There are an increasing number of patients of all ages, who have undergone transcatheter device closure of atrial septal defects or patent foramen ovale. This procedure has been shown to be safe and efficient.Reference Losay, Petit and Lambert1 However, if at a later stage, transseptal puncture is required for an electrophysiology study or ablation of an arrhythmia, it may prove difficult or unsafe to puncture the septum. Indeed, it is uncertain whether puncture can be performed through the device at all. In addition, it is possible that devices may induce arrhythmias as a result of scarring and slow conduction within the artria.
A recent paper reported on two patients in whom transseptal puncture was performed for catheter ablation after the patients had undergone closure of patent foramen ovale with Amplatzer devices.Reference Zaker-Shahrak, Fuhrer and Meier2 The septum was punctured safely just below the edges of the devices. We are not aware of any published reports on the safety and feasibility of transseptal puncture following catheter closure of atrial septal defects. With passage of time, such procedures are likely to be required with increasing frequency.
We report on a 37-year-old woman, who developed atrial arrhythmias following closure of atrial septal defect with an Amplatzer septal occluder device and speculate that the device may have created the substrate for the arrhythmia.
Case report
A 37-year-old woman underwent catheter closure of atrial septal defect closure with a 30-millimetre Amplatzer septal occluder device 4 years before at another institution. The indication for closure of the defect was increasing shortness of breath and a dilated right heart. There was no history of arrhythmias before the device closure. After 3 months of the procedure, she presented with symptomatic palpitations and an electrocardiogram suggestive of atrial flutter. This was successfully cardioverted, but she had further recurrences requiring repeated cardioversions. She subsequently underwent right atrial flutter isthmus ablation 2 years later with creation of bidirectional isthmus block. The electrophysiological study at the time suggested that this was a atypical flutter with a probable left-sided circuit, which was not isthmus dependent. She reverted back into atrial flutter a week after this procedure and continued to have symptomatic palpitations, which were increasingly difficult to control pharmacologically, despite the trial of treatment with metoprolol, atenolol, amiodarone, and verapamil. She was referred to our centre for a further electrophysiological study and possible ablation. Initial electrophysiological study showed a tachycardia based in the left atrium with a cycle length of 240 milliseconds, so a left-sided ablation was planned under transoesophageal echocardiographic guidance to exclude thrombus in the left atrial appendage and to identify a safe area for the transseptal puncture. The transoesophageal echocardiogram showed that although the left atrial disc of the device covered a large area of the atrial septum, there was a small rim of atrial septum inferior to the device available for safe puncture. This rim was used as a target for the puncture, which was performed successfully with a Brockenbrough needle within an SL1 sheath (Fig 1).
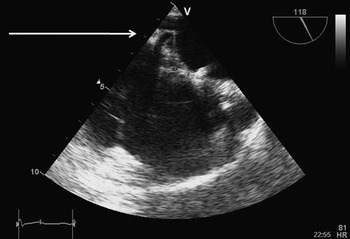
Figure 1 Transoesophageal echocardiographic still frame image showing the Amplatzer device in situ. The Brockenbrough needle is seen tenting the atrial septum just below the device.
Initially, the patient was in sinus rhythm, but it was possible to induce tachycardia with atrial pacing manouvres. The CARTO XP system (Biosense Webster, CA, USA) was used to map the left atrium during the tachycardia using an irrigated tip catheter (Navistar Thermocool F-curve, Biosense Webster, California, United States of America). The CARTO system was used so that only one transseptal puncture would be required for the mapping. The arrhythmia was unstable and degenerated into atrial fibrillation, which recurred despite repeated cardioversion. A wide area of circumferential ablation of the right and left pulmonary veins terminated the atrial fibrillation. Following this, the tachycardia was inducible and stable with a cycle length of 240 milliseconds. The CARTO activation map suggested a focal atrial tachycardia with 2:1 block arising from the edge of the Amplatzer septal occluder near the mitral valve ring. There was also evidence of extensive scarring around the device on the voltage map. Radiofrequency ablation in this region terminated the tachycardia, the lesion being at the earliest activation on the CARTO map 41 milliseconds ahead of the P-wave. Figure 2 shows the fragmented endocardial electrogram at the time of breaking the atrial tachycardia and the artefact produced by the ablation catheter slipping through the septum back into the right atrium is seen. Subsequently, the tachycardia was no longer inducible.

Figure 2 The fragmented endocardial electrogram at the time of breaking the atrial tachycardia (200 millimetres per second). An arrow points to the artefact produced by the ablation catheter slipping through the septum back into the right atrium.
The patient was discharged in stable sinus rhythm. Before discharge, an echocardiogram showed an unchanged appearance of the Amplatzer septal occluder and no inter-atrial shunt. After 10 weeks of the procedure, the patient reports no symptoms or recurrence of arrhythmias.
Discussion
Transient atrioventricular block may occur at the time of closure of atrial septal defect with devices;Reference Suda, Raboisson, Piette, Dahdah and Miro3 however, little is known about arrhythmias in the longer term. In a cohort of 65 consecutive patients undergoing device closure of atrial septal defects, no significant arrhythmias were reported on 24-hour Holter monitoring at a mean of 4 months with a range from 1 to 12 months.Reference Celiker, Ozkutlu, Karakurt and Karagoz4 Transseptal puncture for catheter ablation because of atrial fibrillation has been reported in two patients, who had undergone closure of patent foramen ovale with the Amplatzer devicesReference Zaker-Shahrak, Fuhrer and Meier2. We are not aware of any reports on transseptal punctures following device closure of atrial septal defects.
The left atrial disc diameter of the 30-millimetre Amplatzer septal occluder is 44 millimetres. Thus, it covers a large area of the atrial septum. Before the attempted ablation, there was concern that there may be insufficient remaining atrial septum for safe transseptal puncture. However, transoesophageal assessment and guidance was crucial as it showed a small area of atrial septum suitable for the puncture, which was thus successfully performed with a standard Brockenbrough needle and an SL1 sheath. We had considered using a radiofrequency transseptal needle (Bayliss Medical Company Inc., Montreal, Canada) to facilitate the transseptal puncture. In order to assess the safety of using such a heated needle through or near a device, an ex vivo experiment was performed with an Amplatzer device placed in a saline bath. On contact between the needle heated with radiofrequency and the wire mesh of the device, there was some sparking and damage to the device and it was felt to be unsafe for use in the patient. Later, we used radiofrequency energy to ablate near the device; this was felt to be safe as there was no direct contact with the wire mesh and the device was likely to be fully endothelialised. Although the standard method of puncture was successful in our patient, generally these may prove more difficult without transoesphageal guidance, especially when large occluders are used to close these defects. Therefore, the value of transoesophageal guidance cannot be underestimated.
Our patient also introduces the possibility that the tachycardia arising from close to the edge of the device was possibly related to scar formation and anisotropic conduction secondary to the device implant.
Conclusion
This case highlights the possibility of arrhythmias arising secondary to device closure of atrial septal defects and successful ablation. In the future, there is likely to be an increasing need for left atrial access after the implantation of devices. Such access will not be needed through the mesh of the devices but around their edges under transoesophageal echocardiographic guidance.




