When milk fat is oxidized pronounced off-flavours can develop which involves a significant risk of consumer rejection and problems for retailers. The oxidative process also reduces the nutritional quality of the milk and it results in a shorter shelf life of the products (Richardson & Korycka-Dahl, Reference Richardson, Korycka-Dahl and Fox1983).
Off-flavour in milk is a phenomenon which is not fully understood and the problem often arises in herds that are well managed and high producing (Nicholson & Charmley, Reference Nicholson and Charmley1991; Barrefors et al. Reference Barrefors, Granelli, Appelqvist and Bjoerck1995). The awareness of oxidative flavour as a quality defect of milk is limited and estimates of prevalence are scarce, because many countries lack routine monitoring of off-flavours. In Sweden, sensory testing is performed routinely and the flavour of the milk is included in the milk pricing system. Therefore, off-flavour directly affects farmer's income. The current Swedish monitoring system is based on tests from each bulk tank of milk that is transported to the dairy unit. Studies including heritability measures indicate that occurrence of spontaneous oxidized flavour (SOF) is partly under genetic control (Kratzer et al. Reference Kratzer, Foreman, Freeman, Bird, Rosenberger and Nelson1967; Neimann-Soerensen et al. Reference Neimann-Soerensen, Nielsen, Poulsen and Jensen1973; Juhlin et al. Reference Juhlin, Fikse, Örde-Öström, Barrefors and Lundén2010). This means that the problem may increase through unintended selection of bulls that carry genotypes that predispose for milk susceptible to development of SOF.
The oxidation of milk fat results from presence of pro- and antioxidants and their substrate; unsaturated fatty acids. In contrast to other types of oxidized milk flavours, SOF develops without exposure to light and without addition of prooxidants. The process of oxidation is located at the double bonds of the unsaturated milk fatty acids and the initiation sets off a chain reaction which accumulates aldehydes and ketones in milk, giving it a flavour which is untypical for fresh milk (Shipe et al. Reference Shipe, Bassette, Deane, Dunkley, Hammond, Harper, Kleyn, Morgan, Nelson and Scanlan1978; Frankel, Reference Frankel1991).
Studies have shown that the susceptibility of milk to develop SOF increases with elevated concentration of long chain unsaturated milk fatty acids (Sidhu et al. Reference Sidhu, Brown and Johnson1975; Granelli et al. Reference Granelli, Barrefors, Bjorck and Appelqvist1998; Timmons et al. Reference Timmons, Weiss, Palmquist and Harper2001). However, because intake of polyunsaturated fatty acids (PUFA) have been associated with improved blood cholesterol profile in humans significant efforts have been made during the last decade to increase PUFA in cow's milk fat. It is well known that cow diets have a significant impact on the fatty acid profile in milk; one example is that inclusion of unsaturated fat in cow diets can lead to increased PUFA in milk. An important and often neglected downside of increased PUFA levels is the association with an increased risk for SOF.
The most potent prooxidant in milk is copper and it has a strong effect on SOF development (Haase & Dunkley, Reference Haase and Dunkley1970; Bruhn & Franke, Reference Bruhn and Franke1975; Timmons et al. Reference Timmons, Weiss, Palmquist and Harper2001). Copper occurs naturally in milk but the concentration varies between cows and also depends on the diet and level of supplementation (Dunkley et al. Reference Dunkley, Franke, Robb and Ronning1968). The variation between cows suggests that there may be a genetic component controlling milk copper content but this has not been studied in detail. There have been reports indicating a relatively high heritability in milk copper in dairy cows (Neimann-Soerensen et al. Reference Neimann-Soerensen, Nielsen, Poulsen and Jensen1973; Juhlin, Reference Juhlin2010). Genetic control of copper metabolism has also been suggested in other species (Shim & Harris, Reference Shim and Harris2003).
Also antioxidants affect the oxidative process. α-Tocopherol (vitamin E) is considered as one of the most important antioxidants in cow milk and dietary supplementation of α-tocopherol has been shown to improve milk oxidative stability in several studies (Lundin & Palmquist, Reference Lundin and Palmquist1983; St-Laurent et al. Reference St-Laurent, Hidiroglou, Snoddon and Nicholson1990; Charmley & Nicholson, Reference Charmley and Nicholson1994; Al-Mabruk et al. Reference Al-Mabruk, Beck and Dewhurst2004). However, dietary supplementation of vitamin E did not consistently increase the α-tocopherol concentration in milk and only a small proportion of the α-tocopherol in feed is secreted into milk (Hidiroglou, Reference Hidiroglou1989; Jensen et al. Reference Jensen, Johannsen and Hermansen1999; Shingfield et al. Reference Shingfield, Salo-Väänänen, Pahkala, Toivonen, Jaakkola, Piironen and Huhtanen2005). There are also contradicting studies which failed to show an antioxidative capacity of α-tocopherol in milk (Timmons et al. Reference Timmons, Weiss, Palmquist and Harper2001; Havemose et al. Reference Havemose, Weisbjerg, Bredie, Poulsen and Nielsen2006).
Identifying the factors behind the development of SOF is a pre-requisite for implementation of management and breeding measures to reduce the off-flavour and, in the end, benefit the perceived quality of milk. The aim of this study was to examine the relative importance of PUFA, α-tocopherol, and copper concentration in milk, and their interaction, for the occurrence of SOF, and to evaluate the genetic impact on the variation in SOF.
Material and Methods
Milk samples
A set of 132 milk samples from a previous nutrition study (Agenäs et al. Reference Agenäs, Holtenius, Griinari and Burstedt2002) was made available for this study. The milk samples came from 44 cows of the Swedish Red breed, from a herd with an on-going selection trial including two selection lines where cows were bred with bulls with breeding values for either high or low milk fat content but similar level of milk energy production (Näslund et al. Reference Näslund, Fikse, Pielberg and Lundén2008). The milk samples were obtained when the cows were mid-lactating (194±49 days lactation) and the cows were milked twice daily at 05.30 and 16.00 in a milking parlour. Average milk yield during the experimental period was 28·9±6·6 kg, milk protein content 3·4±0·3%, milk fat content 4·6±0·7% and logSCC 4·2±0·9 (means±sd, n=132). Determination of milk fat, protein and lactose content in milk and milk fatty acid profile was described in detail in Agenäs et al. (Reference Agenäs, Holtenius, Griinari and Burstedt2002).
In the study by Agenäs et al. (Reference Agenäs, Holtenius, Griinari and Burstedt2002), two dietary treatments with high (7% of total DM) or low (0%) inclusion of soya oil were applied during transition from indoor feeding to pasture. Milk samples were obtained at the end of the indoor period (day 1), during adaptation to pasture (day 8) and when cows were fully adapted to pasture (day 29).
Determination of α-tocopherol in milk
Alpha-tocopherol was determined in the lipids without further work-up by direct injection of the lipid sample (10 ml) into HPLC following a modified method by Dutta et al. (Reference Dutta, Appelqvist, Helmersson, Kebedu and Alemaw1994). Isocratic elution is carried out with a mixture of heptane: tert–butylmethylether: tetrahydrofuran: methanol (79: 20: 0, 98: 0, 02 v/v/v/v) at a flow rate of 1·2 ml/minute. A HPLC column (LiChroCART 250-4), packed with Lichrospher 100 NH2, particle size 5 μm and coupled to a guard column LiChroCART 4-4 (Merck KgaA, Darmstadt, Germany), was used for the analysis. Tocopherols were detected by L-4250 fluorescence detector Varian LC 9070 (Walnut Creek, CA, USA) at wavelengths of 294 nm and 320 nm for excitation and emission, respectively. Integration of peaks was accomplished by a HP 3396A Integrator (Hewlett-Packard, Avondale, USA). Quantification of tocopherols was done by using external standard method with reference samples of tocopherols (Merck, Darmstadt, Germany).
Determination of copper in milk
For the analysis of copper in milk combustion of milk samples (5 g) was performed by automatic wet digestion according to a standard program. A mixture of 65% supra pure nitric acid, 70% supra pure perchloric acid and 95% supra pure sulphuric acid was used. The digestion was performed in quartz-glass tubes overnight, using an automated system for control of time and temperature (Foss Tecator Digestion System, Model 40, Foss Tecator, Höganäs, Sweden). The acid residue in the digestion tube was diluted with 1 m-nitric acid to 10 ml. Analysis of copper was performed using an inductively coupled plasma atomic emission spectrometer, ICP-AES, (model JY 238, JY Horiba, division Jobin Yvon, Longgjumeau, France). Analytical copper line used was 324·75 nm. Four different concentrations of copper were used for preparation of the calibration curve, i.e. blank, 0·05, 0·10 and 0·20 mg/ml. The limit of detection (3s) in 5 g sample for copper was 0·002 mg/kg.
Quality control was regularly performed using Community Bureau of Reference Certified Reference Material 063R skim milk powder. The mean±sd for n=28 was 0·58±0·02 mg/kg dry mass. The certified value was 0·602±0·032 mg/kg dry mass. The evaluation of uncertainty was performed according to EURACHEM/CITAC Guide, 2000. The uncertainty values are reported as ± the expanded uncertainty calculated, using a coverage factor k=2, which gives a level of confidence of approximately 95%. Typical values of uncertainties at three different copper levels in milk (low, medium and high) are 0·01±0·005, 0·05±0·007, and 0·36±0·034 mg/kg.
Identification of milk with SOF
Aliquots of morning and evening milk were tested for sensory quality by trained judges who are a part of the testing system used by Swedish dairy companies. In this test each sample was evaluated according to a protocol/instruction manual which carefully describes the sensory parameters to be considered, how to handle samples, and also how judges should be trained. Two judges tested each milk sample, independently of each other. Odour and taste were scored according to a standard of the expected quality characteristics of normal Swedish milk and deviations from the standard as described in the protocol/instruction manual (personal communication Gerd Virdeskog, Eurofins Steins Laboratory AB). The milk samples were classified as either ‘normal’, ‘moderate off-flavour’ (class 1B), or ‘pronounced off-flavour’ (class 2). To classify a milk sample as belonging to class 1B, one of the two judges must scent and/or taste an abnormal odour/flavour in the milk, whereas if both persons characterize the off-flavour as pronounced the milk was assigned to class 2. The judges are trained in recognizing the off-flavours considered in the Swedish test system. Statistics regarding the tests performed throughout the year are analyzed in order to investigate the judges performance and sensitivity to the off-flavours. Throughout the year the laboratory organizes sessions with all judges using known samples of different grades of off-flavour, in order to re-calibrate the judges and to ensure the quality of the tests.
Statistical Analysis
Copper and α-tocopherol:
The distribution of copper concentration in milk was skewed to the right and a test for normality, using the UNIVARIATE procedure (SAS Inst. Inc., Cary, NC), indicated that a log transformation (with base e) of copper concentration reduced the skewness in order to create a normally distributed variable (results not shown).
A mixed effects model was used to analyse the effects of various factors that were expected to contribute to the variation in copper and α-tocopherol in milk. Fixed effects of selection line, period, feed, milk composition parameters and their interactions were tested for in the models, keeping only significant (P<0·05) factors in the final model. Data were analysed using the MIXED procedure (SAS Inst. Inc., Cary, NC) and the following models were obtained:
where: yijk=the test-day record; μ=the overall mean; period=the effect of period, (indoors, transition or outdoors); period-feed=the effect of period and feed (high or low in fat content) within the transition and outdoor period of the experiment; b 1, b 2=regression coefficients; fat=the fat concentration; fat yield=the fat yield; milk yield=the daily milk yield; aj=random additive genetic effect of cow; eijk=the random residual effect.
Spontaneous Oxidized Flavour (SOF):
Analyses of SOF were performed using the GLIMMIX procedure (SAS Inst. Inc., Cary, NC) considering SOF as a multinomial ordinal response variable with three levels (1, 2, and 3, corresponding to ‘normal’, class 1B, and class 2, respectively).
Like Timmons et al. (Reference Timmons, Weiss, Palmquist and Harper2001), only one measurement of fat at a time was included as explanatory variable in the model, and the analysis were repeated for various fat measurements (PUFA, polyunsaturated index (PI), C18:2 n-6, C18:3 n-3 and CLA). Since we expected an effect of α-tocopherol it was also included in the initial model. Effects were only kept if they exceeded the 5% significance threshold, yielding the following model:
where: πijk=the probability that the test-day SOF falls in category r (r=1, 2, 3); μ=the overall mean; aj=random additive genetic effect of cow; pek=the random environmental effect of cow.
Correlations between fatty acids, groups of fatty acids, copper and α-tocopherol were examined using the CORR procedure (SAS Inst. Inc., Cary, NC) and the high correlations between individual fatty acids and indices of fatty acids were a complicating factor building a satisfying model that included the effect of several (indices of) fatty acids to describe SOF. Forward selection and backward elimination for model building in procedure GLIMMIX were tested. However, we reverted to principal component regression to handle the co-linearities between explanatory variables.
Eleven dependant variables (α-tocopherol, mg/kg; α-tocopherol yield, mg; α-tocopherol/PUFA; Copper, mg/kg, ln; Copper yield, mg; Copper/PUFA; C18:2 n-6; CLA; C18:3 n-3; PUFA; PI; all fatty acids are given as g/100 g fatty acids) were subject to a principal component analysis using procedure PRINCOMP (SAS Inst. Inc., Cary, NC). The six principal components with the largest eigenvalues (explaining more than 95% of the variation) from this analysis were subsequently used as explanatory variables in an analysis of SOF using procedure GLIMMIX, and only the significant ones were kept.
The variation left after adjusting for the fixed effects is partitioned into between and within cow variation in the proc MIXED and GLIMMIX analyses. The variation between cows was modelled as the genetic effect, considering pedigree relationships between cows up to two generations. Correlations between repeated observations within a cow were handled by the UN(1) structure for repeated measures. The variances of these random effects (σE2 and σG2, respectively) were estimated by the MIXED and GLIMMIX procedures and used to calculate the heritability, i.e. the proportion of the total variation attributable to genetic variation among individuals:
Results and discussion
Copper concentration
The mean copper concentrations found in this study as presented in Table 1 were within the range of what has been reported in previous studies (Bruhn & Franke, Reference Bruhn and Franke1975; Ford et al. Reference Ford, Schroeder, Bland, Blease and Scott1986; Sol Morales et al. Reference Sol Morales, Palmquist and Weiss2000; Timmons et al. Reference Timmons, Weiss, Palmquist and Harper2001; Havemose et al. Reference Havemose, Weisbjerg, Bredie, Poulsen and Nielsen2006). Copper content was highest during the indoor period, lower during transition to pasture thereafter it increased again; 84·9±7·2, 52·7±5·6 and 71·4±4·6 mg/kg (lsm±se), respectively, with significant differences between all three levels (P<0·05). Pasture has in several studies been shown to lower the copper concentration in milk (Murthy et al. Reference Murthy, Rhea and Peeler1972; Ford et al. Reference Ford, Schroeder, Bland, Blease and Scott1986; Nicholson et al. Reference Nicholson1993) and it is common to give mineral supplements during this period to prevent depletion of individual minerals. The lowest copper concentrations were, however, found in milk from cows during the transition period (Table 1). During this period the cows suffered from a nutrient deficiency (Agenäs et al. Reference Agenäs, Holtenius, Griinari and Burstedt2002) and one might hypothesize that the changes in the metabolism that caused a lower milk yield and altered milk composition also affected milk copper concentrations. However, we cannot suggest any biological explanation for this pronounced lowering of the copper concentration. Dietary fat intake did not affect copper content in milk.
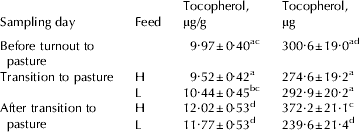
Table 1. Average composition and yield of morning milk from 44 multiparous cows of the Swedish Red breed fed high or low fat concentrate, measured during the indoor period (Day 1), at transition to pasture (Day 8), and the first period on pasture (Day 29)
Values are means±sd for n=44

† Cows were fed low (L) or high (H) amount of soya oil during transition to pasture and at pasture
PUFA=polyunsaturated fatty acids (C18:2 n-6+C18:3 n-3+CLA);
PI=polyunsaturated index (C18:2+(C18:3 n-3×2))
The genetic variance of milk copper concentration was 0·07 and the residual variances for each period were 0·19, 0·32 and 0·09 respectively, resulting in heritabilities of 0·26, 0·17 and 0·44 for the three periods. This would indicate that during transition environmental factors contribute to the variation in milk copper concentration to a higher degree, compared with when cows were at pasture. The estimate obtained from cows on pasture was within the range of the heritability of 0·44±0·06 reported by Neimann-Soerensen et al. (Reference Neimann-Soerensen, Nielsen, Poulsen and Jensen1973) and 0·42 which we recently have found in another material (Juhlin, Reference Juhlin2010). The importance of these findings is that ignoring milk copper concentration in breeding decisions may result in undesirable changes in the level of milk copper concentration.
α-Tocopherol concentration and yield
The mean α-tocopherol concentration found in this study was 1·1±0·3 μg/g (Table 1), which was in agreement with the findings by Jensen et al. (Reference Jensen, Johannsen and Hermansen1999) and Schingfield et al. (2005). The genetic variance of milk α-tocopherol concentration was 2·40 and the residual variances for each period were 1·93, 1·36 and 3·77 respectively, resulting in heritabilities of 0·56, 0·64 and 0·39 for the three periods. The mean α-tocopherol yield was 37·7 mg/d (Table 1) and the genetic variance was 0·003, residual variances for each period 0·003, 0·002 and 0·002 respectively, resulting in heritabilities of 0·48, 0·62 and 0·54 for the three periods. This indicates that the genetic variation contributes to a considerable part of the variation in α-tocopherol secretion into milk, which is in accordance with the results by Jensen et al. (Reference Jensen, Johannsen and Hermansen1999) who reported an effect of sire on variation in milk α-tocopherol in Holstein dairy cows.
Higher intake of α-tocopherol is commonly believed to result in a higher output of α-tocopherol yield in milk (Thompson et al. Reference Thompson, Henry and Kon1964; Schingoethe et al. Reference Schingoethe, Parsons, Ludens, Tucker and Shave1978; Focant et al. Reference Focant, Mignolet, Marique, Clabots, Breyne, Dalemans and Larondelle1998). Fresh grass is rich in vitamins A, E, and β-carotene and our study showed that α-tocopherol in milk increased when cows were turned out to pasture (Table 2). The highest concentrations were found at pasture in the group that received the high fat diet. Others have also found increased milk α-tocopherol when oilseeds were fed (Focant et al. Reference Focant, Mignolet, Marique, Clabots, Breyne, Dalemans and Larondelle1998; Sol Morales et al. Reference Sol Morales, Palmquist and Weiss2000).
Table 2. Variation in milk α-tocopherol concentration and yield in milk from 44 multiparous cows of the Swedish Red breed fed low-fat (L) or high-fat (H) concentrates during transition to pasture and at pasture
Values are lsm±se for n=44
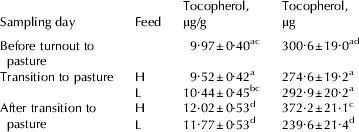
a–d Values within column with different superscript differ (P<0·05)
Oxidative off-flavour (SOF)
A total of 11·4% of the samples in this study showed off-flavour, out of these 8·4% had a moderate off-flavour (class 1B) and 3% had a pronounced off-flavour (class 2). In a recently completed study in Sweden the corresponding figures were 11·7% and 7·1%, respectively, giving a total of 18·8% (Juhlin et al. Reference Juhlin, Fikse, Örde-Öström, Barrefors and Lundén2010) whereas in the Swedish routine testing the corresponding figures for 2002 were 0·28% and 0·39%, respectively (Lindberg et al. Reference Lindberg, Andersson, Lundén, Nielsen, Everitt, Bertilsson and Gustafsson2004). These figures are obtained from sensory tests performed on herd level and the deviation from data on individual cows indicates that the problem with SOF may be underestimated. A study by Nicholson & Charmley (Reference Nicholson and Charmley1991) showed that SOF is detectable in bulk tank milk only when more than 30% of the cows in a herd are affected.
Copper content in milk and substrate availability are clearly associated with SOF (Fig. 1). Due to limitations in the dataset, with few samples with high PUFA and copper concentration, Fig. 1a & b only show the relationship for 80% of the samples. When including only one fat variable at a time in the statistical model the variables PUFA and PI were the most significant (Table 3), which was expected since they directly indicate substrate availability for oxidation in milk.

Fig. 1. The association of (a) PUFA content and (b) copper content with the risk of developing spontaneous oxidized flavour (SOF) in milk analysed from 44 multiparous cows of the Swedish Red breed, Estimates are expressed as odds ratios with 95% confidence intervals using the mean concentration as reference.
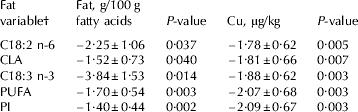
Table 3. Models describing the relationship between oxidative off-flavour (SOF) and concentrations of certain fatty acids and copper in milk from 44 multiparous cows of the Swedish Red breed
Values are regression coefficients±se
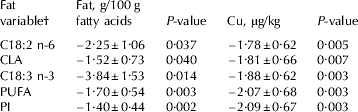
† Fatty acids and groups of fatty acids are expressed as g/100 g fatty acids;
PUFA=polyunsaturated fatty acids (C18:2 n-6+C18:3 n-3+CLA);
PI=polyunsaturated index (C18:2+(C18:3 n-3×2))
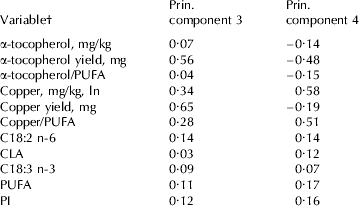
The fourth principal component is a combination of α-tocopherol yield on one hand and copper concentration and copper/PUFA on the other hand, with opposite weights (weights: −0·48, 0·58 and 0·51 respectively; Table 4). The weights on fat components were small and positive, ranging between 0·07 and 0·17 (Table 4). The association of the fourth principal component with SOF development would indicate that substrate and copper content have a positive influence on SOF development whereas the α-tocopherol yield as an antioxidant has a negative influence on the production of SOF, confirming our expectation. Interpretation of the association with the third principal component was more challenging because both pro- and antioxidants received positive weights; results of the principal component regression should be interpreted with caution because of the complexity behind the development of SOF and the difficulties in describing the process when handling a large set of correlated variables.
Table 4. Weights of variables for principal components significantly (P<0·01) contributing to the variation in spontaneous oxidized flavour development
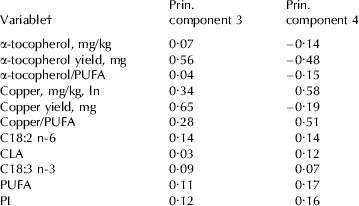
† Fatty acids and groups of fatty acids are expressed as g/100 g fatty acids;
PUFA=polyunsaturated fatty acids (C18:2 n-6+C18:3 n-3+CLA);
PI=polyunsaturated index (C18:2+(C18:3 n-3×2))
Timmons et al. (Reference Timmons, Weiss, Palmquist and Harper2001) found a statistical interaction between copper and PUFA concentrations, suggesting that high concentrations of copper or PUFA alone are not always sufficient for SOF to develop in the milk. The corresponding interaction was not statistically significant in this study. However, for all fat components included in our analyses the pattern looked similar to those reported by Timmons et al. (Reference Timmons, Weiss, Palmquist and Harper2001), indicating that SOF did not develop at low milk substrate concentrations even when copper was present at high concentrations, and vice versa.
The principal component regression suggested an association of α-tocopherol with the development of SOF, but it did not reach significance when it was included as a main effect in the GLIMMIX analysis. Improvement of milk flavour scores as a result of varying rates of vitamin E supplementation have been reported by several authors (Atwal et al. Reference Atwal, Hidiroglou and Kramer1991; Nicholson & St. Laurent, 1991; Charmley & Nicholson, Reference Charmley and Nicholson1994; Al-Mabruk et al. Reference Al-Mabruk, Beck and Dewhurst2004). The estimated heritability of SOF ranged from 0·12–0·21 depending on the component of fat entered into the model. However, the se of the estimated variances was rather large. The value is comparable with preliminary results in a recently finished study (Juhlin, Reference Neimann-Soerensen, Nielsen, Poulsen and Jensen2010) where a heritability of 0·15 was found for SOF and Kratzer et al. (Reference Kratzer, Foreman, Freeman, Bird, Rosenberger and Nelson1967) reported a heritability of 0·26±0·17. In a study by Neimann-Soerensen et al. (Reference Neimann-Soerensen, Nielsen, Poulsen and Jensen1973) the repeated estimates of the heritability of TBA-value, which reflects the degree of oxidation in milk, was in the range of 0·17±0·04 to 0·47±0·06. This result suggests that factors other than levels of substrate and pro- and antioxidants influence the risk of developing SOF, and that these factors are partly of genetic nature. Therefore, ignoring SOF development in breeding decisions may lead to unintentional changes in the frequency of SOF, highlighting the importance of routine monitoring of SOF.
In conclusion, a clear association between concentrations of both copper and PUFA in milk and the risk for developing SOF was found whereas the association between α-tocopherol and SOF was not statistically significant. The interactions between substrate and pro- and antioxidants are complex and need further investigation. Heritability estimates suggest that occurrence of SOF is partly under genetic control which indicates that milk quality may be compromised if breeding bulls are selected that carry genotypes predisposing for milk prone to develop SOF.
The Swedish Farmer's Foundation for Agricultural Research is acknowledged for financial support for this study.