Introduction
Mature seeds of many temperate tree species are dormant and only germinate after moistened seeds are exposed to cold (5°C, cold stratification). Changes in the metabolism or perception of plant hormones are frequently reported during such cold stratification (Corbineau et al., Reference Corbineau, Bianco, Garello and Côme2002; Jacobsen et al., Reference Jacobsen, Pearce, Poole, Pharis and Mander2002; Schmitz et al., Reference Schmitz, Abrams and Kermode2002). Cold stratification may also activate phytase (Andriotis et al., Reference Andriotis, Smith and Ross2004), lipases (Li and Ross, Reference Li and Ross1990a; Zarska-Maciejewska, Reference Zarska-Maciejewska1992) and proteases (Forward et al., Reference Forward, Tranbarger and Misra2001), whereas ultrastructural studies have shown a gradual decline of seed reserves during this treatment (Dawidowicz-Grzegorzewska, Reference Dawidowicz-Grzegorzewska1989; Wang and Berjak, Reference Wang and Berjak2000; Andriotis et al., Reference Andriotis, Smith and Ross2004).
Lipid mobilization during cold stratification is accompanied by the development of enzymatic activities related to gluconeogenesis, such as isocitrate lyase (Noland and Murphy, 1984; Li and Ross, Reference Li and Ross1990a), fructose 1,6-bisphosphatase (Li and Ross, Reference Li and Ross1988) and starch synthase (Li and Ross, Reference Li and Ross1990b), which facilitate accumulation of carbohydrates (Dawidowicz-Grzegorzewska, Reference Dawidowicz-Grzegorzewska1989; Li and Ross, Reference Li and Ross1990b). Lack of reserve mobilization and the inactivity of enzymes related to gluconeogenesis in warm-incubated, moistened dormant seeds led researchers to propose that metabolic inhibition (Ross, Reference Ross and Murray1984; Lewak et al., Reference Lewak, Bogatek, Zarska-Maciejewska, Viemont and Crabbe2000) prevents dormant seeds from utilizing their food reserves, and cold conditions allow germination by activating hydrolases involved in reserve mobilization. However, further studies are needed because some results do not support this proposition (Downie and Bewley, Reference Downie and Bewley2000).
Incubating moistened seeds in warm conditions simulates accelerated ageing accompanied by oxidative stress, and finally leads to seed deterioration (Li and Ross, Reference Li and Ross1990a; McDonald, Reference McDonald1999). Accordingly, in moistened seeds, cold conditions may have a beneficial role in the promotion of germination by activation of cellular repair mechanisms (Wang and Berjak, Reference Wang and Berjak2000) and suppression of oxidative damage.
Walnut kernels differ from other stratification-requiring tree seeds, in that storage protein mobilization occurs under both cold and warm conditions (Einali and Sadeghipour, Reference Einali and Sadeghipour2007). However, under warm conditions, kernels deteriorate, whereas cold stratification leads to enhanced kernel germination. Thus, it was hypothesized that cold conditions direct the products of protein mobilization to unknown metabolic pathways needed for germination. Storage lipids constitute the major (about 70% dry weight) food reserve of walnut kernels (Savage et al., Reference Savage, McNeil and Dutta2001). Lipid mobilization and subsequent gluconeogenesis of the lipolytic products fulfil the major carbon and energy demands of seeds following germination (Cooper and Beevers, Reference Cooper and Beevers1969), and may be important processes during seed dormancy removal by cold (Li and Ross, Reference Li and Ross1990a, Reference Li and Rossb; Zarska-Maciejewska, Reference Zarska-Maciejewska1992). To understand the beneficial effects of cold conditions in the alleviation of dormancy in walnut kernels, lipid mobilization and gluconeogenesis were studied during dormancy removal by cold, as well as seeds that deteriorated due to moist, warm conditions.
Materials and methods
Plant material, stratification protocol and germination studies
Freshly harvested seeds of Persian walnut (Juglans regia L.) were procured from the Gorgan Office of Natural Resources during October of 2004, 2005 and 2006. Kernels not older than 8 months after harvest were used for stratification studies. After soaking in tap water for 24 h, nuts were surface sterilized with 0.5% (w/v) sodium hypochlorite solution for 15 min, followed by washing four times in distilled water. To stratify kernels, every 10 d, lots of 75 nuts (in triplicates of 25) were wrapped in four layers of moistened cheesecloth covered with polythene bags and incubated at 5°C in a refrigerator for up to 60 d. The stratified and non-stratified nuts, the latter imbibed for 24 h only, were then transferred into sand, irrigated to keep them moist, and their germination was recorded for 40 d in a temperature-controlled culture room at 27°C in darkness. Non-stratified nuts kept at 27°C in sand are referred to as warm-incubated kernels. Kernels with an average radicle length of 10 mm were considered as germinated, and they were evident as bulges on the sand surface. After isolating kernels from both cold-stratified and warm-incubated nuts, axes and cotyledons were excised with a razor blade from those that did not show any visible sign of germination and were used for subsequent biochemical analyses.
Preparation of tissue homogenate
Cotyledonary or axial tissues were ground and homogenized in cold homogenization buffer, consisting of 0.1 M Tris buffer pH 7.5, 0.4 M sucrose, 10 mM KCl, 1 mM MgSO4, 1 mM EDTA, 1 mM phenylmethylsulphonyl fluoride (PMSF), 0.1% (v/v) 2-mercaptoethanol and 0.6% (w/v) polyvinylpolypyrrolidone (PVPP). The homogenization buffer also contained 0.1% (v/v) Triton X-100 when extracting isocitrate lyase. The ratio of homogenization buffer to tissue was 6:1 when extracting isocitrate lyase and NADP+-isocitrate dehydrogenase, and 2:1 in other experiments. The homogenate was filtered through five layers of muslin cloth and centrifuged at 10,000 g for 30 min at 4°C. The oil body layer (top layer of the homogenate after centrifugation) was collected with a spatula and used for subsequent analyses. The total soluble protein (TSP) present in the 10,000 g supernatant was used for protein and enzymatic analyses. When using TSP for the assay of walnut kernel fatty acyl-ester hydrolase activity, PMSF and 2-mercaptoethanol were excluded from the homogenization buffer (Teissere et al., Reference Teissere, Borel, Caillol, Nari, Gardies and Noat1995).
Assay of isocitrate lyase activity
Isocitrate lyase (EC 4.1.3.1) activity was assayed according to the method of Cooper and Beevers (Reference Cooper and Beevers1969) with minor modifications. The reaction mixture in a final volume of 1.2 ml consisted of 50 mM Tris buffer (pH 8.0), 5 mM MgSO4, 50 mM 2-mercaptoethanol, 20 mM phenyl hydrazinium hydrochloride, 13 mM dl-isocitrate and aliquots up to 100 μl from the TSP fraction. The reaction was started following the addition of dl-isocitrate, and the increase in the absorbance at 324 nm, due to the formation of glyoxylate–phenylhydrazone complex, was recorded. Enzyme activity was expressed as nmoles glyoxylate produced per min per g fresh weight of tissue [nmol (min.g fresh weight)− 1], assuming an extinction coefficient (ɛ324) of 17 (mM. cm)− 1 for the glyoxylate–phenylhydrazone complex.
Assay of NADP+-isocitrate dehydrogenase activity
The activity of NADP+-isocitrate dehydrogenase (EC 1.1.1.42) was determined as described by Chen et al. (Reference Chen, Le Marechal, Vidal, Jacquot and Gadal1988) with slight modifications. The reaction mixture in a final volume of 1 ml consisted of 50 mM Tris buffer (pH 8), 5 mM MgSO4, 0.2 mM dl-isocitrate, 0.1 mM NADP+ and aliquots up to 100 μl from the TSP fraction as an enzyme source. The oxidation of isocitrate to α-ketoglutarate was started following the addition of enzyme extract, and the increase in absorbance at 340 nm, resulting from the reduction of NADP+, was recorded for 5 min. Enzyme activity was expressed as nmoles isocitrate oxidized per min per g tissue fresh weight [nmol (min.g fresh weight)− 1], assuming an extinction coefficient (ɛ340) of 6220 (M.cm)− 1 for the NADPH product (Tian et al., Reference Tian, Bryk, Itoh, Suematsu and Nathan2005).
Fatty acyl-ester hydrolase activity assay
Fatty acyl-ester hydrolase (EC 3.1.1.1) activity was measured spectrophotometrically, according to the method of Winkler and Stuckmann (1979). Thirty milligrams of p-nitrophenyl palmitate (pNPP) were dissolved in 10 ml of isopropanol and mixed with 90 ml of 55 mM Tris buffer (pH 8.0), containing 0.11% (w/v) gum arabic and 0.23% (w/v) sodium deoxycholate. Freshly prepared substrate solution (2.4 ml) was mixed with aliquots (up to 100 μl) of TSP in a final volume of 2.5 ml. The enzyme-catalysed release of nitrophenyl anions at 37°C was monitored at 410 nm within 2–4 min after starting the reaction. Enzyme activity was expressed as the nmoles nitrophenyl anions released per min per g tissue fresh weight [nmol (min.g fresh weight)− 1], assuming an extinction coefficient (ɛ410) of 15,000 (M.cm)− 1.
Other analytical methods
Extraction and quantification of total lipids from walnut kernels were carried out according to the method of Hara and Radin (Reference Hara and Radin1978). The defatted powder obtained following tissue total lipid extraction was used for extraction and measurement of starch, reducing and non-reducing sugars. Starch content was quantified according to the method of McCready et al. (Reference McCready, Guggolz, Silviera and Owens1950). Reducing and non-reducing sugars were determined by the methods of Miller (Reference Miller1959) and Handel (Reference Handel1968), respectively. Soluble protein in the 10,000 g supernatant of the tissue extract was assayed by the Bradford (Reference Bradford1976) method. The extent of lipid peroxidation was measured according to Du and Bramlage (Reference Du and Bramlage1992). Tissue hydrogen peroxide content was quantified by the method of Jana and Choudhuri (Reference Jana and Choudhuri1981).
Statistical analysis
Statistically significant differences at the 5% level were determined by the Duncan method and Nested Design Analysis (SAS software 2001, SAS Institute Inc., Cary, North Carolina, USA).
Results
Effect of cold stratification on germination of walnut kernels
Figure 1A shows the time course of germination of both cold-stratified and warm-incubated kernels. Warm-incubated kernels achieved 30% germination after 20 d culture at 27°C. Cold stratification of kernels for 30 d greatly enhanced their germination to more than 70% after a subsequent 20 d at 27°C, with lower enhancement after shorter cold periods. A maximum germination percentage of 45% was obtained for warm-incubated kernels that were kept moist for 40 d at 27°C. There were no significant changes in germination percentages of kernels cold stratified for more than 30 d. Kernels cold stratified for 30 d also displayed the highest germination rate (Fig. 1B). No germination occurred at 5°C during 60 d of cold stratification, and it started only after subsequently incubating kernels at 27°C.

Figure 1 (A) Time course of germination at 27°C of walnut kernels non-stratified (closed diamonds) and cold stratified for 10 d (open squares), 20 d (closed triangles) and 30 d (open triangles). (B) Effect of stratification on germination percentage (closed squares) and germination rate (open diamonds) of walnut kernels. Every 10 d for a period of 60 d, seeds imbibed for 24 h were surface sterilized and incubated at 5°C. Moist-chilled seeds were then simultaneously transferred to a sand medium at 27°C, and germination was recorded for a period of 40 d. Each point represents the mean value of triplicate experiments, each consisting of 25 seeds ± SE.
Changes in total lipid content of cold-stratified and warm-incubated walnut kernels
Total lipids constitute about 65% of walnut kernel dry matter. Following imbibition, a significant decrease in lipid content occurred in cold-stratified cotyledons, but not in the embryonic axes (Fig. 2A). The rate of lipid mobilization was very high, i.e. 9.5 mg (g dry weight)− 1 in warm-incubated cotyledons (Fig. 2B), so that about 30% of the initial tissue lipid content was consumed within 20 d. In contrast, lipid content of axes did not change significantly.
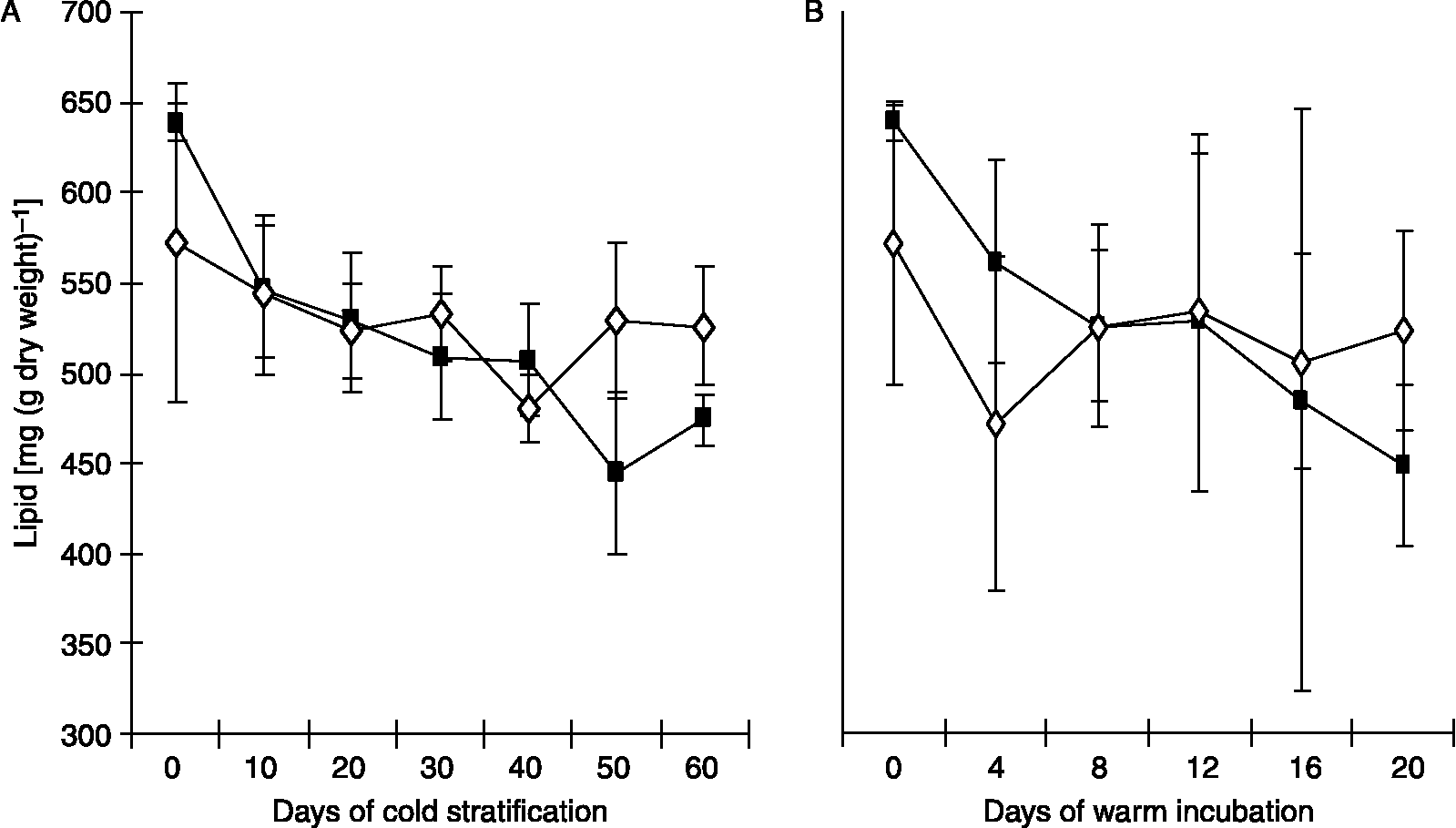
Figure 2 Changes in total lipid content of cotyledons (closed squares) and axes (open diamonds) of cold-stratified (A) and warm-incubated (B) walnut kernels. Each point represents the mean value of three separate extractions ± SE.
Changes in starch and soluble sugar contents of cold-stratified and warm-incubated walnut kernels
The alleviation of apple seed dormancy is accompanied by activation of the gluconeogenesis pathway (Lewak et al., Reference Lewak, Bogatek, Zarska-Maciejewska, Viemont and Crabbe2000). Accordingly, changes in starch, non-reducing and reducing sugars were investigated in cold-stratified and warm-incubated walnut kernels (Fig. 3). Starch content of cotyledons increased significantly (about twofold) within 30 d of cold stratification, and from this time onward it remained more or less unchanged (Fig. 3A). The starch content of the embryonic axes remained unchanged throughout this period. Despite some alterations in starch content, changes were not significant in warm-incubated axes and cotyledons (Fig. 3B).

Figure 3 Changes in starch (A, B), non-reducing sugars (C, D) and reducing sugars (E, F) in cotyledons (closed square) and axes (open diamond) of walnut kernels during cold stratification (A, C, E) or warm incubation (B, D, F). The bars show standard errors of the means of the measured parameters of three separate tissue samples.
A twofold increase in non-reducing sugars occurred in cotyledons from 20 to 40 d of kernel incubation at cold temperatures (Fig. 3C). Cold-stratified axes showed fluctuations in non-reducing sugar content. Neither cotyledons nor axes of warm-incubated kernels accumulated non-reducing sugars (Fig. 3D).
The amount of reducing sugars remained significantly higher in cold-stratified kernels compared with warm-incubated ones (Fig. 3E, F). A significant decline in the amount of reducing sugars, by about 70%, occurred in cotyledons within the first 4 d in kernels incubated at warm temperatures (Fig. 3F).
Changes in walnut kernel fatty acyl-ester hydrolase activity in cold-stratified and warm-incubated walnut kernels
Fatty acyl-ester hydrolases are necessary to hydrolyse acyl-glycerols produced following lipase action in germinated oilseeds (Teissere et al., Reference Teissere, Borel, Caillol, Nari, Gardies and Noat1995). This enzyme activity declined significantly in both cotyledons and axes of cold-stratified kernels (Fig. 4A), whereas it remained variably higher under warm conditions (Fig. 4B). Using α-naphthyl acetate as a substrate to detect fatty acyl-ester hydrolase of walnut kernels in the total soluble protein fraction after separation by SDS-PAGE (Gabriel, Reference Gabriel1971), we found a 64 kDa protein, which displayed a more intense activity in extracts of warm-incubated kernels compared with those from cold-stratified ones (not shown).

Figure 4 Changes in fatty acyl-ester hydrolase activity in cotyledons (closed squares) and axes (open diamonds) of cold-stratified (A) or warm-incubated (B) walnut kernels. Values are means of three separate extractions ± SE.
Isocitrate lyase activity in cold-stratified and warm-incubated walnut kernels
The development of isocitrate lyase (ICL) activity is a marker of gluconeogenesis in both germinated and cold-stratifying oilseeds (Cooper and Beevers, Reference Cooper and Beevers1969; Li and Ross, Reference Li and Ross1990a). Enzyme activity increased in cotyledons and axes of imbibed kernels, with maximum enzyme activity in the cotyledons after 50 d of cold treatment (Fig. 5A). ICL activity also developed in both cotyledons and axes of warm-incubated kernels, so that maximum enzyme activity occurred after 12–16 d (Fig. 5B).

Figure 5 Changes in isocitrate lyase activity in cotyledons (closed squares) and axes (open diamonds) of cold-stratified (A) or warm-incubated (B) walnut kernels. Values are means of three separate extractions ± SE.
Hydrogen peroxide contents in cold-stratified and warm-incubated walnut kernels
Seed deterioration is accompanied by the production of reactive oxygen species (McDonald, Reference McDonald1999). The beneficial effects of cold stratification on seed germination (Wang and Berjak, Reference Wang and Berjak2000) might include prevention of a build-up of reactive oxygen species such as hydrogen peroxide. Hydrogen peroxide contents remained constant during cold stratification of kernels (Fig. 6A), whereas in warm-incubated kernels, extensive hydrogen peroxide accumulation occurred after 4 d, and by 20 d was at least threefold greater (Fig. 6B).

Figure 6 Changes in the hydrogen peroxide contents of cotyledons (closed squares) and axes (open diamonds) of cold-stratified (A) or warm-incubated (B) walnut kernels. Each point represents the mean value of three separate extractions ± SE.
Lipid peroxidation in cold-stratified and warm-incubated walnut kernels
Lipid peroxidation is an important factor in seed deterioration (McDonald, Reference McDonald, Benech-Arnold and Sanchez2004). If a cold-stratification stimulus exerts its beneficial effects through the avoidance of membrane damage (Wang and Berjak, Reference Wang and Berjak2000), the extent of lipid peroxidation might be a good measure in evaluating tissue damage to both cold-stratified and warm-incubated walnut kernels. Means of lipid peroxidation in both cotyledons and axes were significantly greater in warm-incubated walnut kernels than in cold-stratified ones when they were evaluated for the whole period of incubation (Fig. 7).

Figure 7 Comparison of lipid peroxidation between cold-stratified and warm-incubated walnut cotyledons (A) and axes (B). Means and significant differences for lipid peroxidation values were obtained following Nested Design Analysis of data obtained from cold-stratified and warm-incubated kernels for 60 d and 20 d, respectively. MDA, malondialdehyde.
NADP+-isocitrate dehydrogenase activity in cold-stratified and warm-incubated walnut kernels
NADP+-isocitrate dehydrogenase is a cytosolic enzyme in plant tissues (Palomo et al., Reference Palomo, Gallardo, Suarez and Canovas1998). Its role in counteracting oxidative stress is well documented in animal cells (Kim and Park, Reference Kim and Park2003), and by producing reducing equivalents of NADPH, it may behave similarly in plants (Corpas et al., Reference Corpas, Barroso, Sandalio, Palma, Lupianez and del Rio1999; del Rio et al., Reference del Rio, Corpas, Sandalio, Palma, Gomez and Barroso2002; Hodges et al., Reference Hodges, Flesch, Galvez and Bismuth2003). Accordingly, changes in the activity of NADP+-isocitrate dehydrogenase in walnut kernels were taken as a measure of their competence to generate reducing equivalents, and hence counteract oxidative stress. In imbibed axes and cotyledons, the mean enzyme activity was high, but showed no statistically significant changes during cold stratification for 60 d (Fig. 8A). Under warm conditions, NADP+-isocitrate dehydrogenase initially increased by about 18% in cotyledons incubated for 8 d, but further incubation in the warm led to a significant decline of about 40% in both kernel tissues (Fig. 8B).

Figure 8 Changes in NADP+-isocitrate dehydrogenase activity in cotyledons (closed squares) and axes (open diamonds) from cold-stratified (A) or warm-incubated (B) walnut kernels. Values are means of three separate extractions ± SE.
Discussion
Walnut kernels are not deeply dormant (Einali and Sadeghipour, Reference Einali and Sadeghipour2007); in the absence of a cold stimulus, germination was between 20 and 45% (Fig. 1). Cold stratification enhanced kernel germination to 70% (Fig. 1), in agreement with data obtained by others (Kaur et al., Reference Kaur, Sharma, Kumar, Sharma and Sharma2006; Sanchez-Zamora et al., Reference Sanchez-Zamora, Cos-Terrer, Frutos-Tomas and Garcia-Lopez2006). Since there was no germination in kernels during 60 d of stratification at 5°C, all the biochemical changes reported here are not a result of germination, and may be related to dormancy release.
Non-stratified kernels (i.e. warm-incubated) germinated after 10 d incubation at 27°C. Most of them retained white, sound-looking tissue for the first 20 d and 55% of them decayed thereafter. Accordingly, samples were taken from these kernels only up to 20 d of incubation, and to exclude any possible interference by germinative or post-germinative events, analyses were carried out exclusively in those not showing any visible signs of germination. Since cotyledons rather than axes appear to be the cold-perceiving organ in walnut kernels (Einali and Sadeghipour, Reference Einali and Sadeghipour2007), the present discussion is based mainly on the changes in this organ.
Cold stratification is accompanied by gluconeogenesis of lipid reserves
Imbibition was sufficient to bring about mobilization of lipids (Fig. 2), and storage proteins (Einali and Sadeghipour, Reference Einali and Sadeghipour2007) in walnut kernels, irrespective of the temperature of incubation, whereas the mobilization of food reserves in other stratification-requiring tree seeds commences only during cold treatment (Dawidowicz-Grzegorzewska, Reference Dawidowicz-Grzegorzewska1989; Li and Ross, Reference Li and Ross1990a; Zarska-Maciejewska, Reference Zarska-Maciejewska1992; Wang and Berjak, Reference Wang and Berjak2000; Andriotis et al., Reference Andriotis, Smith and Ross2004). Lipid mobilization in cold-stratified walnut kernels was accompanied by an increase of isocitrate lyase activity (Fig. 5A) and the accumulation of starch and higher amounts of reducing and non-reducing sugars (Fig. 3A, C, E), which is consistent with the occurrence of gluconeogenesis. Carbohydrate accumulation and the development of isocitrate lyase activity have been reported in some tree seeds during the alleviation of dormancy by cold (Noland and Murphy, Reference Noland and Murphy1984; Dawidowicz-Grzegorzewska, Reference Dawidowicz-Grzegorzewska1989; Li and Ross, Reference Li and Ross1990a, Reference Li and Rossb) as a prerequisite for germination.
Lipid mobilization in walnut kernels under warm conditions might be associated with a non-gluconeogenic operation of the glyoxylate cycle, as occurs in some oilseeds (Eastmond et al., Reference Eastmond, Germain, Lange, Bryce, Smith and Graham2000; Eastmond and Graham, Reference Eastmond and Graham2001). Lack of starch accumulation under warm conditions (Fig. 3), despite isocitrate lyase activity (Fig. 5B), supports this idea. The diversion of lipolytic metabolites to respiratory pathways (Chia et al., Reference Chia, Pike and Rawsthorne2005) is expected in warm-incubated kernels.
Walnut kernel fatty acyl-ester hydrolase as a non-gluconeogenic enzyme
Two lipolytic activities are detectable in imbibed walnut kernels. One of them is a true lipase activity responsible for kernel lipid mobilization (unpublished data), and the other one is a fatty acyl-ester hydrolase activity that declined in cold-stratified kernels, but remained fairly level during warm incubation (Fig. 4A, B). SDS-PAGE revealed a 64 kDa acyl-ester hydrolase activity band with greater expression in warm-incubated kernels. However, this enzyme may not be involved directly in mobilization and gluconeogenesis of storage lipids and, rather, correlates with the deteriorative process in moistened kernels under warm conditions: (1) as lipid mobilization started in cold-stratified kernels, acyl-ester hydrolase activity declined (Fig. 4A); and (2) despite its higher activity in warm-incubated kernels (Fig. 4B), there was no accumulation of either starch or soluble sugars (Fig. 3). In addition, natural ageing of walnut kernels for 1 and 2 years abolished their germination capacity, but was accompanied by greater acyl-ester hydrolase activity (data not shown). Decreased seed viability of Pinus sylvestris L. due to natural ageing was correlated with changes in the composition of polar and non-polar lipids (Tammela et al., Reference Tammela, Hopia, Hiltunen, Vuorela and Nygren2000), implying the involvement of lipolytic enzymes. Acyl-ester hydrolases have also been implied as the initiators of other deteriorative processes, such as senescence in plant organs (Hong et al., Reference Hong, Wang, Hudak, Schade, Froese and Thompson2000; Thompson et al., Reference Thompson, Taylor and Wang2000).
Warm incubation accompanies the build-up of oxidative stress in walnut kernels
Warm-incubated walnut kernels undergo accelerated ageing (Einali and Sadeghipour, Reference Einali and Sadeghipour2007) with a significant accumulation of hydrogen peroxide (Fig. 6), an indicator of oxidative stress leading to increased lipid peroxidation (Fig. 7), a process that would explain non-germinability and deterioration after 20 d in the warmth, since it is known as a primary cause of seed deterioration during ageing (McDonald, Reference McDonald, Benech-Arnold and Sanchez2004).
A significant decline in the activity of NADP+-isocitrate dehydrogenase from the mid-period of kernel incubation at warm temperatures (Fig. 8B) and the maintenance of this activity in cold-stratified kernels (Fig. 8A), implies that the regenerating capacity of reducing equivalents has been compromised. Thus, reduced germination of warm-incubated walnut kernels (Fig. 1) might result from failure to produce sufficient reducing equivalents to counteract oxidative stress. The role of cytosolic NADP+-isocitrate dehydrogenase against oxidative stress has been well documented in animal cells (Kim and Park, Reference Kim and Park2003); a similar role has been implied in plants (Pastori and del Rio, Reference Pastori and del Rio1997; Corpas et al., Reference Corpas, Barroso, Sandalio, Palma, Lupianez and del Rio1999; Hodges et al., Reference Hodges, Flesch, Galvez and Bismuth2003).
In conclusion, enhanced germination capacity of cold-stratified walnut kernels compared to the warm-incubated ones may be attributed partly to alterations in lipid metabolism. Lipid mobilization in cold-stratified walnut kernels is accompanied by increased products of gluconeogenesis. However, in warm-incubated kernels, the decline in lipid content is non-gluconeogenic and suggests deterioration, as it is accompanied by greater activity of a 64-kDa fatty acyl-ester hydrolase, increased lipid peroxidation, hydrogen peroxide accumulation and potential weakening of NADPH regeneration capacity. Whether these differences in walnut metabolism are the direct effects of cold conditions or associated with dormancy removal from walnut kernels needs further investigations. These data provide biochemical evidence in support of Wang and Berjak (Reference Wang and Berjak2000), who consider that the beneficial effects of cold stratification of imbibed seeds are due to its ability to prevent deteriorative processes and activate cellular repair mechanisms. Furthermore, it refines the ideas suggested by earlier work (Einali and Sadeghipour, Reference Einali and Sadeghipour2007) by showing that there is no block to lipid mobilization in walnut kernels after imbibition, and that cold stratification possibly promotes germination by enhancement of kernel gluconeogenic competence.
Acknowledgements
We thank the GUASNR Deputy of Research and Office of Higher Education for financial support to T.N. and F.T. in the form of grants for MSc research projects. Thanks are also due to Ms Mahrokh Sharbatkhory from the University Central Laboratory for her technical assistance.










