was evaluated (Ebrahimi et al., Reference Ebrahimi, Valojerdi and Yazdi2010). These COCs were chosen for the experiment and randomly divided into four groups: control, conventional straw vitrification, cryotop vitrification, and solid surface vitrification.
Vitrification procedure
COCs in each of the vitrification groups were vitrified by one of three different methods: conventional straws, cryotop and solid surface. Equilibration and vitrification were performed at room temperature (25–27 °C). COCs were first equilibrated for 15 min in a solution of 7.5% ethylene glycol (EG, Sigma), 7.5% dimethyl sulphoxide (DMSO, Sigma) and 20% FBS in α-minimal essential medium (MEM) (Gibco), then immersed in a vitrification medium for 1 min. The vitrification medium consisted of 15% EG, 15% DMSO, 0.5 M sucrose and 20% FBS in α-MEM. Thereafter, in the conventional group, 10 COCs were loaded into conventional straws (0.25 ml) with about 2 cm of vitrification medium around the samples as described by Chen et al. (Reference Chen, Lien and Chen2001); whereas in the cryotop groups, five COCs were loaded on a cryotop tip (Kitazato, Japan; Kuwayama & Kato, Reference Kuwayama and Kato2000) with the minimum amount of vitrification medium as possible (about 1 μl). In the conventional and cryotop groups, devices were plunged directly in liquid nitrogen (LN2). In the last group, a modified method of solid surface vitrification was applied by using a cryotop as a carrier. Cryotops were used according to the minimum essential volume rule and due to their success in the vitrification procedure in comparison with the other methods. In the solid surface group, after loading the COCs on a cryotop tip, they were first placed for 15 s on a cold steel surface that was previously cooled with LN2 and then plunged in LN2. Samples in all experimental groups were preserved in LN2 for 1 week (Ebrahimi et al., Reference Ebrahimi, Valojerdi and Yazdi2010).
Warming procedure
After storage in LN2 for 1 week, the conventional straws were warmed by holding the frozen straw for 30 s in room air. The content of the straw was emptied into a central well dish containing α-MEM, 20% FBS and 1 M sucrose solution for 1 min. In the cryotop and solid surface groups, cryotop tips were placed in a central well dish containing the abovementioned solution. COCs were sequentially rehydrated in α-MEM and 20% FBS containing 0.5 and 0.25 M sucrose for 3–5 min in each, respectively, at room temperature and rinsed 5–10 times in α-MEM and 20% FBS (Ebrahimi et al., Reference Ebrahimi, Valojerdi and Yazdi2010).
Viability assessment
Two hours after warming, COCs within all three vitrification groups were examined under the stereomicroscopy with ×20 magnification for survival determination. Viable COCs showed a fine homogenous granular ooplasm, no space was observed between the oolemma and ooplasm with healthy compact cumulus layers surrounding the oocyte. Degenerated COCs showed a dark ooplasm, which shrank in some cases, and a space was observed between the oolemma and ooplasm (Ebrahimi et al., Reference Ebrahimi, Valojerdi and Yazdi2010). Experiments in each vitrified groups, were repeated eight times and compared with the non-vitrified control group.
Electron microscopy procedure
In all experiments, at least three healthy vitrified–warmed and non-vitrified COCs were randomly selected and washed three times in phosphate-buffered solution, then fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) overnight at 4 °C. COCs were subsequently rinsed in the same buffer for 10 min and post-fixed with 1% osmium tetroxide in PBS for 3 h. Samples were washed again in PBS and dehydrated through ascending concentrations of ethanol. COCs were immersed overnight in propylene oxide and embedded in araldite resin (Taab). Resin blocks were sectioned using an ultra microtome (Leica UC6). Semithin sections (500 nm thick) were stained with toluidine blue and examined by light microscopy (Zeiss) and ultrathin sections (70 nm thick) were cut with a diamond knife, mounted on copper grids and stained with alcoholic uranyl acetate and aqueous lead citrate. They were examined and photographed using a TEM-900 electron microscopy (Zeiss).
Statistical analysis
Percentages of healthy COC were confirmed by the Kolmogorov–Smirnov normalization test and statistical analysis was done using one-way analysis of variance (ANOVA) and post Tukey's test. Data were expressed as mean ± SE. A probability of p < 0.05 was considered to be statistically significant.
Results
Post warming viability rate
Two hours post warming the mean percentage of viable COCs in the cryotop (77.97 ± 2.43%) and solid surface (71.13 ± 1.79%) groups were significantly higher than the conventional straw (56.40 ± 4.88%) group (p < 0.05). Better survivability was seen in the cryotop in comparison with the solid surface group but no significant difference was detected.
Ultrastructural changes of healthy COCs
Control group
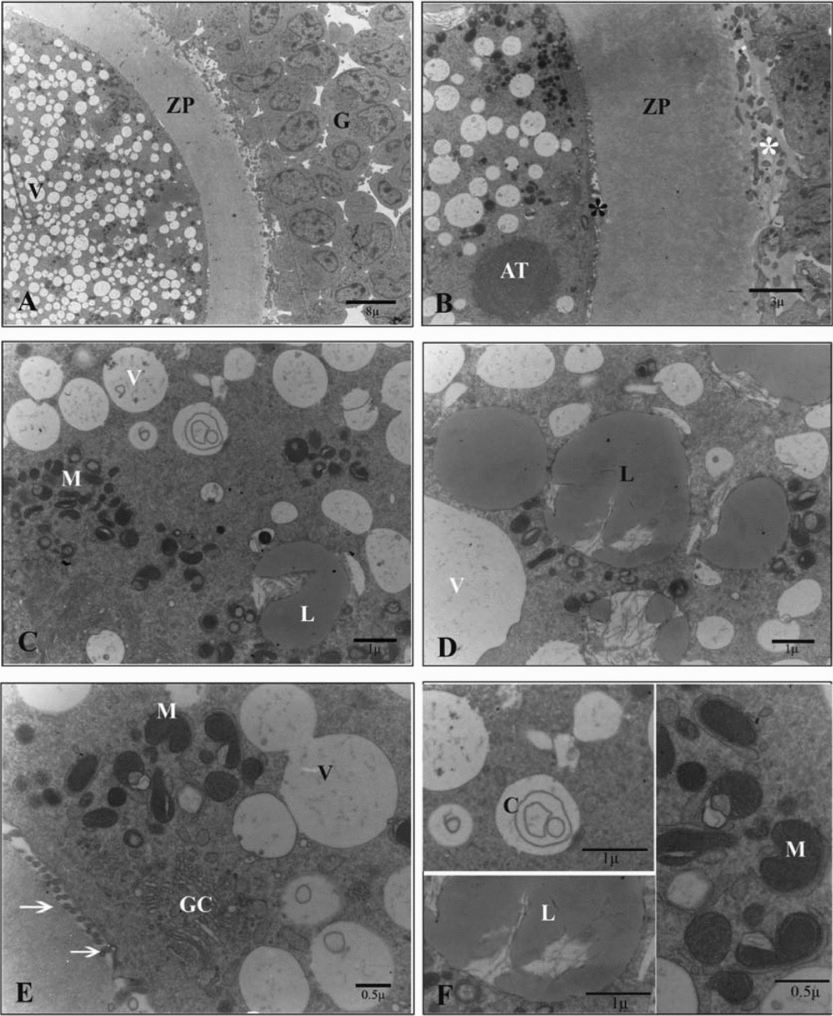
Ultrathin sections of the oocytes in the control group showed numerous small vesicles that were distributed throughout the ooplasm. Vesicles were regular and membrane bound. Some lipid droplets were found in the vesicles that had microfilamentous structures within. Large amounts of round, ellipsoid and hooded mitochondria and well developed Golgi complexes were also observed between the vesicles. In the periphery of the ooplasm, a round structure that seemed to be the aggregation of smooth endoplasmic reticulum tubules was occasionally seen. Oolemma had noticeable microvilli in the perivitelline space (Fig. 1).
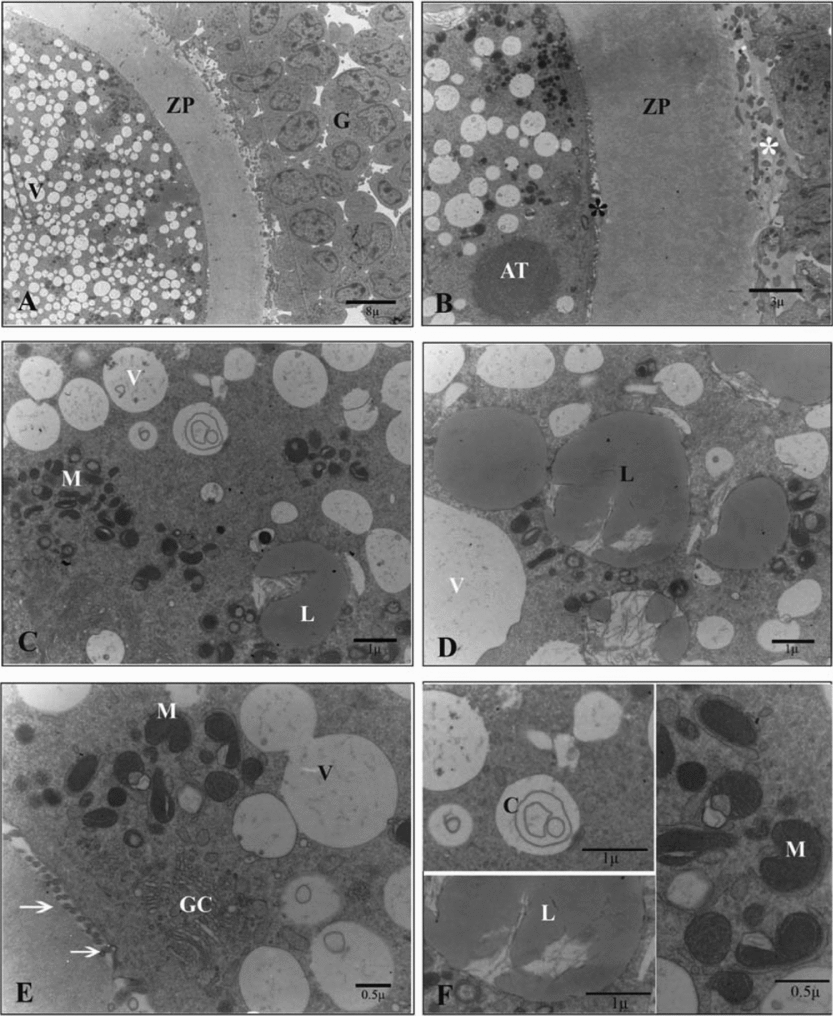
Figure 1 Ultrastructure of the oocyte of the sheep COCs in the control group. (A) G, cumulus cell; ZP, zona pellucida; V, vesicle. (B) AT, aggregation of tubules; *, cumulus cells projections (white) and microvilli (black); ZP, zona pellucida. (C) M, mitochondrion; L, lipid droplet; V, vesicle. (D) L, lipid droplet; V, vesicle. (E) GC, Golgi complex; M, mitochondrion; V, vesicle; arrow, microvilli. (F) C, concentric cristae M, mitochondrion; L, lipid droplet.
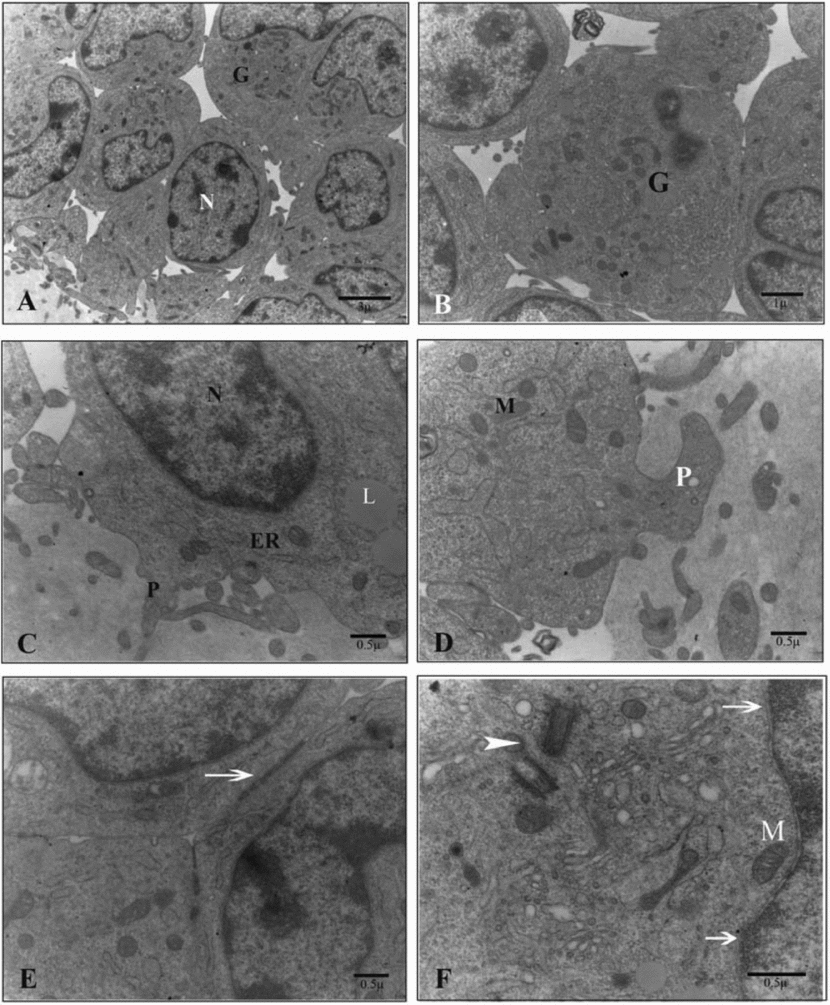
Moreover, compact cumulus layers around the oocyte revealed different types of junctional complexes. There was a clear small space between its inner layer and zona pellucida. This space was occupied by some pedicle-like process of cumulus cells. However, these processes extended, in some part, into the homogenous zona pellucida. Cumulus cells also had a large nucleus with heterochromatin periphery and a cytoplasm that included lipid droplets, endoplasmic reticulum, centrioles and mitochondria (Fig. 2).
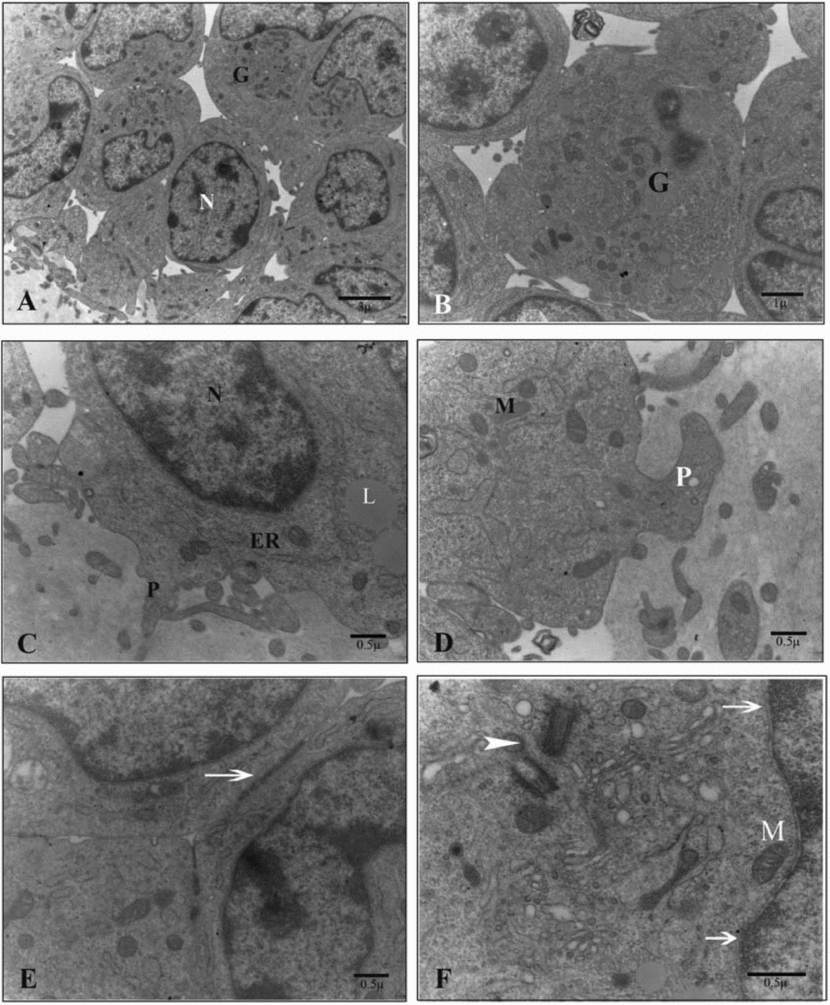
Figure 2 Ultrastructure of the cumulus cells of sheep COCs in the control group. (A) G, cumulus cell; N, nucleus. (B) G, cumulus cell. (C) P, pedicle like projection; ER, endoplasmic reticulum; L, lipid droplet; N, nucleus. (D) M, mitochondrion; P, pedicle like projection. (E) arrow, cell junctions; arrowhead, perpendicular centrioles. (F) arrow, cell junctions; arrowhead, perpendicular centrioles; M, mitochondrion.
Conventional straw group
Ultrathin sections of the oocytes in the conventional straw as compared with the control group showed the extreme space between their oolemma and zona pellucida. Microvilli decreased in the perivitelline space and disorganized ooplasm contained clumped mitochondria that possessed matrixes with different electrodensities that, in some cases, were swollen. Lipid droplets and constructed lipid droplets in the vesicles were obvious in the ooplasm. Large irregular connected vesicles were apparent, some of them seemed to be ruptured and invaded by other cytoplasmic organelles. In some areas the oolemma appeared to be ruptured after this kind of vitrification (Fig. 3).

Figure 3 Ultrastructure of the oocytes of vitrified–warmed sheep COCs in the conventional straw group. (A) G, cumulus cell; ZP, zona pellucida; arrowhead, ruptured oolemma. (B) L, lipid droplet; V, irregular large vesicle. (C) Completely disorganized ooplasm: M, mitochondrion; V, irregular large vesicle. (D) Arrow, distended or ruptured mitochondrion; L, lipid droplet. (E) Disorganized ooplasm: M, mitochondrion; V, irregular large vesicle. (F) M, distended or ruptured mitochondrion.
Although junctional complexes were preserved between cumulus cells, however there was an increase in the spaces between them, particularly between their inner layers and the zona pellucida. Moreover, the zona pellucida lost its homogeneity and cumulus cell projections decreased and in most cases, retracted from the zona pellucida. The cytoplasm of surrounding cumulus cells had different kinds of organelles with large amounts of vacuoles (Fig. 4).

Figure 4 Ultrastructure of the cumulus cells of vitrified–warmed sheep COCs in the conventional straw group. (A) G, cumulus cells; ZP, zona pellucida. (B) V, vacuoles; N, nucleus. (C) G, cumulus cells. (D) N, nucleus; V, vacuoles. (E) Arrowhead, non-uniformity in zona pellucida; V, vacuoles; ZP, zona pellucida. (F) L, lipid droplet; arrow, mitochondrion.
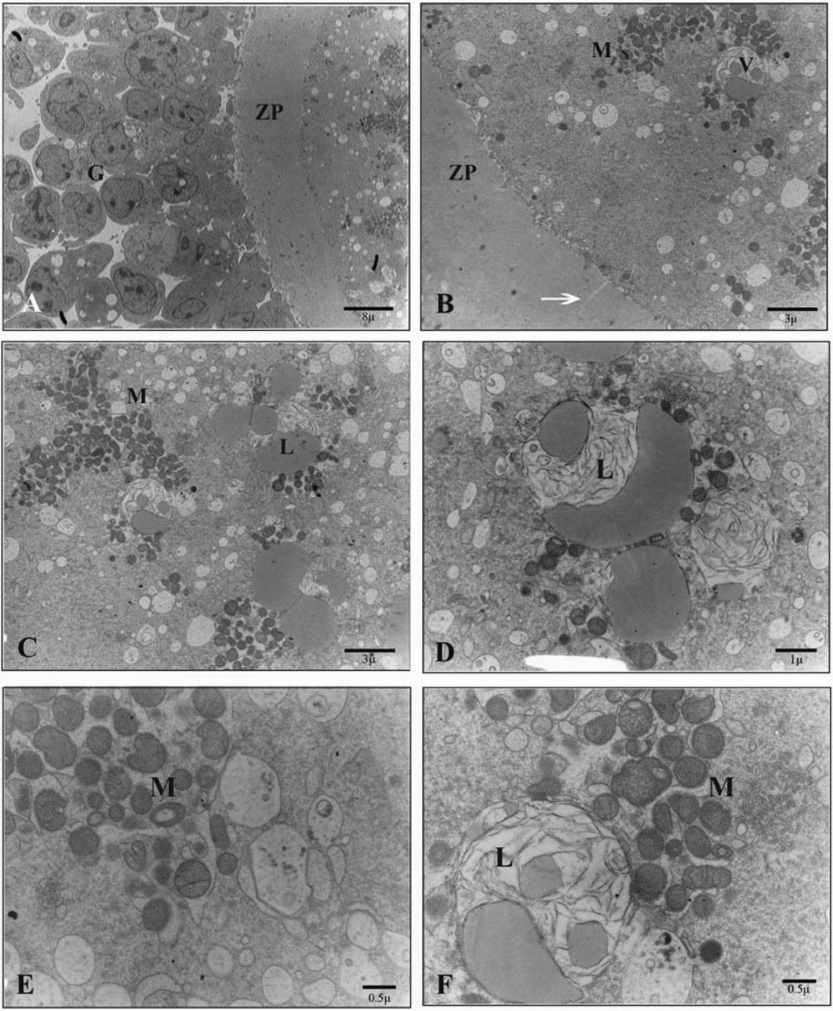
Cryotop group
Ultrathin sections of the oocytes in the cryotop group as compared with the control group showed an extended perivitelline space with numerous microvilli. However, in the well preserved ooplasm, some different sized regular and membranous vesicles, numerous lipid droplets and large amounts of round, ellipsoid and hooded mitochondria, as seen in the control group, were detected (Fig. 5).

Figure 5 Ultrastructure of the oocyte of vitrified–warmed sheep COCs in the cryotop group. (A) G, cumulus cells; V, vesicles; ZP, zona pellucida. (B) M, mitochondrion; L, lipid droplet; *, perivitelline space; V, vesicles. (C) MV, microvilli; V, vesicles. (D) M, aggregation of mitochondrion. (E) L, lipid droplet. (F) Arrow, aggregation of vesicles.
Moreover, similar to the control group, different types of junctional complexes were located between compact cumulus layers, and numerous cell projections in the space between their inner layer and homogenous zona pellucida without any cracks were apparent. In the cytoplasm of cumulus cells, different types of organelles, such as mitochondria, centrioles, endoplasmic reticulum, some vacuoles and lipid droplets were observed. Occasionally, degenerated cells were detected among the healthy cumulus cells (Fig. 6).

Figure 6 Ultrastructure of cumulus cells of vitrified–warmed sheep COCs in the cryotop group. (A) G, cumulus cells; N, nucleus. (B) ZP, zona pellucida; ER, endoplasmic reticulum; P, pedicle-like process. (C) M, mitochondrion; P, pedicle-like process; ZP, zona pellucida. (D) GC, Golgi complex; M, mitochondrion; V, vacuoles. (E) *, cumulus cell membrane expansions. (F) arrow, degenerated cumulus cell.
Solid surface group
Ultrathin sections of the oocytes in the solid surface group as compared with the control group revealed a similar perivitelline space with numerous microvilli. In the ooplasm, however, instead of the normal distribution of mitochondria, batched groups of round, ellipsoid and hooded mitochondria were seen. These mitochondria expanded in some cases. In this group, as found in the cryotop vitrification group, the number of small membranous regular well-preserved vesicles increased but they remained independent and did not show any connections. However, no homogenous distribution of numerous lipid droplets was obvious (Fig. 7).
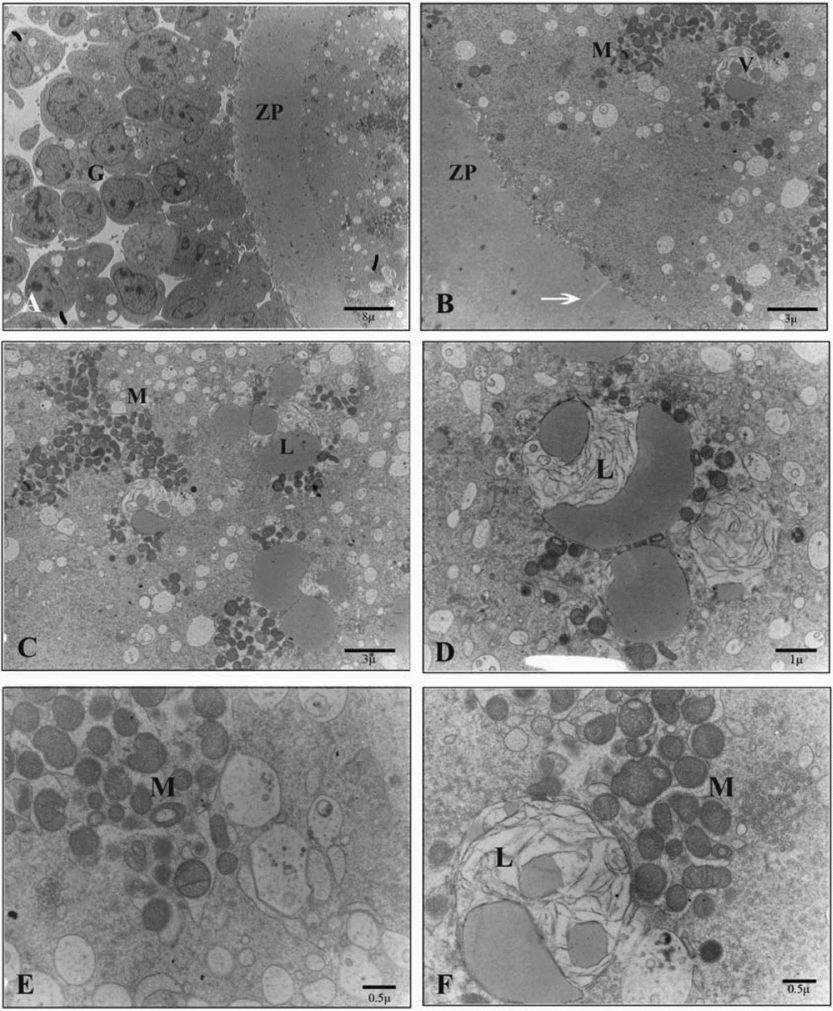
Figure 7 Ultrastructure of the oocyte of vitrified–warmed sheep COCs in the solid surface group. (A) G, cumulus cells; ZP, zona pellucida. (B) V, vesicles; M, mitochondrion; arrow, crack in zona pellucid; ZP, zona pellucida. (C) L, lipid droplet; M, mitochondrion. (D) L, lipid droplet. (E) M, mitochondrion. (F) L, washed or constructing lipid droplet in a vesicles; M, mitochondrion.
Moreover, different types of junctional complexes between cumulus cells and decreased cumulus cell projections in the space between their inner layer and zona pellucida were seen. Cracks were seen in some parts of the zona pellucida. Within the cumulus cells' cytoplasm, some vacuoles, lipid droplets and other organelles such as mitochondria, centrioles and endoplasmic reticulum were observed. Degenerated cells were also noted between the healthy cumulus cells. Also, it must be noted that the outer surface of the zona pellucida became rough in some places (Fig. 8).

Figure 8 Ultrastructure of the cumulus cells of vitrified–warmed sheep COCs in the solid surface group. (A) G, cumulus cells; V, vacuoles; N, nucleus. (B) Arrowhead, degenerated cumulus cell. (C) ZP, zona pellucida; ER, endoplasmic reticulum. (D) M, mitochondrion; L, lipid droplet; GC, Golgi complex. (E) Arrow, centrioles; GC, Golgi complex; L, lipid droplet; M, mitochondrion; V, vacuoles. (F) J, Junctional complex.
Discussion
The most important characteristics of fresh sheep immature oocytes are large amounts of mitochondria, vesicles and lipid droplets distributed throughout the ooplasm (Reader, Reference Reader2007). In immature oocytes, the Golgi complexes are more developed and consist of many dilated lamellae associated with membranous vesicles of variable electrodensity (Hyttel et al., Reference Hyttel, Greve and Callesen1989; Grondahl et al., Reference Grondahl, Hyttel and Grondahl1995). In this study, we examined the ultrastructural changes of sheep COCs following three different methods of vitrification. This research was limited to a population of obviously good quality COCs by phase contrast microscopy. In fact only cryopreserved oocytes that appeared normal and viable after thawing were routinely selected for further analysis.
It has been demonstrated that osmotic damage to cytoplasmic membranes and membranes enveloping intracellular organelles cause various ultrastructural changes after pronuclear and MII oocyte freezing (Hyttel et al., Reference Hyttel, Vajta and Callesen2000; Isachenko et al., Reference Isachenko, Isachenko and Michelmann2001, Reference Isachenko, Selman and Isachenko2003, Reference Isachenko, Montag and Isachenko2004; Rho et al., Reference Rho, Kim and Yoo2002). These ultrastructural changes in MII stage oocytes include the appearance of cracks in the zona pellucida (Fuku et al., Reference Fuku, Liu and Downey1995a), zona hardening by premature release of cortical granules (Pickering et al., Reference Pickering, Braude and Johnson1990; Vincent et al., Reference Vincent, Pickering, Jhonson and Quick1990), disruption of the plasma membrane, destruction of the meiotic spindle (Park et al., Reference Park, Son and Lee1997), peripheral migration of cortical granules (Schalkoff et al., Reference Schalkoff, Oskowitz and Douglas Powers1989; Fuku et al., Reference Fuku, Xia and Downey1995b), extensive disorganization of the ooplasm, mitochondria and cytoskeleton (Fuku et al., Reference Fuku, Xia and Downey1995b; Diez et al., Reference Diez, Duque and Gomez2005), loss of microvilli (Hyttel et al., Reference Hyttel, Greve and Callesen1989; Asada et al., 2000) and loss of oocyte–corona cell attachments (Hyttel et al., Reference Hyttel, Xu, Smith and Greve1986; Hochi et al., Reference Hochi, Kozawa and Fujimoto1996; Asada et al., 2000). Some of these alterations such as zona hardening (Johnson et al., Reference Johnson, Pickering and George1988), plasma membrane destruction (Eroglu et al., Reference Eroglu, Toner, Leykin and Toth1998), cracks in the zona pellucida (Fuku et al., Reference Fuku, Liu and Downey1995a) and ooplasm disarrangement (Diez et al., Reference Diez, Duque and Gomez2005) are also seen in the GV stage of cryopreserved oocytes. Moreover, the sight of numerous cytoplasmic vacuoles in most of the surrounding cumulus cells, disorganization of actin filaments within the transzonal process of the cumulus cells through which they establish physical contact with the oocyte and interruption of the cumulus cells' projections is also common after vitrification of COCs (Younis et al., Reference Younis, Toner, Albertini and Bigger1996).
Additionally, Yang et al. (Reference Yang, Sun and Liu1994) have suggested that the developmental capacity of cryopreserved immature bovine oocytes is impaired because of severe ultrastructural damage.
According to some research, there are small membrane-bound vesicles and in some cases, a rim of both small and large vesicles located underneath the oolemma after vitrification with the solid surface and open pulled straw (OPS) methods (Hyttel et al., Reference Hyttel, Vajta and Callesen2000). Vacuolization is a non-specific response to cryopreservation that threatens latter maturation and development of the oocyte (Ebner et al., Reference Ebner, Moser and Sommergruber2005). However, we observed vacuolization phenomena after conventional straw vitrification. In the latter group, the amount of large irregular vesicles was considerable and they completely connected to each other to form new large vacuoles. Not only in this group, but also in the two other vitrification groups, surrounding cumulus cells showed some vacuoles in their cytoplasm. Cryotop vitrification had the best condition amongst the groups regarding this variant.
According to the origin of the vesicles, Van Blerkom (Reference Van Blerkom1990) has proposed that vesicles in ooplasm may arise either spontaneously or by fusion of pre-existing vesicles derived from the smooth endoplasmic reticulum and are usually filled with a fluid that is virtually similar to perivitelline fluid. In agreement with his proposal, in our study, some vesicles were filled with structures resembling intermediate filaments and in some of them, lipid droplets were constituted on these filaments. Therefore, we assumed that they were the sites for lipid droplet formation. Another supposition is that these vesicles are the remnants of lipid droplets that washed out during the transmission electron microscopy preparation procedure. However more investigation regarding the nature of the vesicles or the filaments within them is still needed.
Moreover, previous research has shown that the viability of immature ovine oocytes reduced after cryotop vitrification. This finding could result from an extensive loss of cumulus cell plasma membrane integrity as well as from a drastic reduction in gap junction communication between the oocytes and surrounding cells during cryopreservation procedures (Bogliolo et al., Reference Bogliolo, Ariu and Fois2007). Our results showed that cryotop vitrification did not affect plasma membrane integrity of cumulus cells or cause severe changes in the gap junction communications between oocytes and surrounding cumulus cells. However, conventional straw vitrification brought about severe changes in the gap junction communications between cells and caused retraction of the cumulus cells' process from the zona pellucida in a way that the space between the inner layers of cumulus cells and zona pellucida increased. The latter event was seen to a certain degree in the solid surface vitrification group, too.
On top of that, Hochi et al. (Reference Hochi, Kozawa and Fujimoto1996) showed that vitrification induced some ultrastructural changes such as: swelling of the mitochondria associated with reduced matrix density and the presence of vacuoles in the periphery of the ooplasm. Also, induction of ultrastructural modifications in microvilli and mitochondria as well as vacuole formation in the ooplasm of cattle germinal vesicle oocytes were reported by Fuku and colleagues (Reference Fuku, Xia and Downey1995b). In addition, some researchers have shown that elongated mitochondria with coarse surfaces and small drops of cracked lipid droplets appeared in the oocytes after vitrification (Fu et al., Reference Fu, Shi and Zhang2009). Cryodamage targets mitochondria functionality and it has been shown that the rate of oocytes with an abnormal mitochondrial distribution increased after vitrification (Rho et al., Reference Rho, Kim and Yoo2002; Dumollard et al., Reference Dumollard, Duchen and Carroll2007; Shi et al., Reference Shi, Jin and Kim2007; Fu et al., Reference Fu, Shi and Zhang2009; Gualtieri et al., Reference Gualtieri, Iaccarino and Mollo2009).
In this study, mitochondria batched around lipid droplets after vitrification except in the cryotop group. A reduction in mitochondrial matrix density, mitochondrial swelling and rupture of some of the mitochondrial membranes were only seen in the conventional straw vitrification group.
On the other hand, Valojerdi & Salehnia (Reference Valojerdi and Salehnia2005) demonstrated the sensitivity of core actin filaments of microvilli to cryopreservation and a reduction in microvilli abundance after vitrification. Correspondingly, it was shown that sheep oocyte microvilli decreased in some areas after solid surface and conventional straw vitrification methods.
In agreement with previously mentioned results, the cryotop vitrification method approximately preserved the integrity of sheep COCs as found in the non-vitrified control group, but the conventional straw vitrification method completely destroyed the organization of the ooplasm, the surrounding cumulus cells and the gap junction connection between them. According to the survivability assays, the viability rates of vitrified–warmed COCs in the cryotop and solid surface groups were higher than the conventional straw group. When considering the resemblance of the vitrification procedure in the conventional and cryotop groups, this extreme difference could be explained as a result of the small amount of the vitrification medium that was used in the cryotop group that accelerated the cooling speed. Additionally, as in the solid surface vitrification method, the same carrier and the same volume of vitrification medium as in the cryotop vitrification group was employed, therefore some deficiencies observed in the solid surface group could be related to the delayed cooling speed of this procedure due to the use of nitrogen vapour instead of the liquid nitrogen.
In conclusion the cryotop vitrification method did not modify the ultrastructure of healthy vitrified–warmed oocytes and can be used for the cryopreservation of sheep COCs. However more research about the maturation and developmental capacity of these oocytes is necessary in order to determine the best procedure.
Acknowledgements
The authors would like to express thanks to Dr A. Piriyaei and Sh. Pourbeyranvand for their technical assistance in transmission electron microscopy.