Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common childhood-onset neurodevelopmental disorder (Polanczyk et al., Reference Polanczyk, de Lima, Horta, Biederman and Rohde2007; Thomas et al., Reference Thomas, Sanders, Doust, Beller and Glasziou2015). The core symptoms and impairments of ADHD persist into adolescence and adulthood in a sizable proportion of childhood patients (Faraone et al., Reference Faraone, Biederman and Mick2006). ADHD tends to coexist with not only psychiatric disorders (McGough et al., Reference McGough, Smalley, McCracken, Yang, Del'Homme, Lynn and Loo2005) but also somatic diseases (Instanes et al., Reference Instanes, Klungsoyr, Halmoy, Fasmer and Haavik2016). One increasingly studied comorbid somatic condition of ADHD over the past few years is obesity. Given the global epidemic of obesity (Cuschieri and Mamo, Reference Cuschieri and Mamo2016) and its link with a variety of adverse health outcomes, including but not limited to type-2 diabetes mellitus, cardiovascular diseases, and increased mortality (Global BMI Mortality Collaboration, 2016), a better understanding of the relationship between ADHD and obesity may have implications in reducing the public-health burden of obesity and obesity related illness.
Two recent meta-analyses (Cortese et al., Reference Cortese, Moreira-Maia, St Fleur, Morcillo-Penalver, Rohde and Faraone2016; Nigg et al., Reference Nigg, Johnstone, Musser, Long, Willoughby and Shannon2016) support an association between ADHD and obesity in adulthood, without evident sex differences in the strength of the association measured by odds ratio (OR). Life-style factors and common-genetic alterations have been proposed as plausible underlying mechanisms (Cortese and Tessari, Reference Cortese and Tessari2017). In most studies entering the meta-analyses, obesity assessment was based on body mass index (BMI) ⩾30 kg/m2. Studies involving individuals who underwent bariatric surgery, a treatment option for severe and refractory obesity (Nguyen and Varela, Reference Nguyen and Varela2017), were not included in the meta-analyses, despite preceding investigations showing overrepresentation of individuals with ADHD symptoms in young adults seeking bariatric surgery (Gruss et al., Reference Gruss, Mueller, Horbach, Martin and de Zwaan2012). Compared with obesity defined by BMI ⩾30 kg/m2, clinically diagnosed obesity may better reflect the pathological aspect of body fat deposition. Bariatric surgery can neither confound nor mediate the association between ADHD and obesity diagnosed before the surgery. When examining modifying effects of sex on the association between ADHD and obesity, previous studies focused predominantly on the OR scale (Cortese et al., Reference Cortese, Moreira-Maia, St Fleur, Morcillo-Penalver, Rohde and Faraone2016; Nigg et al., Reference Nigg, Johnstone, Musser, Long, Willoughby and Shannon2016), whereas sex may modify the association on the risk difference (RD) scale which is of great public-health importance (Knol and VanderWeele, Reference Knol and VanderWeele2012). Hence, a population-based investigation of the association between ADHD and clinical obesity as well as sex differences in the strength of the association measured by RD is needed.
A register-based family study of 4 72 735 young adult males suggests shared familial liability between ADHD and high BMI (Chen et al., Reference Chen, Kuja-Halkola, Sjolander, Serlachius, Cortese, Faraone, Almqvist and Larsson2017). The finding resonates with a recent genome-wide meta-analysis of clinically diagnosed ADHD in 20 183 cases and 35 191 controls reported a genetic correlation of 0.26 between ADHD and BMI (Demontis et al., Reference Demontis, Walters, Martin, Mattheisen, Als, Agerbo, Belliveau, Bybjerg-Grauholm, Bækved-Hansen, Cerrato, Chambert, Churchhouse, Dumont, Eriksson, Gandal, Goldstein, Grove, Hansen, Hauberg, Hollegaard, Howrigan, Huang, Maller, Martin, Moran, Pallesen, Palmer, Pedersen, Pedersen, Poterba, Poulsen, Ripke, Robinson, Satterstrom, Stevens, Turley, Won, Andreassen, Burton, Boomsma, Cormand, Dalsgaard, Franke, Gelernter, Geschwind, Hakonarson, Haavik, Kranzler, Kuntsi, Langley, Lesch, Middeldorp, Reif, Rohde, Roussos, Schachar, Sklar, Sonuga-Barke, Sullivan, Thapar, Tung, Waldman, Nordentoft, Hougaard, Werge, Mors, Mortensen, Daly, Faraone, Børglum and Neale2017). A large representative study on the association between and familial co-aggregation of ADHD and clinical obesity in individuals of both sexes would add to the existing literature the relative contributions of genetic and environmental factors to the overlap between the two conditions.
In this population-based study, we aim to (1) investigate the association between ADHD and clinical obesity in adolescence and young adulthood using data from the Swedish national registers and (2) estimate the extent to which the association could be attributed to genetic underpinnings shared by ADHD and clinical obesity via assessment of familial co-aggregation of the two conditions and quantitative genetic analysis.
Method
Data sources and study population
The Swedish Medical Birth Register contains data on approximately 98% of all births in Sweden since 1973 (Cnattingius et al., Reference Cnattingius, Ericson, Gunnarskog and Kallen1990). The Swedish Total Population Register provides information on sex, dates of birth, death, and migration for all Swedish residents who were born since 1932 and alive in 1968 (Ludvigsson et al., Reference Ludvigsson, Almqvist, Bonamy, Ljung, Michaelsson, Neovius, Stephansson and Ye2016). By linking these two registers, we identified all individuals delivered in single birth between 31 December 1973 and 1 January 2000. Individuals who had congenital malformation, died, or emigrated before age 13 were excluded, leaving a final study population of 25 38 127 individuals aged 13–40 on 31 December 2013 which was defined as the end of the observational period.
We further identified all possible sibling pairs and cousin pairs nested in the study population via the Multi-Generation Register. The register links individuals born in Sweden since 1932 to their biological parents (Ekbom, Reference Ekbom2011), enabling identification of family members of varying degrees of relatedness. In total, we identified 27 03 662 pairs of full siblings, 4 41 956 pairs of maternal half siblings, 4 72 108 pairs of paternal half siblings, and 1 01 58 536 pairs of full cousins (i.e. children of full siblings), for assessment of family co-aggregation of ADHD and obesity.
Next, we randomly selected one pair of siblings from each nuclear family to obtain a sibling sample consisting of 6 64 721 pairs of full siblings, 68 347 pairs of maternal half siblings, and 69 351 pairs of paternal half siblings. This sample was used for quantitative genetic analysis.
All demographic and health data were collected by the Swedish National Board of Health and Welfare, and pseudonymized by Statistics Sweden, an independent government agency, to mask the identity of individual participants. The study was approved by the regional ethics review board in Stockholm, Sweden.
Ascertainment of ADHD
The Swedish National Patient Register (NPR) and the Prescribed Drug Register (PDR) were used for ascertainment of ADHD. The NPR compiles hospital discharge records of psychiatric inpatient care since 1973, with complete nationwide coverage achieved since 1987 (Ludvigsson et al., Reference Ludvigsson, Andersson, Ekbom, Feychting, Kim, Reuterwall, Heurgren and Olausson2011). From 2001 onward, the register also involves approximately 80% of outpatient visits to specialists (Ludvigsson et al., Reference Ludvigsson, Andersson, Ekbom, Feychting, Kim, Reuterwall, Heurgren and Olausson2011). Diagnoses in the NPR are coded according to the International Classification of Diseases, 8th revision (ICD-8) during 1969–1986, ICD-9 during 1987–1996, and ICD-10 from 1997 onward. The PDR provides information on all drugs prescribed and dispensed to the entire population in Sweden since 1 July 2005, including prescribing dates and active ingredients coded according to the anatomical therapeutic chemical classification system (Wettermark et al., Reference Wettermark, Hammar, Fored, Leimanis, Otterblad Olausson, Bergman, Persson, Sundstrom, Westerholm and Rosen2007). In the current study, individuals with ADHD were ascertained based on having at least one registered diagnosis of ADHD (ICD-9: 314; ICD-10: F90) in the NPR or at least one registered prescription of methylphenidate (N06BA04), amphetamine (N06BA01), dexamphetamine (N06BA02), lisdexamfetamine (N06BA12), or atomoxetine (N06BA09) in the PDR at any point between their third birthday and 31 December 2013.
Ascertainment of clinical obesity
The NPR was also used to identify individuals diagnosed with obesity (ICD-8: 277.99; ICD-9: 278A and 278B; ICD-10: E65 and E66) at any point between their 13th birthday and 31 December 2013 (N = 48 725). In the current study, data on BMI at obesity diagnosis were not available. Nonetheless, we attempted to gain some understanding about the distribution of BMI among males diagnosed with obesity using data from the Swedish Military Service Conscription Register. The register contains information on directly measured weight and height for Swedish males at conscription (approximately 18 years of age) since 1968 (Gale et al., Reference Gale, Batty, McIntosh, Porteous, Deary and Rasmussen2013). The information for females was not available. BMI was calculated as body weight in kg divided by height in m2. Among individuals with both a diagnosis of obesity and information on BMI (N = 4517), 54.2% had BMI ⩾30 kg/m2, 24.7% had BMI ⩾35 kg/m2, and 7.5% had BMI ⩾40 kg/m2 at conscription. Moreover, of all individuals diagnosed with obesity, 21.0% underwent obesity surgery for management of severe obesity according to the NPR. One validation study reported high accuracy of obesity surgery registration in the NPR in 2011, with an estimated positive predictive value of 97% (Tao et al., Reference Tao, Holmberg, Naslund, Naslund, Mattsson, Lagergren and Ljung2016). In Sweden, surgical treatment of obesity should be reserved for individuals with BMI ⩾40 kg/m2, or BMI ⩾35 kg/m2 and obesity related comorbidities (Memarian et al., Reference Memarian, Sundquist, Calling, Sundquist and Li2015). Taken together, individuals with clinical obesity in the current study seemed to suffer from relatively severe level of obesity.
Covariates
Several variables were selected as covariates, including age at the end of observation period, sex, birth order (first, second, third, or fourth or higher), maternal age at delivery (<35 years or ⩾35 years), paternal age at childbirth (<45 years or ⩾45 years), family education, and psychiatric comorbidity. Age at the end of observational period was treated as a categorical variable with each year as a separate category. It served as a proxy for the length of observational period, change in register coverage, and change in public awareness of ADHD and clinical obesity during the study period. Information on education was retrieved from the Longitudinal Integration Database for Health Insurance and Labor Market (Statistics Sweden, 2011). Family education was defined as the highest level of education achieved by either parent and categorized into elementary and upper secondary education (<13 years) or higher education (⩾13 years). Diagnoses of comorbid psychiatric conditions, particularly depression (ICD-8: 296.2, 298.0, 300.4; ICD-9: 296B, 300E; ICD-10: F32–F34), anxiety (ICD-8: 300 except 300.4; ICD-9: 300 except 300E; ICD-10: F40–F42, F44–F45, F48), bipolar disorder (ICD-8: 296.1-3, 296.8; ICD-9: 296A, 296C, 296D, 296E, 296W; ICD-10: F30, F31), and substance use disorder (ICD-8: 303, 304; ICD-9: 303–305; ICD-10: F10–F19) were extracted from the NPR and treated as binary variables (presence or absence).
Statistical analyses
First, we compared the risk of obesity in individuals with ADHD to the risk in those without ADHD. Logistic regression models and regression standardization approach (Sjolander, Reference Sjolander2016) were used to obtain age-adjusted estimates of risk, RD, and risk ratio (RR), together with their respective 95% confidence intervals (CIs). The estimates were further adjusted for birth order, maternal age at delivery, paternal age at childbirth, and family education. Since depression, anxiety, bipolar disorder, and substance use disorder frequently co-occur with ADHD and obesity, the models were further adjusted for these conditions to examine the influence of psychiatric comorbidity on the observed associations. We also rerun the model after excluding individuals with other psychiatric disorder than ADHD (ICD-9: 290–319 except 314; ICD-10: F00–F99 except F90). All the aforementioned analyses were performed in the entire study population, and separately for males and females. Sex differences in the RD and RR were examined by testing the statistical significance of an interaction term of ADHD by sex.
Second, we assessed family co-aggregation of ADHD and obesity in full siblings, half siblings, and full cousins. Specifically, we compared the risk of obesity in relatives of individuals with ADHD to the risk in relatives of individuals without ADHD. To assess the relative importance of genetic and shared environmental influences on the familial co-aggregation, we tested the difference in RD between maternal and paternal half siblings. In Sweden, children continued to live predominantly with their mothers following parental separation during the study period. Maternal half siblings were thus assumed to share more environmental factors than paternal half siblings. Since maternal and paternal half siblings are similar in their genetic sharing (on average 25%), a significantly higher association in maternal half siblings than in paternal half siblings would indicate the importance of shared environmental influence on the familial co-aggregation. On the other hand, non-significant difference in the association between maternal and paternal half siblings, in combination with significant lower RDs compared with full siblings, would highlight the role of genetic factors in the familial co-aggregation. The analyses were adjusted for sex and age of both the index person and the relative, and ADHD status of the relative (Chen et al., Reference Chen, Kuja-Halkola, Sjolander, Serlachius, Cortese, Faraone, Almqvist and Larsson2017).
Finally, we performed quantitative genetic analysis to assess the relative contributions of genetic and environmental factors to the correlation between ADHD and obesity on a liability scale. The joint distribution of the liabilities to ADHD and obesity was assumed to follow a multivariate normal distribution (Neale et al., Reference Neale and Cardon1992). Phenotypic correlation (i.e. cross-disorder within-individual correlation) was calculated for the entire sample of sibling pairs. Within-disorder cross-sibling and cross-disorder cross-sibling correlations were calculated separately in full siblings, maternal half siblings, and paternal half siblings. A bivariate Cholesky decomposition model was fitted to decompose the variance in each condition and the covariance between the two conditions into additive genetic (A), dominant genetic (D), shared environmental (C), and non-shared environmental (E) components. The ADCE model was then compared with ACE model and AE model. Likelihood ratio tests were used to determine the best fitting model (i.e. a model with as few parameters as possible and no significant deterioration in fit). Model fitting was based upon the following assumptions: correlation for additive genetic components is 0.50 in full siblings and 0.25 in half siblings; correlation for dominant genetic components is 0.25 in full siblings and 0.00 in half siblings; correlation for shared environmental components is 1.00 in full and maternal half siblings and 0.00 in paternal half siblings.
All statistical hypotheses were two-sided, with a significance level of 5%. SAS software version 9.4 was used for constructing analytic datasets. Logistic regression and regression standardization were performed using the stdReg package, and quantitative genetic analysis was carried out using the OpenMx package (Neale et al., Reference Neale, Hunter, Pritikin, Zahery, Brick, Kirkpatrick, Estabrook, Bates, Maes and Boker2016) in R software version 3.3 (R Development Core Team, 2012).
Results
Descriptive statistics
Among 25 38 127 individuals included in the study population, 80 009 (3.15%) were diagnosed with ADHD and 48 725 (1.92%) were diagnosed with obesity during the observational period. Other descriptive characteristics of the study population are shown in Table 1.
Table 1. Descriptive characteristics of the study population

Association between ADHD and clinical obesity
Figure 1 illustrates age-adjusted absolute risks of clinical obesity by ADHD status. Generally, the risk of obesity in individuals with ADHD (5.55%) was three times higher than the risk in individuals without ADHD (1.82%), equivalent to an RD of 3.73% (Table 2). Females were more likely to be diagnosed with obesity than males both in individuals with ADHD and in those without (Fig. 1). The RD was higher in females (5.11%) than in males (2.86%), whereas the RR was higher in males (3.74) than in females (2.86). The associations remained statistically significant when the models were additionally adjusted for birth order, parental age at childbirth, and family education (Table 2). So did the associations after further adjustment of depression, anxiety, substance use disorder, and bipolar disorder (Table 2) or excluding individuals with psychiatric disorders other than ADHD (Table 2). Adjustment for each selected comorbid condition at a time attenuated the magnitude of the association to different degrees (Table 2). All RDs and RRs were statistically different between males and females (p < 0.001).

Fig. 1. Risk of obesity in individuals with and without ADHD adjusted for age at the end of observational period.
Table 2. Association between ADHD and obesity in the entire study population
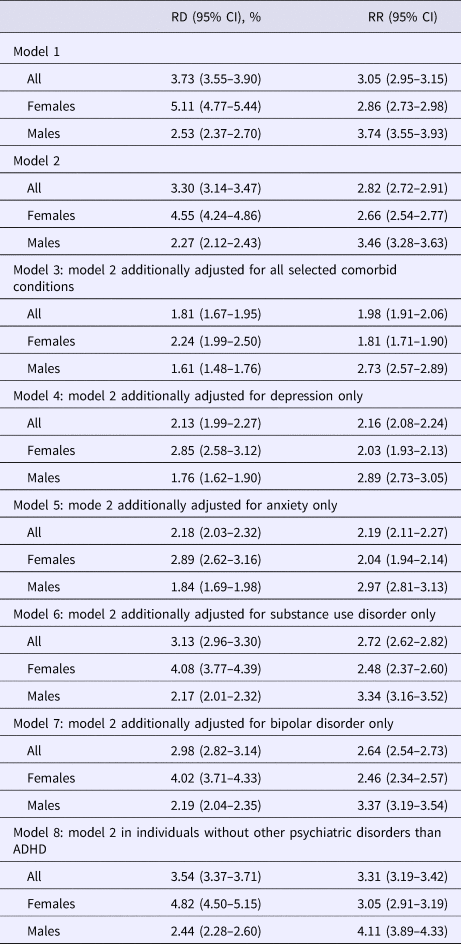
RD, risk difference; RR, risk ratio; CI, confidence interval.
Model 1: adjusted for age at the end observational period.
Model 2: adjusted for age at the end observational period, birth order, maternal age at delivery, paternal age at childbirth, and family education.
Familial co-aggregation of ADHD and clinical obesity
Full siblings of individuals with ADHD showed excess risk for clinical obesity compared with full siblings of individuals without ADHD (RD 1.11%, 95% CI 0.98–1.24%). Significant associations were also observed in half siblings (RD 0.88%, 95% CI 0.71–1.04%) and full cousins (RD 0.67%, 95% CI 0.61–0.74%), with the magnitude being lower than that in full siblings (p < 0.001). Nevertheless, the RDs did not differ significantly (p = 0.556) between maternal and paternal half siblings (Table 3). The corresponding RRs are shown in Table 3.
Table 3. Familial co-aggregation of ADHD and obesity

RD, risk difference; RR, risk ratio; CI, confidence interval.
All models were adjusted for age at the end of observational period and sex of both the index person and the relative, and ADHD status of the relative.
Quantitative genetic analysis
The correlation between the liabilities to ADHD and clinical obesity was estimated to be 0.19 (95% CI 0.18–0.21). Full siblings showed higher within-disorder cross-sibling correlations and cross-disorder cross-sibling correlation than maternal half siblings, indicating genetic influence on not only the variance in each condition but also the covariance between the two conditions (Table 4). Cross-sibling correlations on ADHD did not differ between maternal and paternal half siblings, suggesting that shared environmental influence is of limited importance for the variance in ADHD (Table 4). In contrast, higher cross-sibling correlation on clinical obesity in maternal half siblings than in paternal half siblings points to significant shared-environmental influence on the variance in clinical obesity (Table 4). Finally, cross-disorder cross-sibling correlation was similar in maternal and paternal half siblings, suggesting shared environmental factors playing only a limited role in the covariance between ADHD and clinical obesity (Table 4). According to the results of likelihood ratio tests, the bivariate ACE model was the best fitting model (online Supplementary Table S1). The heritability of ADHD was estimated to be 77% (95% CI 69–85%) and the heritability of obesity was 47% (95% CI 34–59%). The bivariate analysis showed that 7% of the total variance (9% of the genetic variance) in ADHD was in common with clinical obesity (Table 5 and online Supplementary Fig. S1), and 4% of the total variance (9% of the genetic variance) in clinical obesity was in common with ADHD. The genetic correlation between the two conditions was 0.30 (95% CI 0.17–0.44). The environmental overlap between ADHD and clinical obesity was not statistically significant (Table 5).
Table 4. Within-disorder cross-sibling correlations and cross-disorder cross-sibling correlations between ADHD and obesity

r, correlation; CI, confidence interval.
Table 5. Estimated variance explained by genetic and environmental factors from bivariate ACE model

r, correlation; CI, confidence interval.
Proportion of phenotypic correlation explained by shared genetic influence: 0.98 (0.55–1.00).
Discussion
In this population-based register study, we found a statistically significant association between ADHD and clinical obesity in adolescence and young adulthood, while taking into account several selected covariates. Familial co-aggregation of ADHD and clinical obesity was detected in full siblings, half siblings, and full cousins, with the strength of the co-aggregation decreasing with decreasing genetic relatedness. Quantitative genetic analysis suggests the association between ADHD and clinical obesity could be entirely attributed to their genetic overlap.
The age-adjusted risk of clinical obesity was estimated to be 5.6% among individuals with ADHD, much lower than the risks for high BMI reported by the meta-analysis (28.2% in adults with ADHD and 10.8% in children with ADHD) (Cortese et al., Reference Cortese, Moreira-Maia, St Fleur, Morcillo-Penalver, Rohde and Faraone2016). We speculate that the discrepancy is primarily due to the differences in assessment approach for obesity. In most studies entering the meta-analysis, obesity was defined by BMI ⩾30 kg/m2 for adults, and BMI >95 percentile for children and adolescents. Individuals undergoing bariatric surgery were excluded from the meta-analyses but included in our study. Accordingly, in the current study, individuals whose obesity condition captured the attention of health professionals were likely to have high extreme BMI and/or suffered from other medical complications (Fruh, Reference Fruh2017); they mainly represented those actively seeking treatment for obesity or obesity related medical conditions.
The sex differences in absolute risk and RD might to some extent be explained by difference in health care-seeking behavior between males and females. Females visit health-care providers more frequently than males for both mental and physical concerns (Thompson et al., Reference Thompson, Anisimowicz, Miedema, Hogg, Wodchis and Aubrey-Bassler2016). Further, higher RDs in females suggest that proper management of ADHD as well as its comorbid psychiatric disorders, if proven effective in preventing or mitigating clinical obesity, might likely benefit more females with ADHD than males. The estimated RRs appeared to be higher in males than in females because males without ADHD (reference for RR in males) had a relatively lower risk of clinical obesity than females without ADHD (reference for RR in females). It is not entirely clear whether the observed sex differences reflect sex-specific pathophysiological mechanisms underlying the association between ADHD and clinical obesity. Given a lack of evidence for sex-specific effects of common-genetic variants on either ADHD or BMI (Locke et al., Reference Locke, Kahali, Berndt, Justice, Pers, Day, Powell, Vedantam, Buchkovich, Yang, Croteau-Chonka, Esko, Fall, Ferreira, Gustafsson, Kutalik, Luan, Magi, Randall, Winkler, Wood, Workalemahu, Faul, Smith, Hua Zhao, Zhao, Chen, Fehrmann, Hedman, Karjalainen, Schmidt, Absher, Amin, Anderson, Beekman, Bolton, Bragg-Gresham, Buyske, Demirkan, Deng, Ehret, Feenstra, Feitosa, Fischer, Goel, Gong, Jackson, Kanoni, Kleber, Kristiansson, Lim, Lotay, Mangino, Mateo Leach, Medina-Gomez, Medland, Nalls, Palmer, Pasko, Pechlivanis, Peters, Prokopenko, Shungin, Stancakova, Strawbridge, Ju Sung, Tanaka, Teumer, Trompet, van der Laan, van Setten, Van Vliet-Ostaptchouk, Wang, Yengo, Zhang, Isaacs, Albrecht, Arnlov, Arscott, Attwood, Bandinelli, Barrett, Bas, Bellis, Bennett, Berne, Blagieva, Bluher, Bohringer, Bonnycastle, Bottcher, Boyd, Bruinenberg, Caspersen, Ida Chen, Clarke, Daw, de Craen, Delgado, Dimitriou, Doney, Eklund, Estrada, Eury, Folkersen, Fraser, Garcia, Geller, Giedraitis, Gigante, Go, Golay, Goodall, Gordon, Gorski, Grabe, Grallert, Grammer, Grassler, Gronberg, Groves, Gusto, Haessler, Hall, Haller, Hallmans, Hartman, Hassinen, Hayward, Heard-Costa, Helmer, Hengstenberg, Holmen, Hottenga, James, Jeff, Johansson, Jolley, Juliusdottir, Kinnunen, Koenig, Koskenvuo, Kratzer, Laitinen, Lamina, Leander, Lee, Lichtner, Lind, Lindstrom, Sin Lo, Lobbens, Lorbeer, Lu, Mach, Magnusson, Mahajan, McArdle, McLachlan, Menni, Merger, Mihailov, Milani, Moayyeri, Monda, Morken, Mulas, Muller, Muller-Nurasyid, Musk, Nagaraja, Nothen, Nolte, Pilz, Rayner, Renstrom, Rettig, Ried, Ripke, Robertson, Rose, Sanna, Scharnagl, Scholtens, Schumacher, Scott, Seufferlein, Shi, Vernon Smith, Smolonska, Stanton, Steinthorsdottir, Stirrups, Stringham, Sundstrom, Swertz, Swift, Syvanen, Tan, Tayo, Thorand, Thorleifsson, Tyrer, Uh, Vandenput, Verhulst, Vermeulen, Verweij, Vonk, Waite, Warren, Waterworth, Weedon, Wilkens, Willenborg, Wilsgaard, Wojczynski, Wong, Wright, Zhang, Brennan, Choi, Dastani, Drong, Eriksson, Franco-Cereceda, Gadin, Gharavi, Goddard, Handsaker, Huang, Karpe, Kathiresan, Keildson, Kiryluk, Kubo, Lee, Liang, Lifton, Ma, McCarroll, McKnight, Min, Moffatt, Montgomery, Murabito, Nicholson, Nyholt, Okada, Perry, Dorajoo, Reinmaa, Salem, Sandholm, Scott, Stolk, Takahashi, Van't Hooft, Vinkhuyzen, Westra, Zheng, Zondervan, Heath, Arveiler, Bakker, Beilby, Bergman, Blangero, Bovet, Campbell, Caulfield, Cesana, Chakravarti, Chasman, Chines, Collins, Crawford, Cupples, Cusi, Danesh, de Faire, den Ruijter, Dominiczak, Erbel, Erdmann, Eriksson, Farrall, Felix, Ferrannini, Ferrieres, Ford, Forouhi, Forrester, Franco, Gansevoort, Gejman, Gieger, Gottesman, Gudnason, Gyllensten, Hall, Harris, Hattersley, Hicks, Hindorff, Hingorani, Hofman, Homuth, Hovingh, Humphries, Hunt, Hypponen, Illig, Jacobs, Jarvelin, Jockel, Johansen, Jousilahti, Jukema, Jula, Kaprio, Kastelein, Keinanen-Kiukaanniemi, Kiemeney, Knekt, Kooner, Kooperberg, Kovacs, Kraja, Kumari, Kuusisto, Lakka, Langenberg, Le Marchand, Lehtimaki, Lyssenko, Mannisto, Marette, Matise, McKenzie, McKnight, Moll, Morris, Morris, Murray, Nelis, Ohlsson, Oldehinkel, Ong, Madden, Pasterkamp, Peden, Peters, Postma, Pramstaller, Price, Qi, Raitakari, Rankinen, Rao, Rice, Ridker, Rioux, Ritchie, Rudan, Salomaa, Samani, Saramies, Sarzynski, Schunkert, Schwarz, Sever, Shuldiner, Sinisalo, Stolk, Strauch, Tonjes, Tregouet, Tremblay, Tremoli, Virtamo, Vohl, Volker, Waeber, Willemsen, Witteman, Zillikens, Adair, Amouyel, Asselbergs, Assimes, Bochud, Boehm, Boerwinkle, Bornstein, Bottinger, Bouchard, Cauchi, Chambers, Chanock, Cooper, de Bakker, Dedoussis, Ferrucci, Franks, Froguel, Groop, Haiman, Hamsten, Hui, Hunter, Hveem, Kaplan, Kivimaki, Kuh, Laakso, Liu, Martin, Marz, Melbye, Metspalu, Moebus, Munroe, Njolstad, Oostra, Palmer, Pedersen, Perola, Perusse, Peters, Power, Quertermous, Rauramaa, Rivadeneira, Saaristo, Saleheen, Sattar, Schadt, Schlessinger, Slagboom, Snieder, Spector, Thorsteinsdottir, Stumvoll, Tuomilehto, Uitterlinden, Uusitupa, van der Harst, Walker, Wallaschofski, Wareham, Watkins, Weir, Wichmann, Wilson, Zanen, Borecki, Deloukas, Fox, Heid, O'Connell, Strachan, Stefansson, van Duijn, Abecasis, Franke, Frayling, McCarthy, Visscher, Scherag, Willer, Boehnke, Mohlke, Lindgren, Beckmann, Barroso, North, Ingelsson, Hirschhorn, Loos and Speliotes2015; Martin et al., Reference Martin, Walters, Demontis, Mattheisen, Lee, Robinson, Brikell, Ghirardi, Larsson, Lichtenstein, Eriksson, Werge, Mortensen, Pedersen, Mors, Nordentoft, Hougaard, Bybjerg-Grauholm, Wray, Franke, Faraone, O'Donovan, Thapar, Børglum and Neale2017) and that sex-limitation model in quantitative genetic analysis using siblings is not yet a standard method, we did not explore sex differences in the subsequent analyses.
Consistent with earlier research, adjustment for comorbid psychiatric disorders including depression, anxiety, bipolar disorder, and substance use disorder somewhat attenuated the association between ADHD and obesity (Cortese and Tessari, Reference Cortese and Tessari2017). Genetic overlap across these psychiatric disorders, ADHD, and obesity may, in part, account for this attenuation (Lee et al., Reference Lee, Ripke, Neale, Faraone, Purcell, Perlis, Mowry, Thapar, Goddard, Witte, Absher, Agartz, Akil, Amin, Andreassen, Anjorin, Anney, Anttila, Arking, Asherson, Azevedo, Backlund, Badner, Bailey, Banaschewski, Barchas, Barnes, Barrett, Bass, Battaglia, Bauer, Bayes, Bellivier, Bergen, Berrettini, Betancur, Bettecken, Biederman, Binder, Black, Blackwood, Bloss, Boehnke, Boomsma, Breen, Breuer, Bruggeman, Cormican, Buccola, Buitelaar, Bunney, Buxbaum, Byerley, Byrne, Caesar, Cahn, Cantor, Casas, Chakravarti, Chambert, Choudhury, Cichon, Cloninger, Collier, Cook, Coon, Cormand, Corvin, Coryell, Craig, Craig, Crosbie, Cuccaro, Curtis, Czamara, Datta, Dawson, Day, De Geus, Degenhardt, Djurovic, Donohoe, Doyle, Duan, Dudbridge, Duketis, Ebstein, Edenberg, Elia, Ennis, Etain, Fanous, Farmer, Ferrier, Flickinger, Fombonne, Foroud, Frank, Franke, Fraser, Freedman, Freimer, Freitag, Friedl, Frisen, Gallagher, Gejman, Georgieva, Gershon, Geschwind, Giegling, Gill, Gordon, Gordon-Smith, Green, Greenwood, Grice, Gross, Grozeva, Guan, Gurling, De Haan, Haines, Hakonarson, Hallmayer, Hamilton, Hamshere, Hansen, Hartmann, Hautzinger, Heath, Henders, Herms, Hickie, Hipolito, Hoefels, Holmans, Holsboer, Hoogendijk, Hottenga, Hultman, Hus, Ingason, Ising, Jamain, Jones, Jones, Jones, Tzeng, Kahler, Kahn, Kandaswamy, Keller, Kennedy, Kenny, Kent, Kim, Kirov, Klauck, Klei, Knowles, Kohli, Koller, Konte, Korszun, Krabbendam, Krasucki, Kuntsi, Kwan, Landen, Langstrom, Lathrop, Lawrence, Lawson, Leboyer, Ledbetter, Lee, Lencz, Lesch, Levinson, Lewis, Li, Lichtenstein, Lieberman, Lin, Linszen, Liu, Lohoff, Loo, Lord, Lowe, Lucae, MacIntyre, Madden, Maestrini, Magnusson, Mahon, Maier, Malhotra, Mane, Martin, Martin, Mattheisen, Matthews, Mattingsdal, McCarroll, McGhee, McGough, McGrath, McGuffin, McInnis, McIntosh, McKinney, McLean, McMahon, McMahon, McQuillin, Medeiros, Medland, Meier, Melle, Meng, Meyer, Middeldorp, Middleton, Milanova, Miranda, Monaco, Montgomery, Moran, Moreno-De-Luca, Morken, Morris, Morrow, Moskvina, Muglia, Muhleisen, Muir, Muller-Myhsok, Murtha, Myers, Myin-Germeys, Neale, Nelson, Nievergelt, Nikolov, Nimgaonkar, Nolen, Nothen, Nurnberger, Nwulia, Nyholt, O'Dushlaine, Oades, Olincy, Oliveira, Olsen, Ophoff, Osby, Owen, Palotie, Parr, Paterson, Pato, Pato, Penninx, Pergadia, Pericak-Vance, Pickard, Pimm, Piven, Posthuma, Potash, Poustka, Propping, Puri, Quested, Quinn, Ramos-Quiroga, Rasmussen, Raychaudhuri, Rehnstrom, Reif, Ribases, Rice, Rietschel, Roeder, Roeyers, Rossin, Rothenberger, Rouleau, Ruderfer, Rujescu, Sanders, Sanders, Santangelo, Sergeant, Schachar, Schalling, Schatzberg, Scheftner, Schellenberg, Scherer, Schork, Schulze, Schumacher, Schwarz, Scolnick, Scott, Shi, Shilling, Shyn, Silverman, Slager, Smalley, Smit, Smith, Sonuga-Barke, St Clair, State, Steffens, Steinhausen, Strauss, Strohmaier, Stroup, Sutcliffe, Szatmari, Szelinger, Thirumalai, Thompson, Todorov, Tozzi, Treutlein, Uhr, van den Oord, Van Grootheest, Van Os, Vicente, Vieland, Vincent, Visscher, Walsh, Wassink, Watson, Weissman, Werge, Wienker, Wijsman, Willemsen, Williams, Willsey, Witt, Xu, Young, Yu, Zammit, Zandi, Zhang, Zitman, Zollner, Devlin, Kelsoe, Sklar, Daly, O'Donovan, Craddock, Sullivan, Smoller, Kendler and Wray2013; Demontis et al., Reference Demontis, Walters, Martin, Mattheisen, Als, Agerbo, Belliveau, Bybjerg-Grauholm, Bækved-Hansen, Cerrato, Chambert, Churchhouse, Dumont, Eriksson, Gandal, Goldstein, Grove, Hansen, Hauberg, Hollegaard, Howrigan, Huang, Maller, Martin, Moran, Pallesen, Palmer, Pedersen, Pedersen, Poterba, Poulsen, Ripke, Robinson, Satterstrom, Stevens, Turley, Won, Andreassen, Burton, Boomsma, Cormand, Dalsgaard, Franke, Gelernter, Geschwind, Hakonarson, Haavik, Kranzler, Kuntsi, Langley, Lesch, Middeldorp, Reif, Rohde, Roussos, Schachar, Sklar, Sonuga-Barke, Sullivan, Thapar, Tung, Waldman, Nordentoft, Hougaard, Werge, Mors, Mortensen, Daly, Faraone, Børglum and Neale2017; van Hulzen et al., Reference van Hulzen, Scholz, Franke, Ripke, Klein, McQuillin, Sonuga-Barke, Kelsoe, Landen, Andreassen, Lesch, Weber, Faraone, Arias-Vasquez and Reif2017). It is also possible that ADHD, if left untreated or treated improperly, might give rise to psychiatric disorders, which are in turn associated with clinical obesity. Nevertheless, the statistically significant association between ADHD and clinical obesity in individuals without any other psychiatric disorders indicates that alternative explanations for the association should be considered.
In the current study, relatives of individuals with ADHD showed an excess risk for clinical obesity compared with relatives of individuals without ADHD, after adjustment for ADHD status in the relative. The RDs decreased with decreasing relatedness between the index person and the relative, indicating a shared familial liability to ADHD and clinical obesity. Furthermore, there was no significant difference in RD between maternal and paternal half siblings, suggesting that shared environmental factors play a very limited role in the familial co-aggregation of ADHD and clinical obesity. Hence, the shared familial liability was mainly attributable to the genetic overlap between the two conditions. It is noteworthy that among relatives of individuals without ADHD, half siblings appeared to be at a more elevated risk of clinical obesity than full siblings and cousins. This is not surprising given that having a half sibling might serve as a proxy of exposure to family dysfunction and that the latter was found to be associated with obesity (Halliday et al., Reference Halliday, Palma, Mellor, Green and Renzaho2014). Half siblings therefore seem to be a high-risk group that may open new opportunities for research in influence of gene-environment interplay on clinical obesity and its association with other conditions.
The genetic overlap between ADHD and clinical obesity was further supported by the results from quantitative genetic analysis showing that the correlation between the two conditions could be entirely attributed to the shared genetic risk factors. The findings, together with the findings from individual-level and familial liability analyses, confirm the critical role of genetic effects in occurrence ADHD and clinical obesity and have implications in treatment planning. Since patients with clinical obesity are likely to be genetically predisposed to ADHD, early detection and proper management of ADHD symptoms may help improve patient adherence to treatment of obesity and obesity related conditions. The moderate genetic correlation estimated in the current study was similar to the common-genetic variant correlation between ADHD and BMI reported in a recent genome-wide meta-analysis of ADHD (Demontis et al., Reference Demontis, Walters, Martin, Mattheisen, Als, Agerbo, Belliveau, Bybjerg-Grauholm, Bækved-Hansen, Cerrato, Chambert, Churchhouse, Dumont, Eriksson, Gandal, Goldstein, Grove, Hansen, Hauberg, Hollegaard, Howrigan, Huang, Maller, Martin, Moran, Pallesen, Palmer, Pedersen, Pedersen, Poterba, Poulsen, Ripke, Robinson, Satterstrom, Stevens, Turley, Won, Andreassen, Burton, Boomsma, Cormand, Dalsgaard, Franke, Gelernter, Geschwind, Hakonarson, Haavik, Kranzler, Kuntsi, Langley, Lesch, Middeldorp, Reif, Rohde, Roussos, Schachar, Sklar, Sonuga-Barke, Sullivan, Thapar, Tung, Waldman, Nordentoft, Hougaard, Werge, Mors, Mortensen, Daly, Faraone, Børglum and Neale2017). Future studies in independent samples and using different measurements may eventually advance our knowledge in the precise etiology of both ADHD and obesity.
Our study was subject to several limitations. First, the validity of ADHD diagnosis in the NPR has not yet been evaluated. Nevertheless, a prior study of 19 150 twins in Sweden found a high consistency between ADHD diagnosis and parent-rated level of ADHD symptoms. Specifically, 70% twins with an ADHD diagnosis also screened positive for ADHD by their parents (Skoglund et al., Reference Skoglund, Chen, D'Onofrio, Lichtenstein and Larsson2014). Further evidence for the validity of register-based diagnoses of ADHD comes from a recent genome-wide association study (GWAS) (Demontis et al., Reference Demontis, Walters, Martin, Mattheisen, Als, Agerbo, Belliveau, Bybjerg-Grauholm, Bækved-Hansen, Cerrato, Chambert, Churchhouse, Dumont, Eriksson, Gandal, Goldstein, Grove, Hansen, Hauberg, Hollegaard, Howrigan, Huang, Maller, Martin, Moran, Pallesen, Palmer, Pedersen, Pedersen, Poterba, Poulsen, Ripke, Robinson, Satterstrom, Stevens, Turley, Won, Andreassen, Burton, Boomsma, Cormand, Dalsgaard, Franke, Gelernter, Geschwind, Hakonarson, Haavik, Kranzler, Kuntsi, Langley, Lesch, Middeldorp, Reif, Rohde, Roussos, Schachar, Sklar, Sonuga-Barke, Sullivan, Thapar, Tung, Waldman, Nordentoft, Hougaard, Werge, Mors, Mortensen, Daly, Faraone, Børglum and Neale2017), which found a very high genetic correlation (r g 1.17, s.e. 0.20) between ADHD diagnosed with research interviews and ADHD defined by diagnoses recorded in the Danish national health-care registers, which are similar to the registers in Sweden. In the current study, individuals with ADHD were ascertained via the presence of at least one registered diagnosis of ADHD in the NPR or prescription of ADHD medication in the PDR. According to the treatment recommendations from the Swedish Medical Products Agency, pharmacotherapy should be reserved for individuals with severe ADHD, or those with less severe ADHD who failed to respond to non-pharmacological options (Swedish Medical Products Agency, 2016). Thus, the identified individuals with ADHD most likely represented relatively severe ADHD cases. Second, we did not examine to what degree different symptom patterns of ADHD relate to clinical obesity due to the lack of measurements on quantitative traits of inattention, hyperactivity, and impulsivity. Third, no prior study assessed the validity of obesity diagnosis in the NPR, and information on BMI at obesity diagnosis was not available. The identified individuals diagnosed with obesity mainly represented those who were referred for obesity treatment due to other reasons, such as infertility, cardiovascular diseases, and surgery, and motivated to change their weight problems. However, the largely overlapping samples of individuals with obesity diagnosis, BMI ⩾30 kg/m2 at conscription, and obesity surgery indicate that the obesity diagnosis in the NPR seemed to capture a large number of individuals with very high BMI. Fourth, individuals failing to seek medical care would not be included in the NPR or PDR. Meanwhile, hospital visits due to either ADHD or obesity could increase the chance of detecting the other condition in the same patient, leading to an overestimated association between ADHD and clinical obesity. Finally, in the quantitative genetic analysis, the assumptions for shared environmental effects (i.e. correlation for shared environmental components is 1.00 in full and maternal half siblings and 0.00 in paternal half siblings) were not tested but partially based on the fact that children live predominantly with their mothers after parental separation during the study period in Sweden. Nonetheless, since previous twin studies consistently reported limited role of shared environmental effects in the variance of ADHD, a slight violation of these assumptions should not dramatically influence the estimated shared environmental correlation.
In conclusion, our findings support an association between ADHD and clinical obesity in adolescence and young adulthood, which is predominantly attributable to the genetic overlap between the two conditions. Children with ADHD should be monitored for weight gain so that preventive measures can be taken for those on a suboptimal trajectory.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718002532.
Acknowledgements
This work was supported by Shire International GmbH. Although employees of the Sponsor were involved in the editing and fact checking of information, the content of this manuscript, the design of the work, the interpretation of the data, and the decision to submit the manuscript for publication in Psychological Medicine was made by the authors independently. This work was also supported by the Swedish Research Council (grant #2014-3831), the Swedish Initiative for Research on Microdata in the Social And Medical Sciences (SIMSAM) framework (grant #340-2013-5867), and the European Union's Horizon 2020 research and innovation programme (grant #667302). Dr Faraone is supported by the K.G. Jebsen Centre for Research on Neuropsychiatric Disorders, University of Bergen, Bergen, Norway, the European Union's Seventh Framework Programme for research, technological development and demonstration (grant #602805), the European Union's Horizon 2020 research and innovation programme under grant agreements (grant #667302 and 728018), and the National Institute of Mental Health (grant #5R01MH101519 and U01 MH109536-01).
Conflict of interest
In the past year, Dr Faraone received income, potential income, travel expenses continuing education support and/or research support from Otsuka, Lundbeck, KenPharm, Rhodes, Arbor, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA, Sunovion, Genomind and NeuroLifeSciences. With this institution, he has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. Dr Larsson has served as a speaker for Eli-Lilly and has received research grants from Shire.








