INTRODUCTION
Central neurocytoma was first described in 1982 by Hassoun et al.Reference Hassoun, Gambarelli, Grisoli, Pellet, Salamon, Pellissier and Toga1 as a well-differentiated tumour of neuronal origin and is now classified as a grade II brain tumour according to the World Health Organization classification. Since the central neurocytoma was described, no more than 1000 patients were reported.
This midline tumour is characterised by greater frequency among young adults, specific radiologic features, and good postoperative prognosis due to its benign clinical course.Reference Chen, Shen, Wang, Lu and Lee2
Neurocytoma can arise from the septum pellucidum, fornix, or the walls of the lateral ventricles (subependymal layer). It may stem from bipotential precursor cells of the periventricular germinal matrix, which are capable of both neuronal and glial differentiation.Reference Paek, Shin, Kim, Park, Son and Kim3 However, there are also rare cases of central neurocytoma with craniospinal dissemination,Reference Takao, Nakagawa and Ohtomo4 and a growing list of unusual sites in which neurocytomas have been found. This seems to refute the notion that they originate from subependymal progenitor cells.Reference Kowalski, Prayson and Lee5
The incidence of central neurocytoma is estimated to be only 0.1–0.5% of all primary central nervous system tumours.Reference Kowalski, Prayson and Lee5,Reference De Lope, De La Lama, López-Ariztegui, Martínez, Conde, Fiaño and Vázquez6,Reference Rades and Schild7,Reference Leenstra, Rodriguez, Frechette, Giannini, Stafford, Pollock, Schild, Scheithauer, Jenkins, Buckner and Brown8 There is no predilection for either sex.Reference De Lope, De La Lama, López-Ariztegui, Martínez, Conde, Fiaño and Vázquez6
The typical clinical manifestation is consistent with the signs and symptoms associated with the increased intracranial pressure. These tumours may also be associated with intratumoural haemorrhage, inappropriate secretion of hormones, and epilepsy when a large intraparenchymal component is present.Reference Yuzo, Noboru, Takashi, Shin-ichi and Hiromu9,Reference Terakawa, Tsuruno, Ishibashi, Okada, Shimotake and Murata10
On light microscopy, they are composed of small round cells with rosette-like structures, alternating a fibrillar matrix with cellular areas. Ultrastructurally, neurites and neurosecretory granules are found in the perinuclear cytoplasm.Reference Chen, Shen, Wang, Lu and Lee2,Reference Kowalski, Prayson and Lee5 Immunohistochemically, they stain with markers of neuronal differentiation, such as synaptophysin and neuron-specific enolase. Glial fibrillary acidic protein (GFAP) staining can be found in central neurocytoma. It is unclear whether the GFAP-positive cells represent neoplastic cells or reactive astrocytes.Reference Chen, Shen, Wang, Lu and Lee2 Ki-67 antigens react with MIB-1 monoclonal antibodies and a MIB-1 labelling index of >2% is a relevant prognostic factor of central neurocytoma for both local control and overall survival.Reference Chen, Shen, Wang, Lu and Lee2,Reference Leenstra, Rodriguez, Frechette, Giannini, Stafford, Pollock, Schild, Scheithauer, Jenkins, Buckner and Brown8 No chromosomal deletions on 1p or 19q have been identified.Reference Leenstra, Rodriguez, Frechette, Giannini, Stafford, Pollock, Schild, Scheithauer, Jenkins, Buckner and Brown8
Neurocytomas can be divided in two major groups, typical (well-differentiated) and atypical neurocytomas. About 20% of the tumours show a more aggressive behaviour and are considered atypical. Atypical lesions are characterised by a MIB-1 labelling index ≥3% and atypical histology features such as necrosis, increased mitotic activity, and vascular proliferation.Reference Rades and Schild7
Computed tomography (CT) scans typically demonstrate an iso- or slightly hyperdense well circumscribed mass within the body of the lateral ventricles near the foramen of Monro. Areas of hypodensity represent cystic degeneration. Approximately 51% of central neurocytoma demonstrate calcification on CT. The tumours usually have a broad-based attachment to the superior and lateral wall of the ventricle. Obstruction of the interventricular foramen of Monro by the tumour mass usually results in hydrocephalus. Contrast enhancement is mild to moderate for most central neurocytoma.Reference De Lope, De La Lama, López-Ariztegui, Martínez, Conde, Fiaño and Vázquez6 Magnetic Resonance (MR) imaging usually reveals a mass that is isointense with cortex in T1- and T2-weighted images. There is usually moderate enhancement after the administration of gadolinium.Reference De Lope, De La Lama, López-Ariztegui, Martínez, Conde, Fiaño and Vázquez6
For most patients with a newly diagnosed intraventricular mass, the first choice of treatment is surgery. The surgical approaches for these lateral ventricular tumours include transcortical–transventricular and interhemispheric transcallosal–transventricular routes.
Reports on chemotherapy for central neurocytoma have been more limited. Various combinations of carmustine, lomustine, prednisolone, vincristine, and cisplatin have been used to treat relapses from central neurocytoma, even primary tumours, but the responses to these agents have not been well documented.Reference Chen, Shen, Wang, Lu and Lee2,Reference Brandes, Amistà, Gardiman, Volpin, Danieli, Guglielmi, Carollo, Pinna, Turazzi and Monfardini11
The 10-year overall survival and local control were 90–97% and >70% respectively for typical tumours compared with 63–70% and 46% for those with atypical tumours.Reference Rades and Schild7,Reference Leenstra, Rodriguez, Frechette, Giannini, Stafford, Pollock, Schild, Scheithauer, Jenkins, Buckner and Brown8
CASE REPORT
We reported a case of 22-year-old man with a history of bifrontal headache for a few days. His medical history was unremarkable. He didn’t take any drugs.
Neurological and physical examinations were normal. Analytic exams were normal as well. CT and MR showed an intraventricular enlarge mass lesion, 46 mm in maximum diameter, located inside the left ventricle with slightly left ventricular dilatation (Figure 1).
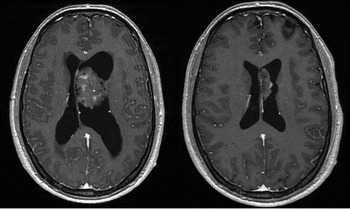
Figure 1. T1-weighted magnetic resonance in both images. Left side: Intraventricular enlarge mass with slightly ventricular dilatation. Right side: Recurrent tumour with enlargement of the septum pellucidum without contrast enhancement.
In February 2008 the patient underwent a transfrontal surgery with complete macroscopic resection of the tumour. Neurocytoma was diagnosed based on strong immunohistochemical staining for synaptophysin and no reactivity for GFAP. MIB-1 labelling index was relatively high with a value of 2–4%. Necrosis was not identified.
Magnetic resonances which were performed 3 and 6 months after the surgery showed a recurrent tumour with progressive growth (Figure 1).
Patient underwent a second surgery in November of 2008, with incomplete resection. Postoperatively his neurological status remained at the baseline. Histological and immunohistochemical staining were the same with a Ki-67 of 3–4%.
He received postoperative radiosurgery by the relapsing disease on December of 2008 (Figure 2).

Figure 2. Radiosurgery planning with 3D reconstruction of the patient, and axial, sagital and coronal views. MR, magnetic resonance.
Patient was treated with 6 MV energy photons from Clinac 600 C/D (Varian Medical Systems, Palo Alto, CA); using m3 micromultileaf collimator (BrainLAB, Heimstetten, Germany), with dynamic arctherapy technique (five arcs) to encompass the PTV (Planning Target Volume). The PTV was CTV (Clinical Target Volume) plus 2 mm. A 16 Gy dose was administered to isodose that encompasses the PTV (90%), conformity index was 1.91.
Patient remained stable and was followed with serial MR. However, in the following 11 months, the MR showed a growth of the lesion with peripheral enhancement of contrast (Figure 3). Methionine-PET was negative for progression and the pathological image improved in the next MR (Figure 4). Patient didn’t need corticosteroids. Finally the diagnosis was radionecrosis (Figure 3). As far as we know, this is the first description in the literature of radionecrosis in a neurocytoma treated with radiosurgery.

Figure 3. T1-weighted magnetic resonance in both images. Left side: Growth of the lesion with peripheral enhancement of contrast. Right side: Progressive improves of the previous lesion.

Figure 4. Methionine-Positron Emission Tomography without contrast enhancement in the magnetic resonance’s lesional area, and in the surgical bed.
One and a half year after radiosurgery he remains clinically and radiographically stable.
DISCUSSION
Nowadays the surgery is the gold standard treatment for the central neurocytoma, but in only 50% of the cases it is possible to obtain a complete resection. However, although central neurocytoma typically follows a benign clinical course, local relapse occurs in 33% after complete resection and 50% after incomplete resection.Reference Leenstra, Rodriguez, Frechette, Giannini, Stafford, Pollock, Schild, Scheithauer, Jenkins, Buckner and Brown8 Also, at the time of recurrence, initially benign neurocytomas may manifest severe complications such as craniospinal dissemination or intraventricular haemorrhage. Additionally, further operative procedures for relapses impose significant risks to the patients. That is why it is important to know the role of the radiotherapy as adjuvant treatment, especially for incomplete resection or for the atypical forms of the central neurocytoma. Most of the published articles are case reports, which make it difficult to define the best therapy available. The accumulative retrospective review data with or without meta-analysis appears to be the only way to obtain a relatively large cohort of patients with this rare tumour and make reasonable treatment recommendations.
There are two situations to analyse, complete or incomplete resection.
Radiotherapy after complete resection remains controversial since most patients have long-term tumour control without radiotherapy. Rades et al.Reference Rades and Schild7 reviewed the largest series of literature patients ever reported with central neurocytoma and demonstrated by a meta-analysis, that adjuvant radiotherapy after complete resection did not result in improved local control or increased survival. They also found that complete vs incomplete resection, and typical lesions vs atypical; were associated with better local control and overall survival.Reference Rades and Schild7
The use of irradiations for the residual tumours after incomplete resection is also controversial. The same group found that incomplete resection followed by radiotherapy was superior to incomplete resection alone with significantly better overall survival in both, typical and atypical central neurocytoma, but not in children (≤18 years).Reference Rades and Schild7
In this line, Schild et al.Reference Schild, Scheithauer, Haddock, Schiff, Burger, Wong and Lyons12 demonstrated that the 5-year local control rate for residual central neurocytoma was 100% with radiotherapy, compared to 50% without radiotherapy (p < 0.02). Leenstra et al. showed as well, that postoperative radiotherapy in incomplete resection, improves local control at 10 years (75% with radiotherapy vs 51% without radiotherapy, p = 0.045).Reference Leenstra, Rodriguez, Frechette, Giannini, Stafford, Pollock, Schild, Scheithauer, Jenkins, Buckner and Brown8
Other conclusions of the Rades et al. meta-analysis were that after incomplete resection, radiotherapy improve local control in all groups (typical, atypical, ≤18 years, >18 years). After incomplete resection, doses ≤54 Gy for typical lesions and >54 Gy in atypical lesions appeared beneficial. In children there is not differences between ≤50 or >50 Gy. Because psychomotor retardation and secondary malignancies are more important issues in children, postoperative radiotherapy might be restricted to limited situations such as incomplete resection atypical lesions or recurrent tumours.Reference Rades and Schild7
On the other hand, radiosurgery seems more advantageous than adjuvant radiotherapy in the treatment of central neurocytoma because these tumours are mostly well circumscribed in the ventricles. Radiosurgery is more precise, requires less treatment time, and reduces the volume of surrounding normal brain tissue that is irradiated such as the fornix, thalamic and basal ganglia nuclei, and the deep frontal lobe.Reference Chen, Shen, Wang, Lu and Lee2,Reference Rades and Schild13 A reduction of the treatment volume may result in less toxicity. Patients with a typical neurocytoma have an excellent prognosis and live long enough to be at risk for late radiation effects such as cognitive dysfunction or secondary malignancies.
The first typical neurocytoma patient treated with radiosurgery was described in 1997. Rades and Schild review the value of the postoperative radiosurgery in the incomplete resection of typical neurocytoma and compared it with the role of conventional radiotherapy. They compared 121 patients, 59 with incomplete resection alone, 41 with incomplete resection and conventional radiotherapy and 21 of them treated with incomplete resection and radiosurgery. This review of the literature suggests a significant benefit for radiosurgery in the treatment of patients with incomplete resection typical neurocytomas, the results of which were comparable to those of conventional radiotherapy. The 5-year-local control after incomplete resection was 51%. Local control was significantly better after incomplete resection + radiotherapy (87%, p = 0.001) and after incomplete resection + radiosurgery (100%, p = 0.004). The difference between incomplete resection + radiotherapy and incomplete resection + radiosurgery was not statistically significant (p = 0.45).Reference Rades and Schild13
The mean dose used in most articles was 15 Gy and the literature mode dose is 16 Gy to the encompass isodosis.Reference Leenstra, Rodriguez, Frechette, Giannini, Stafford, Pollock, Schild, Scheithauer, Jenkins, Buckner and Brown8,Reference Rades and Schild13,Reference Kim, Paek, Jeong, Chung, Han, Park, Jung and Kim14,Reference Yen, Sheehan, Patterson and Steiner15
Since this review, few publications have been published.Reference Leenstra, Rodriguez, Frechette, Giannini, Stafford, Pollock, Schild, Scheithauer, Jenkins, Buckner and Brown8,Reference Yen, Sheehan, Patterson and Steiner15 Even with limited data and follow-up, it appears that for small, residual, or recurrent typical or atypical tumours, radiosurgery is a reasonable alternative to conventional radiotherapy in selected patients.Reference Rades and Schild13,Reference Bertalanffy, Roessler, Koperek, Gelpi, Prayer and Knosp16
Occasionally, irradiations (conventional radiotherapy and radiosurgery) have been used as primary treatment after histological or radiological diagnoses. Experience is limited and the reported follow-up duration has been brief.Reference Leenstra, Rodriguez, Frechette, Giannini, Stafford, Pollock, Schild, Scheithauer, Jenkins, Buckner and Brown8,Reference Kim, Paek, Jeong, Chung, Han, Park, Jung and Kim14
CONCLUSION
Typical neurocytomas have a significantly better prognosis than atypical lesions. Complete resection is associated with better outcome than incomplete resection in all groups of neurocytomas, and should be performed, whenever safely possible.
After complete resection, postoperative irradiation is not required, because radiotherapy does not significantly improve outcome.
After incomplete resection, postoperative irradiation significantly improves overall survival and local control for both typical and atypical neurocytomas. In adults, a total dose of 50–54 Gy is sufficient for typical lesions, whereas atypical lesions require 56–60 Gy. After incomplete resection in children, postoperative radiotherapy significantly improves local control, but not overall survival. For this and the importance of long-term toxicity, postoperative radiotherapy might be restricted to limited situations such as incomplete resection atypical lesions or recurrent tumours. Doses of 50 Gy appear appropriate, but radiosurgery also should be valued as an optional treatment.
On the other hand, radiosurgery could be a promising option for recurrent tumours or when postoperative irradiation is needed because of its advantages over conventional radiotherapy. Or even in patients with inoperable conditions or who refuse surgery. It enables a rich and safe dose because of high dose gradient and peritumoural structures preservation. The recommended dose would be 15–16 Gy. More studies are needed to demonstrate it.






