Introduction
Accurate target localisation is an important consideration during radiotherapy, especially for stereotactic body radiation therapy (SBRT). SBRT is a technique more commonly used for treating early stage non-small-cell lung cancers and metastatic lung tumours.Reference Baumann, Nyman and Hoyer1, Reference Timmerman, Paulus and Galvin2 The large dose per fraction of SBRT treatments requires very conformal dose distribution including a rapid fall-off outside the target volume accompanied by small setup margins in order to minimise damage to normal tissues. Accordingly, the accuracy of dose delivery, conformity of dose distribution and accurate target volume localisation using image-guided techniques are crucial in delivering safe and effective SBRT. Despite the various efforts to achieve accurate positioning, patient setup uncertainties still exist and pose a challenge for SBRT treatment. Conventionally, geometric corrections are applied to translational offsets (x, y, z) and limited rotational (yaw) offset without the ability in the rotational offsets in roll and pitch as the conventional treatment couch does not provide rotational corrections for all axes.Reference Yang, Catalano, Kelsey, Yoo, Yin and Cai3 With the recent technological advances in radiation therapy delivery, the 6 degrees (6D) of freedom treatment couches are commercially available and in clinical use which allow pitch and roll angle adjustments up to 3° for more accurate patient alignment. Multiple studies have reported on the application of couch rotation and evaluated the dosimetric differences in treatment setup errors using commercially available systems in conventional, SBRT and stereotactic radiosurgery patients.Reference Peng, Liu, Chen, Amdur, Vanek and Li4–Reference Dhabaan, Schreibmann and Siddiqi11
Despite the 6D couch motion benefits in patient setup, based on the centre of rotation respect to the target, the final adjustment may involve an extra translational couch shift as well. These adjustments need to be confirmed with image guided radiotherapy which require extra radiation dose and time.
The accuracy of all of these components should be evaluated by precise machine quality assurance (QA).Reference Wilbert, Guckenberger and Polat8, Reference Dhabaan, Schreibmann and Siddiqi11–Reference Belcher, Liu, Grelewicz, Pearson and Wiersma13 If it is not handled and confirmed accurately, the consequences in patient setup can be drastic as errors in the couch rotation in setting up SBRT patients can cause reduced target coverage and increased organ at risk (OAR) dose, especially in irregularly shaped targets. Therefore, it is important to investigate the need for these shifts to see if there are significant benefits in using these systems for various tumour sizes and shapes in different treatment scenarios. Although some studiesReference Peng, Liu, Chen, Amdur, Vanek and Li4–Reference Dhabaan, Schreibmann and Siddiqi11 have reported the advantages of using couch rotation for setup, only a few studiesReference Garibaldi, Piperno and Ferrari9–Reference Dhabaan, Schreibmann and Siddiqi11 investigate the dosimetric benefit of it. The purpose of this study is to investigate the necessity of rotational couch motion for lung SBRT by analysing the dose variations caused by patient rotation. In this study, we analysed and quantified the magnitude of rotational setup errors and their resultant dosimetric impact on lung SBRT by rotating the three-dimensional (3D) dose array calculated from the original computed tomography (CT) image set. The original 3D dose array, calculated using an Eclipse™ (Varian Medical Systems, Palo Alto, CA, USA) treatment planning system (TPS), has been rotationally shifted (±1°, ±3°, ±5° for pitch, roll and yaw) and overlaid on the original CT image, to investigate dosimetric effects. The simulated dose distributions of 18-rotational plans were compared with those of the original plan using dose–volume histogram (DVH).
Material and Methods
20 patients treated with lung SBRT, with a lesion size <5 cm, have been included for retrospective dosimetric analysis. Among selected patients, six of them had a tumour located in the left lower lobe, five in the right upper lobe, five in the right lower lobe and four in the left upper lobe. Dose prescriptions were 48 Gy (12 Gy per fraction) or 50 Gy (10 Gy per fraction), among the 20 patients: 14 were treated with static-gantry intensity modulated radiation therapy with 8–11 beams and 6 with volumetric modulated arc therapy using 2 or 4 arcs.
The gross tumour volume (GTV) was contoured on the maximum intensity projection from four-dimensional CT of end-of-exhale respiratory phase image as well as the 3D scans. The internal target volume (ITV) was obtained by combining the both GTVs. The planning target volume (PTV) was defined as the ITV with 5 mm isotropic margin.
To simulate the rotational setup errors, the 3D dose array of original plans was calculated and then exported from Eclipse™ TPS. The rotational shift of ±1°, ±3°, ±5° for pitch, roll and yaw were applied to the planning 3D dose array using an in-house programme. The rotationally shifted 3D dose was imported into the TPS and overlaid to the original CT images. To investigate the dosimetric effects of rotational errors, for each patient the DVH parameters of the simulated dose distributions of 18-rotational plans of each patient were compared to those of the original plan for targets and OARs (Figure 1). Paired t-test was used for comparisons of the target coverage and OARs dose between the original and rotated plans. The p-value <0·05 was considered statistically significant and performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
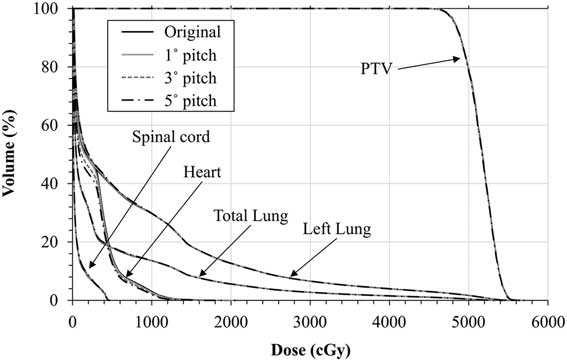
Figure 1 Dose–volume histogram comparisons between the original, 1°, 3° and 5° rotational shifted along x direction (pitch) plans for of planning target volume (PTV) and organs at risk for a target located in left lower lobe of lung.
For better illustration and analysis, a RayStation TPS (RaySearch Medical Laboratories, Stockholm, Sweden) was used to compare DVH parameters and isodose distributions. Figure 2 shows isodose-line comparisons between the original plan, 3° and 5° rotational shifted plans along x (pitch), y (roll) and z (yaw) directions for a target located in the lower lobe of the left lung.
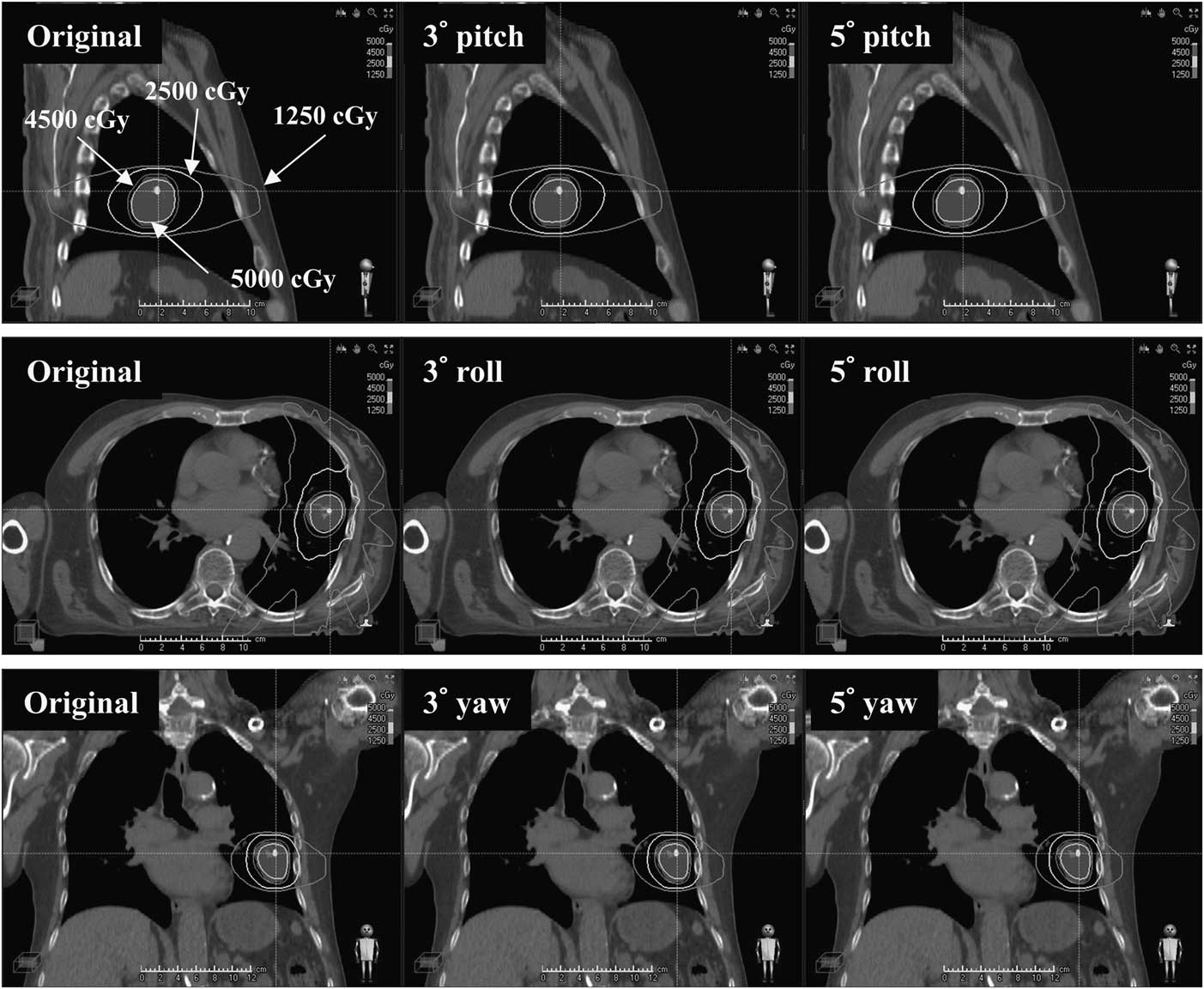
Figure 2 Isodose-line comparisons between the original plan, 3° and 5° rotational shifted plans along x (pitch), y (roll) and z (yaw) axes for a target located at lower lobe of left lung.
Results
Change of target coverage
Figure 3 shows the mean of absolute differences of all 20 patients in target dose parameters due to rotational shifts. No significant dosimetric differences have been observed in the target coverage: D 99, D 95 and mean dose (D mean) of PTV and conformity index (CI). For all of the cases up to 5° of couch angles (yaw, pitch and roll), maximum dose differences were less than 2·16, 2·93 and 0·93% for D 99, D 95 and D mean, respectively. Variations of CI were also <0·05 with an overall average value: 0·010±0·005. All rotational offsets in every direction were found statistically significant with <0·05 p-value.

Figure 3 The mean difference between original plan and rotational shift plans (in x, y and z direction) for planning target volume (PTV) D 99, D 95, mean dose (D mean) and conformity index (CI). For all of the cases up to 5° no significant dosimetric differences have been observed in D 99, D 95, D mean and CI of PTV.
Table 1 shows the mean (of absolute) and maximum differences of PTV parameters (D 99, D 95, D mean and CI) between original plan and rotationally shifted plans in x (pitch), y (roll) and z (yaw) directions.
Table 1 The mean and maximum differences between original plan and rotational shift plans (in x, y and z direction) for planning target volume (PTV) D 99, D 95, mean dose (D mean) and conformity index (CI)

Change of doses to organs at risk
Figure 4 shows the mean of absolute differences of all 20 patients in OARs dose parameters due to rotational shifts. None of the OAR doses exceeded the dose limit. Maximum differences were 179 cGy, 0·36%, 0·2% for the maximum dose (D max) to the heart and V 20 of the ipsilateral and the total lungs, respectively. The maximum dose differences of the spinal cords for most cases were <150 cGy except in one case having 171 and 193 cGy for 3° and 5° rotation shifts, respectively, when the target is located close to the cord. For some OARs shows insignificant rotational offsets (spine D max 3° and 5° yaw offset/heart D max 3° and 5° pith, roll, yaw offset/For total lung D mean 3° and 5° pith, roll, yaw offset). All other rotational offsets were found statistically significant.

Figure 4 The difference between original plan and rotational shift plans (in x, y and z direction) for V 20 of ipsilateral and total lungs, maximum dose (D max) to the spine and heart. For all of the cases up to 5° no significant dosimetric differences have been observed in maximum dose of the heart and V 20 of ipsilateral and total lung.
Table 2 shows the average and maximum difference between original plan and rotationally shifted plans in x (pitch), y (roll) and z (yaw) directions of OAR parameters: spine (D max), heart (D max and D mean), ipsilateral and total lungs (D mean and V 20).
Table 2 The mean and maximum differences between original plan and rotational shift plans (in x, y and z direction) for spine (D max), heart (D max and D mean), ipsilateral and total lungs (D mean and V 20)

Abbreviation: OAR, organ at risk.
Discussion
SBRT is commonly used to deliver a high dose to small, deep-seated tumours in early localised lung cancer.Reference Timmerman, Kavanagh, Cho, Papiez and Xing14 Compared to conventional treatment, SBRT treatment tumour position must be more accurately assessed due to the higher dose per fraction and sharp dose falloff. This can be achieved with accurate patient setup using advanced image guidance and precise couch motion. The 6D couch allows for additional rotational adjustments (pitch and roll) to patient setup accuracy. The application of 6D couch shifts has been reported by multiple studies.Reference Peng, Liu, Chen, Amdur, Vanek and Li4–Reference Dhabaan, Schreibmann and Siddiqi11 It has been shown that in large targets with irregular or elliptical shapes, the target coverage decreased significantly when rotational errors of 5° or more were present.Reference Peng, Liu, Chen, Amdur, Vanek and Li4 The study of non-stereotactic treatments of different sites showed that the system was most beneficial for head and neck patients due to the large treatment volume.Reference Kovacs, Iseli, Lang, Malla and Winter6 A lung SBRT study showed that the mean 3D vector (translational and rotational) can be significantly reduced to about one-third of the initial setup after 6D automatic correction.Reference Garibaldi, Piperno and Ferrari9
The ability of extra rotational motions of the couch may, however, introduce potential uncertainties which could lead to dosimetric differences especially on large and irregularly shaped targets unless the setup accuracy is verified by the comprehensive and adequate QA procedures, such as a verification of cone-beam computed tomography after rotational shifts. Target coverage may not be susceptible to rotations unless the shape is too elongated. The 3° rotational adjustment can result in about 1-mm shift at the tip of the 4-cm-length target (which is well within margins used for the clinical target volume) which may or may not produce significant dosimetric benefits for the ordinary circumstances. This concern requires the investigation of 6D couch motion benefits on a case by case basis. Based on our study couch rotations should be discouraged if it’s not necessary to decrease imaging repetition and dose. A simulation method to test the dose variation due to the rotational couch shifts and necessity of using it with its potential source of error can be helpful to exclude the unnecessary use of 6D couch.
However, in few cases where the OARs were close to the target volume, the dosimetric changes after translational corrections were still considerable. These might be due to the irregular shape of each isodose plan, and those were patient specific. Moreover, because the target volume has a steep gradient, the dosimetric changes due to the rotational errors were more sensitive when the OARs near the target volume than the far. Therefore, translational corrections alone are not recommended and corrections in all six degrees of freedom should be employed, for OARs near the target volume.
We propose a simulation method to analyse the dosimetric effects of the patient rotational adjustments. Although the commercial 6D couches are limited to 3° pitch and roll, we evaluated the dosimetric effects of them up to 5° adjustments. Our study showed that it would be unnecessary to perform rotational shift up to 5° for lung SBRT treatments (particularly for relatively round targets) and the translational shift is sufficient for such cases and give comparable dosimetric results. This tool can be applied to determine the rotational susceptibility of the dose variations on a case by case basis.
Conclusion
A simulation method to test the dose variation due to the rotational couch shifts has been suggested and tested. It has been found to be unnecessary to perform rotational shift up to 5° for lung SBRT treatments; the translational shift is sufficient for the cases used in this study. This method may be applied and tested after every planning to rule out exceptionally extreme cases.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.








