Introduction
Intracytoplasmic sperm injection (ICSI) is an assisted reproductive technique that consists of injecting a spermatozoon into the ooplasm of an oocyte arrested in metaphase II (MII). ICSI has been applied in different species as in Uehara and Yanagimachi (Reference Uehara and Yanagimachi1976) who injected golden hamster oocytes with sperm nuclei of the same species or human spermatozoa, obtaining pronuclear formation. The parameters generally used to determine the efficiency of an ICSI experiment are the oocyte activation rate, often measured as the percentage of oocytes that release two polar bodies (PBs), the fertilization rate, often measured as the percentage of oocytes that release two PBs and form two pronuclei (PNs) and the cleavage, development to blastocysts, pregnancy and birth rates (Ng et al., Reference Ng, Martelli, Liow, Herbert and Oh2002; Galli et al., Reference Galli, Vassiliev, Lagutina, Galli and Lazzari2003; Nakai et al., Reference Nakai, Ito, Suzuki, Fuchimoto, Sembon, Suzuki, Noguchi, Kaneko, Onishi, Kashiwazaki and Kikuchi2016; Canel et al., Reference Canel, Suvá, Bevacqua, Arias, Felmer and Salamone2018; Zhu et al., Reference Zhu, Cui and Dai2018), being the rate of development to blastocysts one of the most important parameters, as at this stage embryos are transferred to recipient females, studied in vitro or cryopreserved.
It has been proposed that ICSI can serve as a tool to help endangered species (Salamone et al., Reference Salamone, Canel and Rodríguez2017), as these populations have low genetic diversity and low sperm quality, especially after cryopreservation procedures (Baqir et al., Reference Baqir, Orabah, Al-Zeheimi, Al-Shakaili, Al-Rasbi, Gartley and Mastromonaco2018; Cai et al., Reference Cai, An, Liu, Yie, Zhang, Li, Chen, Wang, Morrell and Hou2018; Franklin et al., Reference Franklin, Waddell and Goodrowe2018). It also constitutes a useful technique for the production of genetically modified embryos by coupling with the sperm-mediated gene transfer technique (ICSI–SMGT) (Perry et al., Reference Perry, Wakayama, Kishikawa, Kasai, Okabe, Toyoda and Yanagimachi1999; Pereyra-Bonnet et al., Reference Pereyra-Bonnet, Fernández-Martín, Olivera, Jarazo, Vichera, Gibbons and Salamone2008; Sánchez-Villalba et al., Reference Sánchez-Villalba, Arias, Loren, Fuentes, Pereyra-Bonnet, Salamone and Felmer2018). Conversely, ICSI offers greater potential for research on early embryonic development with respect to other embryo production techniques such as in vitro fertilization (IVF), as it allows several experiments to be performed with only one sperm sample (Takahashi et al., Reference Takahashi, Hanazawa, Inoue, Sato, Sedohara, Okahara, Suemizu, Yagihashi, Yamamoto, Eto, Konno, Okano, Suematsu and Sasaki2014). These applications make ICSI a promising technique for the improvement of animal production systems.
Currently, its main utility has been focused to overcome severe infertility problems associated with the male factor in humans, achieving pregnancies and offspring from severely impaired sperm samples (Palermo et al., Reference Palermo, Joris, Devroey and Van Steirteghem1992). In animals such as mice, high blastocyst formation rates have been observed (∼50−70%) with the birth of live offspring (Kimura and Yanagimachi, Reference Kimura and Yanagimachi1995; Hu et al., Reference Hu, Shen, Zheng, Wang, Liu, Jin and Lei2012). Successful results have also been achieved in equine ICSI reaching clinical and commercial applications and being the only technique available for the in vitro production of embryos, albeit its low efficiency (Salamone et al., Reference Salamone, Canel and Rodríguez2017). However in bovine, despite the efforts of different research groups, the efficiency of this technique is far from optimal. This aspect was confirmed in a previous study by our research group in which IVF and ICSI experiments were carried out in bovine under the same laboratory conditions and we found cleavage (89%) and blastocyst (36%) formation rates superior for embryos generated by IVF compared with ICSI (64% and 22%, respectively) (Arias et al., Reference Arias, Risopatrón, Sánchez and Felmer2015).
This species is one of the most important for the food industry, both for its meat, which is one of the most produced worldwide, and for its milk (Tapia, Reference Tapia2020), therefore the standardization of ICSI for the production of bovine embryos, would allow the generation of offspring from individuals with characteristics of interest for the industry, but that have low sperm quality, in terms of sperm viability and/or motility (Awda et al., Reference Awda, Miller, Montanholi, Voort, Caldwell, Buhr and Swanson2013; Kaya and Memili, Reference Kaya and Memili2016), which are considered the most important parameters in the evaluation of sperm quality (Morrell and Rodriguez-Martinez, Reference Morrell and Rodriguez-Martinez2009). In addition, a greater number of embryos can be generated by ICSI from a single semen sample, which can be optimized by cutting the straw in parts, further allowing the generation of offspring with the desired sex by coupling this technique with cell sorting. It should also be noted that the use of other assisted reproductive techniques, such as IVF or artificial insemination (AI), require a much higher number of spermatozoa with normal morphology and correct functionality, while ICSI has been successful in several species, even using suboptimal semen quality (Unnikrishnan et al., Reference Unnikrishnan, Kastelic and Thundathil2021).
In fact, viable embryos and offspring have been produced by ICSI with spermatozoa with damaged plasma and acrosome membranes by treating sperm with membrane-destabilizing compounds including DTT, lysolecithin, Triton X-100, or alkali, among others (Morozumi and Yanagimachi Reference Morozumi and Yanagimachi2005; Morozumi et al., Reference Morozumi, Shikano, Miyazaki and Yanagimachi2006; Li et al., Reference Li, Mizutani, Ono and Wakayama2009; Seita et al., Reference Seita, Ito and Kashiwazaki2009; Zambrano et al., Reference Zambrano, Águila, Arias, Sánchez and Felmer2016). The later treatment was effective in generating high rate of blastocysts and normal offspring after ICSI, and was also efficient in the generation of transgenic mice by ICSI–sperm-mediated gene transfer (Li et al., Reference Li, Mizutani, Ono and Wakayama2010). Bovine blastocysts have also been produced by injection of heat or freeze-dried spermatozoa, which is equivalent to using dead sperm (Lee and Niwa, Reference Lee and Niwa2006; Keskintepe et al., Reference Keskintepe, Pacholczyk, Machnicka, Norris, Curuk, Khan and Brackett2002).
Although it has been reported that the rates of major malformations in children born after intracytoplasmic morphologically selected sperm injection (IMSI) are significantly lower (1.33%) than those born after ICSI (3.8%) (Cassuto et al., Reference Cassuto, Hazout, Bouret, Balet, Larue, Benifla and Viot2014), the use of normal and teratozoospermic spermatozoa in different species has also revealed no differences in parameters such as cleavage rate, blastocyst formation, pregnancy, and risk of birth defects (Prochowska et al., Reference Prochowska, Niżański, Partyka, Kochan, Młodawska, Nowak, Skotnicki, Grega and Pałys2019; Zhou et al., Reference Zhou, Huang, Jiang, Ji, Gong, Fan and Zhu2021). Similarly, human ICSI with spermatozoa with different abnormalities, such as severe asthenoteratospermia, oligoasthenospermia and multiple morphological abnormalities of the flagellum, yielded fertilization, blastocyst development and clinical pregnancy rates of more than 65%, 50%, and 50%, respectively, and healthy births (Wu et al., Reference Wu, Wang, Cheng, Gao, Liu, Zhang, Jiang, Li, Zhu, Lv, Liu, Tan, Zhang, Wang, Ni, Chen, Song, Zhou and Wei2020). Therefore, the relationship between morphological abnormal sperm and post-ICSI success is not entirely conclusive.
Some limitations to the success of ICSI in bovine are related to oocyte factors, such as failures in oocyte activation and sperm factors, including inadequate or lack of sperm capacitation prior to injection, the presence of sperm membranes in the ooplasm, defective decondensation of sperm chromatin, and technical factors, such as the injection method. Accordingly, different strategies have been devised in order to increase the efficiency of this technique in bovine, such as the use of exogenous oocyte activators, sperm capacitation pretreatments, use of detergents or compounds capable of dissolving the sperm membranes, incubation with reducing agents to facilitate sperm chromatin decondensation, implementation of ICSI variants such as piezo-directed injection, among others.
Oocyte factors
During natural fertilization, artificial insemination (AI) or IVF, different events occur such as the fusion of the plasma membranes of the sperm and oocyte, the entry of the intracellular contents of the sperm into the ooplasm, including sperm–oocyte activating factor (SOAF), and the solubilization of the postacrosomal region of the perinuclear theca (PA-PT) (Sutovsky et al., Reference Sutovsky, Manandhar, Wu and Oko2003; Yanagimachi, Reference Yanagimachi2005). Importantly, although phospholipase Cζ (PLCζ) has been widely accepted as a SOAF, because it qualifies as such, other candidate proteins have also been proposed (Yeste et al., Reference Yeste, Jones, Amdani, Coward, Nagy, Varghese and Agarwal2019). It has been observed, for example, that postacrosomal WW domain-binding protein (PAWP/WBP2NL) elicits calcium oscillations that activate mammalian oocytes (Aarabi et al., Reference Aarabi, Balakier, Bashar, Moskovtsev, Sutovsky, Librach and Oko2014). However, other reports have pointed out that this protein is unable to achieve this effect (Nomikos et al., Reference Nomikos, Sanders, Kashir, Sanusi, Buntwal, Love, Ashley, Sanders, Knaggs, Bunkheila, Swann and Lai2015), therefore the identity of SOAF remains controversial (Yeste et al., Reference Yeste, Jones, Amdani, Coward, Nagy, Varghese and Agarwal2019) (Figure 2).
SOAF entry into the ooplasm leads to the formation of inositol triphosphate (IP3) and diacylglycerol (DAG) from inositol bisphosphate (PIP2), which is thought to originate from cytoplasmic vesicles (Sanders et al., Reference Sanders, Ashley, Moon, Woolley and Swann2018; Yeste et al., Reference Yeste, Jones, Amdani, Coward, Nagy, Varghese and Agarwal2019). IP3 is taken up by its receptor (IP3R) in the smooth endoplasmic reticulum (SER), triggering the release of intracellular calcium [(Ca2+)i] in the form of repetitive oscillations that increase IP3 production, creating a positive feedback (Sanders et al., Reference Sanders, Ashley, Moon, Woolley and Swann2018; Yeste et al., Reference Yeste, Jones, Amdani, Coward, Nagy, Varghese and Agarwal2019). The (Ca2+)i oscillations activate the oocyte through different events such as cortical granule exocytosis (CGE), to prevent polyspermy, activation of calcium/calmodulin-dependent protein kinase II (CaMKII), which modulates signalling pathways that downregulate maturation-promoting factor (MPF), an heterodimer complex constituted by CDK1/p34cdc2 (the catalytic subunit) and cyclin B (the regulatory subunit), and the decreased MAPK activity, possibly also related to decreased MPF (Ducibella et al., Reference Ducibella, Huneau, Angelichio, Xu, Schultz, Kopf, Fissore, Madoux and Ozil2002; Madgwick et al., Reference Madgwick, Levasseur and Jones2005; Yanagimachi, Reference Yanagimachi2005; Kubiak et al., Reference Kubiak, Ciemerych, Hupalowska, Sikora-Polaczek and Polanski2008; Suvá et al., Reference Suvá, Canel and Salamone2019). Although it was believed until recently that the downregulation of MPF occurs in the bovine oocyte by proteasome-mediated degradation of cyclin B, allowing the exit from the MII stage, new findings from our group indicated that downregulation of MPF would occur by specific inhibition of CDK1 without degradation of cyclin B (Valencia et al., Reference Valencia, Pérez, Matus, Felmer and Arias2021) (Figure 2).
It is also worth mentioning that (Ca2+)i and DAG activate protein kinase C (PKC), which has also a role in CGE through phosphorylation of myristoylated alanine-rich C-kinase substrate (MARCKS) and subsequent disassembly of filamentous actin (F-actin), and in other subsequent processes such as the reorganization of cytoplasmic structures like endoplasmic reticulum, changing its location from cortical to medullar, through the polymerization of F-actin (Tsaadon et al., Reference Tsaadon, Kaplan-Kraicer and Shalgi2008; Yeste et al., Reference Yeste, Jones, Amdani, Coward, Nagy, Varghese and Agarwal2019; Feitosa et al., Reference Feitosa, Lopes, Visintin and Assumpção2020). A schematic representation of the main events that occur during fertilization in mammals is depicted in Figure 2.
In the bovine species, most oocytes subjected to ICSI fail to activate because sperm injection alone is not sufficient to initiate (Ca2+)i oscillations and the other aforementioned events, unlike observations in other species such as human and mouse (Malcuit et al., Reference Malcuit, Maserati, Takahashi, Page and Fissore2006; Salamone et al., Reference Salamone, Canel and Rodríguez2017). For this reason, bovine oocytes subjected to ICSI require external activation stimuli to initiate embryonic development.
Oocyte activation
External or artificial activation can be induced in oocytes in the absence of paternal genome to generate parthenotes that, despite presenting a low developmental capacity, constitute a valuable research tool (Nair et al., Reference Nair, Aboobacker, Mutalik, Kalthur and Adiga2017; Suvá et al., Reference Suvá, Canel and Salamone2019). For example, activation in the establishment of haploid embryonic stem cells (PG-haESCs) can be used in the identification of recessive or X-linked gene functions, in the generation of genetically modified animals (He et al., Reference He, Chen and Gao2019) or in the evaluation of oocyte activation conditions in techniques such as somatic cell nuclear transfer (SCNT) or ICSI (Felmer and Arias, Reference Felmer and Arias2015; Arias et al., Reference Arias, Sánchez and Felmer2016).
Unlike oocytes used for SCNT, which require diploid activation treatments to maintain the 2n chromosome set of the injected cell, for oocytes subjected to ICSI, the most suitable activation treatments are those that allow the extrusion of the second polar body (2nd PB) after the injection of the spermatozoon and reduce the number of parthenogenetic blastocysts that can be transferred to female recipients (Rho et al., Reference Rho, Wu, Kawarsky, Leibo and Betteridge1998a; Suvá et al., Reference Suvá, Canel and Salamone2019).
In general, activation of bovine oocytes has been performed through chemical or physical (electrical) stimuli, using different treatments to induce Ca2+ i oscillations in the ooplasm but, as they are not sufficient to sustain Ca2+ i oscillations and oocyte activation, they are usually combined with other compounds such as inhibitors of MPF activity or protein phosphorylation. Further details regarding oocyte activation treatments with better results after ICSI in cattle are shown in Table 1.
Table 1. Main oocyte activation treatments assessed in bovine ICSI, mechanisms of action and most relevant results
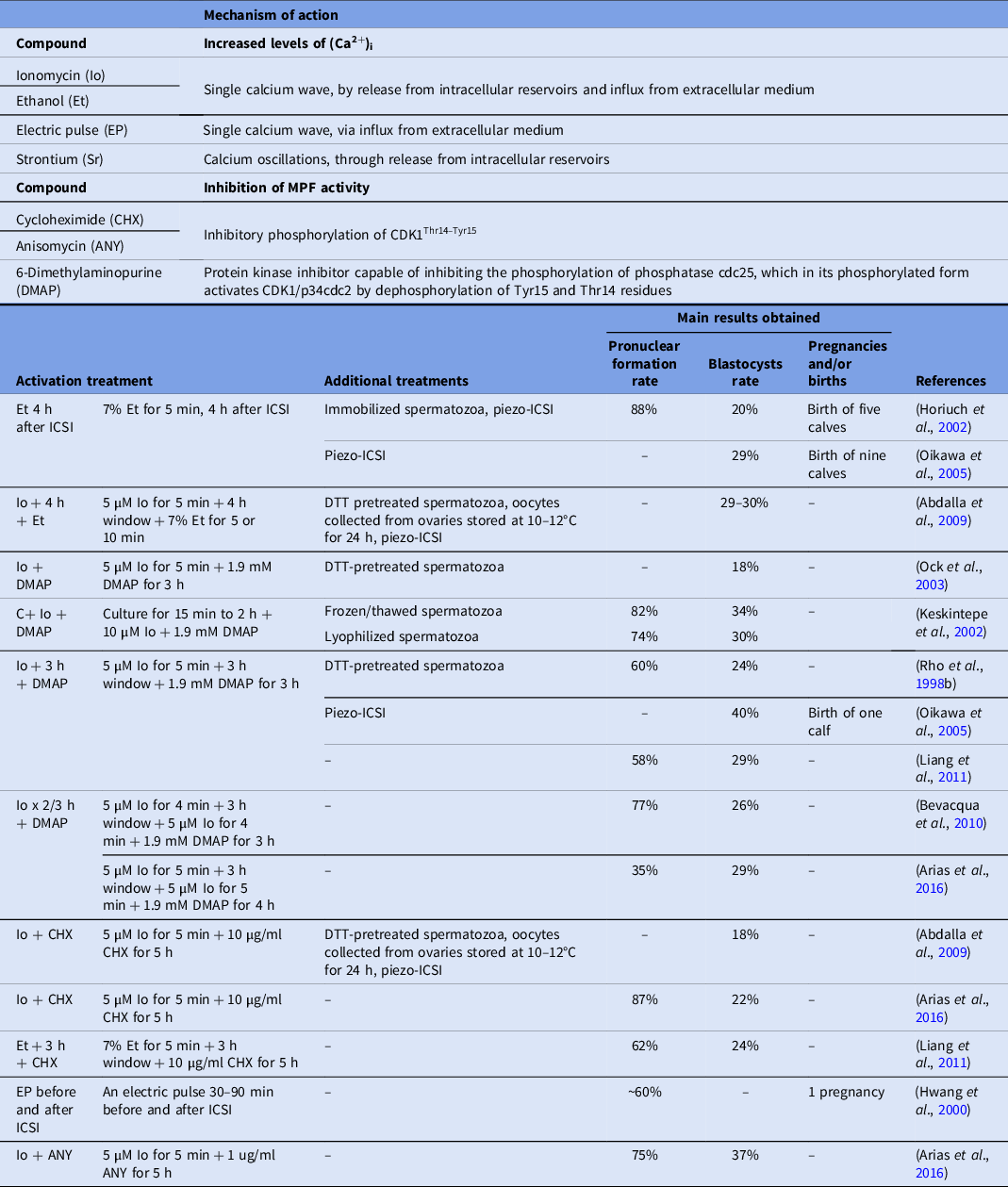
In this regard, the most commonly used activation protocols include the incubation with ionomycin (Io) or ethanol (Et) that, unlike natural fertilization, induces a single Ca2+ wave through its release from intracellular reservoirs and its influx from extracellular medium (Hoth and Penner, Reference Hoth and Penner1992; Shiina et al., Reference Shiina, Kaneda, Matsuyama, Tanaka, Hiroi and Doi1993), in combination with cycloheximide (CHX), a protein synthesis inhibitor, which has recently been shown to inhibit MPF through specific phosphorylation of CDK1Thr14-Tyr15 (Valencia et al., Reference Valencia, Pérez, Matus, Felmer and Arias2021) or 6-dimethylaminopurine (DMAP), a protein kinase inhibitor capable of inhibiting MPF activity indirectly, by inhibiting the phosphorylation of phosphatase cdc25, which in its phosphorylated form activates CDK1/p34cdc2 by dephosphorylation of Tyr15 and Thr14 residues (Alberio et al., Reference Alberio, Zakhartchenko, Motlik and Wolf2001).
Classical oocyte activation treatments
One of the earliest oocyte activation protocols evaluated was 7% Et for different exposure times. In this study, bovine oocytes were in vitro matured for 30 h and parthenogenetically activated with 7% Et for 2 min, obtaining 2PN formation rates greater than 50%, a protocol that would be inappropriate for oocytes subjected to ICSI, because it could generate a high proportion of embryos with more than 2PNs (Minamihashi et al., Reference Minamihashi, Watson, Watson, Church and Schultz1993).
Later, the parthenogenetic activation of bovine oocytes with 7% Et for 5 min, followed by 10 µg/ml CHX for 20 h, generated higher pronuclear formation rates (84%) compared with Et (44%) or cycloheximide (43%) alone (Yang et al., Reference Yang, Presicce, Moraghan, Jiang and Foote1994), supporting the idea that it is more appropriate to use compounds that induce an increase in intracellular calcium in combination with another modulator of oocyte activation. Subsequently, Chen and Seidel (Reference Chen and Seidel1997) did not observe blastocyst formation upon activation of bovine oocytes with 7% Et for 5 min after injection of heparin-capacitated spermatozoa. In contrast, Horiuch et al. (Reference Horiuch, Emuta, Yamauchi, Oikawa, Numabe and Yanagimachi2002) reported that 88% of oocytes fertilized normally and a blastocyst rate of 20% when bovine oocytes were activated with Et 4 h after ICSI (Horiuch et al., Reference Horiuch, Emuta, Yamauchi, Oikawa, Numabe and Yanagimachi2002). These differences may be attributed to the fact that, in this study, the injection was performed with a piezo system using immobilized spermatozoa and that Et was used 4 h after ICSI. Similarly, Fujinami et al. (Reference Fujinami, Hosoi, Kato, Matsumoto, Saeki and Iritani2004) used the piezo system to immobilize and inject spermatozoa into bovine oocytes, and also activated the oocytes with Et 4 h after ICSI, obtaining normal pronuclear formation and blastocyst rates of 91% and 14%, respectively (Fujinami et al., Reference Fujinami, Hosoi, Kato, Matsumoto, Saeki and Iritani2004). Later, relatively high percentage of blastocysts (29–30%) were obtained by Abdalla et al. (Reference Abdalla, Shimoda, Hirabayashi and Hochi2009) using piezo-directed ICSI with DTT-treated spermatozoa and subsequent activation with Io + 4 h + Et, confirming the higher efficiency of this protocol of Et activation (Abdalla et al., Reference Abdalla, Shimoda, Hirabayashi and Hochi2009).
One of the first studies evaluating activation of bovine oocytes with Io was that of Susko-Parrish et al. (Reference Susko-Parrish, Leibfried-Rutledge, Northey, Schutzkus and First1994), who reported that exposure of in vitro matured bovine oocytes to parthenogenetic activation with 5 µM Io for 4 min exerted the greatest effect on the exit of meiosis II, preserving the integrity of the oocytes, thus this compound alone or in combination with other activators, began to be widely used (Susko-Parrish et al., Reference Susko-Parrish, Leibfried-Rutledge, Northey, Schutzkus and First1994). In this way, activation of bovine oocytes injected with frozen–thawed or lyophilized spermatozoa with 10 µM Io for 5 min, resulted in 2PN formation rates of 66% and 56% and blastocyst rates of 19% and 11%, respectively (Keskintepe et al., Reference Keskintepe, Pacholczyk, Machnicka, Norris, Curuk, Khan and Brackett2002). In a different study, 18% of bovine blastocyst rate using piezo-directed ICSI and activation with 5 µM Io in Ca2+- and Mg2+-free medium for 5 min was obtained (Oikawa et al., Reference Oikawa, Takada, Kikuchi, Numabe, Takenaka and Horiuchi2005).
In addition, Susko-Parrish et al. (Reference Susko-Parrish, Leibfried-Rutledge, Northey, Schutzkus and First1994) also evaluated DMAP in parthenogenetic activation of bovine oocytes, in vitro matured for 24 h or more, with 5 µM Io for 4 min followed immediately by 1.9 mM DMAP for 5 h, obtaining a pronuclear formation rate greater than 75% and a blastocyst rate of 21%. However, this protocol (Io followed immediately by DMAP), resulted in a high proportion of embryos that formed 1PN, without extrusion of the 2nd PB (Susko-Parrish et al., Reference Susko-Parrish, Leibfried-Rutledge, Northey, Schutzkus and First1994). Later, Rho et al. (Reference Rho, Wu, Kawarsky, Leibo and Betteridge1998a) observed that bovine oocytes parthenogenetically activated with Io + 3 h + DMAP, showed significantly higher rates of oocytes that formed 1PN and extruded the second polar body (2nd PB) compared with 5 µM Io for 5 min (Io), 5 µM Io for 5 min repeated four times (Io × 4) or Io + DMAP. Moreover, in the same study it was observed that, although the blastocyst rates obtained when treating bovine oocytes with 5 µM Io, Io × 4 or Io + 3 h + DMAP, were significantly lower than those obtained when treated with Io + DMAP, the former were mostly haploid whereas, with the latter treatment, they were mostly mixoploid or polyploid, highlighting the importance of delaying the addition of DMAP (Rho et al., Reference Rho, Wu, Kawarsky, Leibo and Betteridge1998a). In another study from the same group, the inclusion of a 3 h culture period between the Io and DMAP application, generated 60% of 2PNs, 24% blastocysts rate, and 61% of diploid embryos in oocytes subjected to ICSI (Rho et al., Reference Rho, Kawarsky, Johnson, Kochhar and Betteridge1998b). However, controversy exist as in Keskintepe et al. (Reference Keskintepe, Pacholczyk, Machnicka, Norris, Curuk, Khan and Brackett2002) who obtained high proportions of 2PN formation and development to blastocysts after injection of frozen–thawed (82% and 34%, respectively) or lyophilized (74% and 30%, respectively) spermatozoa and activation of bovine oocytes with 10 µM Io and 1.9 mM DMAP, after a culture period of 15 min to 2 h, and without this window time between treatments (Keskintepe et al., Reference Keskintepe, Pacholczyk, Machnicka, Norris, Curuk, Khan and Brackett2002). Subsequently, it was observed that the activation of bovine oocytes with Io + DMAP or Io + 3 h + DMAP, showed higher blastocyst rates (18% and 15%, respectively) compared with 5 µM Io alone (0.6%), although more ploidy defects were detected after performing ICSI in bovines and activation with DMAP immediately after Io, than after 3 h of culture, emphasizing again the importance of the culture period between both treatments to allow the extrusion of the 2nd PB (Ock et al., Reference Ock, Bhak, Balasubramanian, Lee, Choe and Rho2003). Conversely, Abdalla et al. (Reference Abdalla, Shimoda, Hirabayashi and Hochi2009) obtained only 11% blastocyst formation by piezo-ICSI with DTT-treated spermatozoa and activation with Io + 3 h + DMAP (Abdalla et al., Reference Abdalla, Shimoda, Hirabayashi and Hochi2009). Subsequently, Bevacqua et al. (Reference Bevacqua, Pereyra-Bonnet, Fernandez-Martin and Salamone2010) evaluated two new Io activation treatments: (i) Io × 2/3 h + DMAP; and (ii) applying two doses of 5 µM Io for 4 min, separated by 3 h of culture (Io × 2/3 h) in ICSI–SMGT experiments, and obtained 77% and 55% of oocytes that formed 2PNs and extruded the 2nd PB, respectively, and 26% and 9% blastocyst formation, respectively (Bevacqua et al., Reference Bevacqua, Pereyra-Bonnet, Fernandez-Martin and Salamone2010). However, although Arias et al. (Reference Arias, Sánchez and Felmer2016) obtained almost a similar blastocyst formation rate (29%) when activating bovine oocytes after ICSI with a similar treatment (Io × 2/3 h + DMAP, see Table 1), the correct pronuclear formation rate observed was only 35% and with a high percentage of parthenogenetic embryos with this treatment (Arias et al., Reference Arias, Sánchez and Felmer2016).
Another drawback of using protein kinase inhibitors is that these compounds are broad-spectrum drugs with low specificity, and can intervene in several metabolic pathways, potentially affecting subsequent embryonic development (Alberio et al., Reference Alberio, Kubelka, Zakhartchenko, Hajdúch, Wolf and Motlik2000; Fernandes et al., Reference Fernandes, Devito, Martins, Blanco, de Lima Neto, Tsuribe, Gonçalves and da Cruz Landim-Alvarenga2014). For this reason, activation of bovine oocytes was also evaluated in parallel in some groups using CHX as an alternative to DMAP. One of the earliest studies was that of Suttner et al. (Reference Suttner, Zakhartchenko, Stojkovic, Müller, Alberio, Medjugorac, Brem, Wolf and Stojkovic2000), who reported that activating bovine oocytes subjected to ICSI with 5 µM calcium ionophore (CaI), which activates oocytes similarly to Io and Et, for 10 min followed by 5 µM CHX for 5 h, resulted in only 10% and 8% of normal fertilization and blastocysts formation, respectively (Suttner et al., Reference Suttner, Zakhartchenko, Stojkovic, Müller, Alberio, Medjugorac, Brem, Wolf and Stojkovic2000). Subsequently, a bovine blastocyst rate of 18% was obtained when oocytes were subjected to piezo-injection of DTT-treated spermatozoa and activation with Io + CHX and only 1% blastocysts were generated by parthenogenetic activation of bovine oocytes with the same treatment (Abdalla et al., Reference Abdalla, Shimoda, Hirabayashi and Hochi2009). Activation of buffalo oocytes after ICSI with Et + 3 h + CHX generated 62% of oocytes that extruded the 2nd PB and formed 2PNs, similar to activation with Io + 3 h + DMAP (58%), and blastocyst formation rates of 24% and 29%, respectively (Liang et al., Reference Liang, Ye, Laowtammathron, Phermthai, Nagai, Somfai and Parnpai2011). Conversely, activation of bovine oocytes subjected to ICSI with Io + CHX generated 87% correct pronuclear formation and 22% of blastocysts and in contrast with the study reported by Abdalla et al. (Reference Abdalla, Shimoda, Hirabayashi and Hochi2009), a high percentage of parthenogenetic embryos was observed with this treatment (Arias et al., Reference Arias, Sánchez and Felmer2016).
With respect to the generation of live offspring by ICSI in cattle, the activation treatment of Et 4 h after ICSI (Horiuch et al., Reference Horiuch, Emuta, Yamauchi, Oikawa, Numabe and Yanagimachi2002) has given the best results so far, with the birth of five healthy calves. Interestingly, Oikawa et al. (Reference Oikawa, Takada, Kikuchi, Numabe, Takenaka and Horiuchi2005) obtained 40% development to blastocysts with piezo-ICSI in oocytes activated with Io + 3 h + DMAP, one of the highest percentages obtained to date, and 29% with a protocol of activation with Et 4 h after ICSI, but when transferring the embryos to recipient females, they obtained nine calves from the activation protocol with Et and only one with DMAP (Oikawa et al., Reference Oikawa, Takada, Kikuchi, Numabe, Takenaka and Horiuchi2005). On this point, it is important to highlight that this is the first and only report of the birth of a calf by ICSI in cattle with oocytes activated with DMAP, whereas no births with CHX have been reported so far (Salamone et al., Reference Salamone, Canel and Rodríguez2017).
Other oocyte activation treatments
Other less used methods to increase (Ca2+)i levels in bovine oocytes include electrical stimuli, which induce a single transient of this cation in the ooplasm through its influx from the external environment (Fissore and Robl, Reference Fissore and Robl1992), and strontium (Sr) treatment, which generates (Ca2+)i oscillations through its release from intracellular reservoirs (Kline and Kline, Reference Kline and Kline1992; Zhang et al., Reference Zhang, Pan, Yang, He, Huang and Sun2005). In this sense, parthenogenetic activation of bovine oocytes with electrical pulse followed by 7% Et for 5 min resulted in significantly higher rates of pronuclear formation than both treatments separately, or by reversing the order of these stimuli (Yang et al., Reference Yang, Presicce, Moraghan, Jiang and Foote1994). Conversely, it has been reported that parthenogenetic activation of bovine oocytes with 5 µM Io for 8 min followed by 20 mM SrCl2 for 5 h, resulted in pronuclear formation rates of 41% and 19% in medium with high or low Ca2+ concentration, respectively (Fernandes et al., Reference Fernandes, Devito, Martins, Blanco, de Lima Neto, Tsuribe, Gonçalves and da Cruz Landim-Alvarenga2014). However, when the electric pulse (EP) activation treatment was applied before and after ICSI, a percentage of 2PN formation close to 60% was obtained and, although the percentage of morula and blastocyst formation was only 13%, one transferred blastocyst generated a pregnancy (Hwang et al., Reference Hwang, Lee, Yoon, Yoon, Lee and Choi2000). Conversely, after ICSI–SMGT and activation of bovine oocytes with 5 µM Io for 4 min + 20 mM SrCl2 for 5 h, correct pronuclear formation rates of 78% were obtained, although the proportion of blastocysts was only 11% (Bevacqua et al., Reference Bevacqua, Pereyra-Bonnet, Fernandez-Martin and Salamone2010).
A new modulator of MPF activity recently evaluated is anisomycin (ANY), a protein synthesis inhibitor (Joiakim et al., Reference Joiakim, Mathieu, Elliott and Reiners2004), which has been shown to exert a mechanism of MPF inhibition similar to that of CHX, through specific phosphorylation of CDK1Thr14-Tyr15 (Valencia et al., Reference Valencia, Pérez, Matus, Felmer and Arias2021). By activating bovine oocytes subjected to ICSI with Io + ANY, a correct pronuclear formation rate of 75% and blastocyst formation rate of 37% was obtained, being one of highest blastocyst rates recorded so far in bovine ICSI without piezo (Arias et al., Reference Arias, Sánchez and Felmer2016).
Another drug used to inhibit MPF is roscovitine (Rosc), capable of blocking CDK1 kinase activity by preventing its binding to ATP (Mermillod et al., Reference Mermillod, Tomanek, Marchal and Meijer2000). Recently, Suvá et al. (Reference Suvá, Canel and Salamone2019) reported pronuclear formation rates higher than 80% and 2nd PB extrusion rates of 70%, in bovine oocytes parthenogenetically activated with 5 µM Io for 4 min followed by 50 µM Rosc for 5 h alone or in combination with 10 µg/ml CHX. However, low blastocyst rates were generated with these treatments (11% and 6%, respectively) (Suvá et al., Reference Suvá, Canel and Salamone2019). In a different study, blastocyst rates of only 15% were obtained by piezo-ICSI and oocyte activation with 5 µM Io for 8 min + 66 µM Rosc in combination with 5 µg/ml CB (Devito et al., Reference Devito, Fernandes, Blanco, Tsuribe and Landim-Alvarenga2010).
The data reviewed above suggest that, although chemical activation leads to an improvement in the development of ICSI-derived bovine embryos, it is relevant to consider that it can also generate parthenogenetic development, highlighting the importance of confirming correct fertilization with these activation protocols (Arias et al., Reference Arias, Sánchez and Felmer2016).
Sperm factors
To understand the sperm factors involved in the low rates of embryo development in bovine ICSI, it is necessary to first understand some relevant events that occur during sperm maturation and others that occur after natural fertilization. During sperm maturation in the epididymis, sperm undergo the replacement of their canonical histones (H1, H2A, H2B, H3 and H4) by protamines (Barrachina et al., Reference Barrachina, Soler-Ventura, Oliva, Jodar, Zini and Agarwal2018) (Figure 1). Nevertheless, variable rates of 1–15% histone retention have been reported in mammalian spermatozoa (Torres-Flores and Hernández-Hernández, Reference Torres-Flores and Hernández-Hernández2020). In this regard, a recent study showed that the main areas of histone retention are distal intergenic regions, whereas histones with post-translational modifications are predominantly retained in specific genomic components such as CpG-rich promoters and in satellite repeats (Yamaguchi et al., Reference Yamaguchi, Hada, Fukuda, Inoue, Makino, Katou, Shirahige and Okada2018). Additionally, mammalian protamines undergo the oxidation of thiol (S–H) groups on their cysteine residues, forming disulfide (S–S) bonds that stabilize the genetic material (Kosower et al., Reference Kosower, Katayose and Yanagimachi1992) (Figure 1). Conversely, for the mammalian spermatozoon to be able to fertilize the oocyte, it must first undergo a series of biochemical and physiological changes known as ‘sperm capacitation’, which occurs in the female reproductive tract (Yanagimachi, Reference Yanagimachi2005) (Figure 2). In addition to the post-fertilization events mentioned above [release of (Ca2+)i oscillations, CGE, decrease in MPF, etc.], others events also occur, such as the extrusion of the 2nd PB and recruitment of maternal mRNAs that allow protein synthesis (Howlett and Bolton, Reference Howlett and Bolton1985; Hyttel et al., Reference Hyttel, Greve and Callesen1988; Ducibella et al., Reference Ducibella, Huneau, Angelichio, Xu, Schultz, Kopf, Fissore, Madoux and Ozil2002). In turn, S–S bonds of the sperm head are reduced to S–H by reduced glutathione (GSH), an endogenous disulfide bond reducing agent present in the oocyte, whose intracellular levels are higher in mature oocytes (Perreault et al., Reference Perreault, Wolff and Zirkin1984, Reference Perreault, Barbee and Slott1988a). When the bonds are reduced, sperm protamines are replaced by maternal histones, which is accompanied by events such as decondensation of hypercondensed sperm chromatin, solubilization of the subacrosomal region of perinuclear theca (SAR-PT) and pronuclear formation (Sutovsky and Schatten, Reference Sutovsky and Schatten2000; Sutovsky et al., Reference Sutovsky, Manandhar, Wu and Oko2003; Katayama et al., Reference Katayama, Sutovsky, Yang, Cantley, Rieke, Farwell, Oko and Day2005). It is also important to mention that pronuclear formation requires a decrease in MAPK activity, mainly ERK1/2 (Sanders and Swann, Reference Sanders and Swann2016). Studies in cattle have indicated that pronuclear formation occurs in parallel with the release of the 2nd PB (Hyttel et al., Reference Hyttel, Greve and Callesen1988), but this is unclear because in mice this process is initiated once the 2nd PB is released (Howlett and Bolton, Reference Howlett and Bolton1985). Subsequently, both pronuclei migrate until they are adjacent to each other, DNA replication begins, the maternal and paternal chromosomes condense, pronuclear envelope rupture occurs and a single metaphase plate is formed during the first mitosis of the zygote (Howlett and Bolton, Reference Howlett and Bolton1985; Hyttel et al., Reference Hyttel, Greve and Callesen1988) (Figure 3). However, during ICSI non-capacitated sperm is used and, furthermore, no sperm–oocyte fusion occurs, as the spermatozoon is injected directly into the ooplasm with its membranes and acrosomal content, which could affect the release of sperm factors necessary to complete fertilization and the replacement of protamines by maternal histones. Because of this, different sperm capacitation treatments, sperm membrane destabilization, and sperm chromatin decondensation protocols have been evaluated in bovine ICSI. Table 2 summarizes the main studies that have been used to improve the efficiency of ICSI in cattle based on sperm factors and their main results obtained.
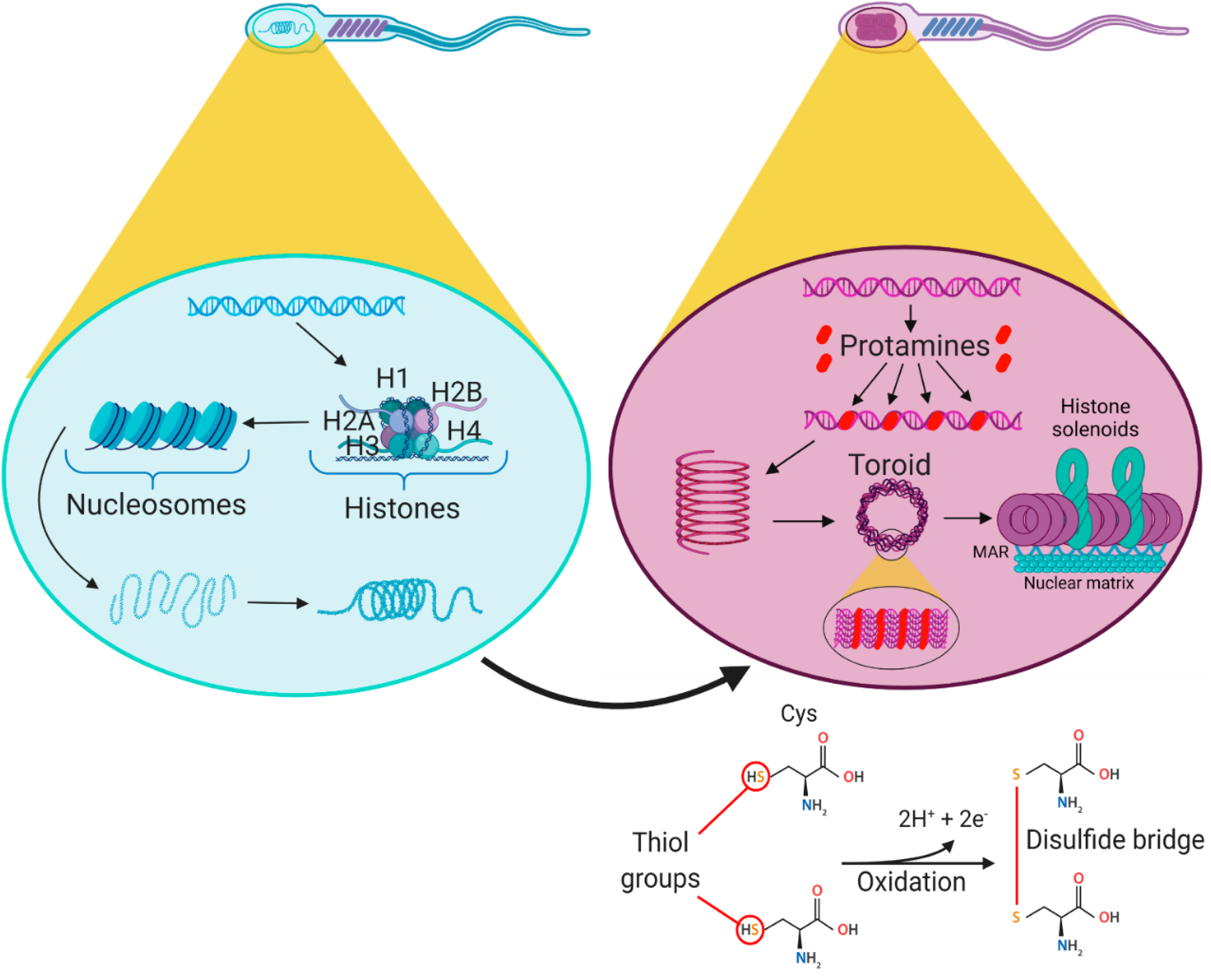
Figure 1. Replacement of histones by protamines during sperm maturation. On the left (light blue spermatozoon), the organization of chromatin bound to histones is depicted. On the right (purple spermatozoon), the organization of chromatin bound to protamines is depicted. DNA bound to protamines forms condensed structures called toroids. Between the toroids is the DNA bound to histones in the form of a solenoid. The matrix-attachment region links the toroids to the nuclear matrix (Singh and Agarwal, Reference Singh and Agarwal2011). Mammalian protamines undergo oxidation of the thiol (S–H) groups of their cysteine residues forming disulphide (S–S) bonds that stabilize the genetic material (lower right) and allow further compaction of the DNA. MAR: matrix-attachment region. Created with BioRender.com

Figure 2. Events occurring during natural fertilization in mammals. Black background numbers: 1. A capacitated and acrosome-reacted spermatozoon is able to cross the zona pellucida and fuse its membrane with that of the oocyte. 2. Release of sperm contents such as the nucleus and SOAF into the oocyte. 3. Solubilization of PA-PT. 4. Formation of IP3 and DAG from PIP2 from intracellular vesicles. 5. Uptake of IP3 by its receptor IP3R in the endoplasmic reticulum. 6. Release of calcium in the form of repetitive oscillations from intracellular pools. 7. The released calcium increases IP3 production generating a positive (+) feedback. 8. Repetitive calcium oscillations induce CGE. 9. Released cortical granules lead to blockade of polyspermy. 10. Released calcium also activates CaMKII. 11. CaMKII phosphorylates Wee1B, activating it. 12. Phosphorylation of residue Tyr15 by Wee1B and phosphorylation of residue Thr14 inactivate CDK1 (Oh et al., Reference Oh, Susor and Conti2011; Valencia et al., Reference Valencia, Pérez, Matus, Felmer and Arias2021) 13. Decreases MPF activity. 14. Released calcium also decreases MAPK activity (Yeste et al., Reference Yeste, Jones, Amdani, Coward, Nagy, Varghese and Agarwal2019). 15. It is possible that the decrease in MAPK activity is involved in the decrease in MPF activity (Valencia et al., Reference Valencia, Pérez, Matus, Felmer and Arias2021). 16. Transition from metaphase to anaphase (shown in the background) due to decreased MPF activity. Grey background numbers: 1. Activation of PKC by DAG and released intracellular calcium. 2. Phosphorylation of MARCKS. 3. Depolymerization of F-actin. 4. Depolymerization of F-actin also contributes to CGE. 5. PKC activates one or more unidentified proteins. 6. This or these proteins lead to F-actin polymerization. 7. Reorganization of the endoplasmic reticulum (ER) from a cortical to a central location by F-actin polymerization. 1st PB: first polar body; CaM: calmodulin; CaMKII: calcium/calmodulin-dependent protein kinase II; CDK1: cyclin-dependent kinase 1; CGE: cortical granule exocytosis; DAG: diacylglycerol; ER: endoplasmic reticulum. F-actin: filamentous actin; IP3: inositol triphosphate; IP3R: inositol triphosphate receptor; MAPK: mitogen-activated protein kinase; MARCKS: myristoylated alanine-rich C-kinase substrate; MPF: maturation-promoting factor; PA-PT: postacrosomal region of the perinuclear theca; PIP2: inositol bisphosphate; PKC: protein kinase C; RER: rough endoplasmic reticulum; SAR-PT: subacrosomal region of perinuclear theca; SER: smooth endoplasmic reticulum; SOAF: sperm–oocyte activating factor. Created with BioRender.com

Figure 3. Events following natural fertilization in mammals. 1. Exit of MII leads to extrusion of the second polar body. 2. Intracellular calcium release triggered early after fertilization also leads to maternal mRNA recruitment. 3. Entry of GSH into the sperm nucleus to reduce S–S to S–H bonds (upper right corner). 4. Transition of protamines to maternal histones. 5. Reduction of disulphide bonds leads to decondensation of hypercondensed chromatin of the spermatozoon, which is also modulated by the decrease in MAPK activity. 6. Male and female pronuclei formation. 7. Solubilization of SAR-PT. 8. Migration of pronuclei towards the zygote centre and DNA replication. 9. DNA condensation and rupture of pronuclear envelopes. 10. Chromosomes of both pronuclei are located in the equatorial plane, forming the first metaphase of the embryo (represented in the background). 1st PB: first polar body; 2nd PB: second polar body; FPN: female pronucleus; GSH: reduced glutathione; MAPK: mitogen-activated protein kinase; MPN: male pronucleus; SAR-PT: subacrosomal region of perinuclear theca. Created with BioRender.com
Table 2. Main treatments assessed in bovine ICSI to induce sperm capacitation, destabilization of plasma and acrosomal membranes, and chromatin decondensation, mechanisms of action and most relevant results

BSPs: bovine seminal plasma proteins; NKA: Na+-K+-ATPase; SOD: superoxide dismutase enzyme.
Sperm capacitation
Despite the above-mentioned criteria, and although it is not entirely clear, the use of non-capacitated sperm prior to ICSI has allowed the generation of high blastocyst rates, close to or more than 50% in some species such as mice (Kimura and Yanagimachi, Reference Kimura and Yanagimachi1995; Hu et al., Reference Hu, Shen, Zheng, Wang, Liu, Jin and Lei2012) and humans (Speyer et al., Reference Speyer, O’Neill, Saab, Seshadri, Cawood, Heath, Gaunt and Serhal2019). In cattle, a possible explanation for the low rates of embryo development to blastocyst by ICSI is that the release of (Ca2+)i oscillations is defective in these oocytes, either because they fail to initiate or they are short in duration (Malcuit et al., Reference Malcuit, Maserati, Takahashi, Page and Fissore2006), as a result of deficient SOAF release to the ooplasm or the lack of other factors absent in the non-capacitated sperm.
One of the most commonly used compounds to capacitate spermatozoa in vitro is heparin, which binds to bovine seminal plasma (BSPs) proteins, and which in turn bind to the sperm membrane. It has been proposed that heparin binding to BSP proteins generates changes in the plasma membrane leading to the loss of BSPs, cholesterol and phospholipids, in addition to increased pH and intracellular (Ca2+)i, culminating in tyrosine phosphorylation of sperm proteins (Parrish, Reference Parrish2014), one of the hallmark of sperm capacitation (Visconti et al., Reference Visconti, Bailey, Moore, Pan, Olds-Clarke and Kopf1995a; Tavalaee et al., Reference Tavalaee, Parivar, Shahverdi, Ghaedi and Nasr-Esfahani2017; Bathala et al., Reference Bathala, Fereshteh, Li, Al-Dossary, Galileo and Martin-DeLeon2018; Matamoros-Volante et al., Reference Matamoros-Volante, Moreno-Irusta, Torres-Rodriguez, Giojalas, Gervasi, Visconti and Treviño2018). However, heparin treatment before ICSI has not shown substantial improvements in terms of blastocyst development. In a previous study, Chen and Seidel (Reference Chen and Seidel1997) performed ICSI with spermatozoa capacitated with 5 U/ml heparin, followed by activation with CaI for 5 min, obtaining only 10–16% of blastocysts. Although this was significantly higher than the control, it was also a low percentage compared with that obtained by other studies (Chen and Seidel, Reference Chen and Seidel1997).
Heparin treatment for bovine sperm capacitation in IVF studies is often combined with d-penicillamine, hypotaurine and epinephrine (PHE). d-Penicillamine is a Zn2+ chelator that reduces the levels of this cation in the spermatozoon, which appears to be an important step for spermatozoa to achieve capacitation and penetrate the zona pellucida (ZP) (Andrews et al., Reference Andrews, Nolan, Hammerstedt and Bavister1994; Kerns et al., Reference Kerns, Zigo, Drobnis, Sutovsky and Sutovsky2018, Reference Kerns, Sharif, Zigo, Xu, Hamilton, Sutovsky, Ellersieck, Drobnis, Bovin, Oko, Miller and Sutovsky2020). Hypotaurine has been shown to exert beneficial effects on sperm motility in vitro, which could be related to inhibition of the Na+-K+-ATPase (NKA), protecting the sperm from damaging amounts of K+, but it has been suggested that this inhibition must be partial, as the activity of this enzyme has also been reported to be necessary to maintain sperm motility (Mrsny and Meizel, Reference Mrsny and Meizel1985; Takei and Hayashi, Reference Takei and Hayashi2020), while other reports indicated that this compound prevents the decreased of sperm motility by reducing superoxide (O2 − and HO2) production and protects the superoxide dismutase (SOD) enzyme from inactivation, thus inhibiting lipid peroxidation (Alvarez and Storey, Reference Alvarez and Storey1983). Epinephrine stimulates the acrosomal reaction presumably via an α1-adrenergic receptor (Meizel, Reference Meizel1985). However, although Galli et al. (Reference Galli, Vassiliev, Lagutina, Galli and Lazzari2003) investigated the effect of sperm capacitation with Hep + PHE prior to piezo-ICSI, they did not observe an improvement in the development to blastocysts (Galli et al., Reference Galli, Vassiliev, Lagutina, Galli and Lazzari2003).
Other inducers more recently used to capacitate sperm are 3-isobutyl-1-methylxanthine (IBMX), capable of increasing cAMP concentration in the cell (Parsons et al., Reference Parsons, Ramkumar and Stiles1988; Tscharke et al., Reference Tscharke, Kind, Kelly, Kleemann and Len2020) and methyl-β-cyclodextrin (MβCD), a compound that promotes cholesterol efflux from the sperm membrane (Yoshimoto et al., Reference Yoshimoto, Takeo, Irie and Nakagata2017). Both compounds increased intracellular cAMP concentration and plasma membrane cholesterol efflux, two essential events for proper mammalian sperm capacitation (Visconti et al., Reference Visconti, Moore, Bailey, Leclerc, Connors, Pan, Olds-Clarke and Kopf1995b, 1999; Bromfield et al., Reference Bromfield, Aitken, Gibb, Lambourne and Nixon2014; Bernecic et al., Reference Bernecic, Gadella, de Graaf and Leahy2020). In a different study, although sperm incubation with IBMX and MβCD increased intracellular Ca2+ levels, plasma membrane fluidity and tyrosine phosphorylation, only MβCD treatment showed a higher blastocyst rate (24%) compared with the control (Águila et al., Reference Águila, Zambrano, Arias and Felmer2017). Nevertheless, sperm capacitation in medium containing MβCD has also demonstrated some negative effects, increasing the rate of DNA fragmentation at the pronuclear stage and the rate of chromosome aberration at the blastocyst stage (Kato and Nagao, Reference Kato and Nagao2015).
It should also be mentioned that an interesting modification to the sperm preparation protocols for ICSI is energy restriction followed by the incorporation of pyruvate and lactate into the medium. This treatment allowed the restoration of the motility of bovine sperm and, after their use in piezo-ICSI, higher cleavage and blastocyst formation rates were observed compared with embryos generated without the energy restriction protocol (Navarrete et al., Reference Navarrete, Águila, Martin-Hidalgo, Tourzani, Luque, Ardestani, Garcia-Vazquez, Levin, Buck, Darszon, Buffone, Mager, Fissore, Salicioni, Gervasi and Visconti2019).
Importantly, studies with capacitated bovine sperm prior to ICSI are limited, and those that exist are inconclusive regarding the benefits of the tested compounds on embryonic development after sperm injection.
Destabilization of sperm membranes
As mentioned above, during natural fertilization the plasma membranes of both gametes fuse, allowing the release of sperm factors into the ooplasm necessary for oocyte activation and the entry of chromatin decondensation factors into the sperm nucleus. The transfer of these compounds can be affected if the plasma membrane of the injected spermatozoon is very stable and the ability of the ooplasm to degrade that membrane is low, causing the degradation process to occur slowly or not at all (Morozumi et al., Reference Morozumi, Shikano, Miyazaki and Yanagimachi2006). In a previous study, it was observed that, in the presence of dithiothreitol (DTT), an agent used to reduce disulfide bonds (Perreault et al., Reference Perreault, Barbee, Elstein, Zucker and Keefer1988b), human sperm nuclei decondensed only if the tails had been previously damaged with an injection pipette (Dozortsev et al., Reference Dozortsev, Rybouchkin, De Sutter and Dhont1995), supporting the idea that damage to the plasma membrane is necessary for both the release of oocyte activating factor and the accessibility of nuclear decondensation factors to sperm chromatin. In fact, most of the studies carried out in bovine ICSI include the immobilization of the spermatozoon prior to injection. For conventional ICSI, this is accomplished by scraping the tail of the spermatozoon to the bottom of the plate prior to injection and for piezo-directed ICSI, it is accomplished by applying electrical pulses to the tail of the spermatozoon. In this sense, spermatozoon tail scoring generated higher rates of nuclear decondensation or MPN formation (Wei and Fukui, Reference Wei and Fukui1999), and by means of piezoelectric pulses, higher rates of cleavage and blastocysts were observed, compared with dead sperm by repeated freezing–thawing (Horiuch et al., Reference Horiuch, Emuta, Yamauchi, Oikawa, Numabe and Yanagimachi2002). In addition, when sperm were immobilized by piezoelectric pulses, higher fertilization rates were obtained compared with immobilization by tail scoring (Katayose et al., Reference Katayose, Yanagida, Shinoki, Kawahara, Horiuchi and Sato1999). These results could be explained by the damage to the plasma membrane immediately before injection that could facilitate the interaction between ooplasmic and sperm factors necessary for the development of the embryo (Horiuchi and Numabe, Reference Horiuchi and Numabe1999), unlike spermatozoa subjected to repeated freeze-thawing, which may loss important factors for the activation of the oocyte, due to a longer period of time outside the oocyte with the plasma membrane damaged.
The presence of the acrosome and its content of hydrolyzing enzymes in the ooplasm, may also be detrimental to oocyte activation and pronuclear formation after ICSI. These enzymes are released during the acrosomal reaction and are necessary for sperm penetration through the ZP (Liu and Baker, Reference Liu and Baker1993; Yanagimachi, Reference Yanagimachi2005; Hirose et al., Reference Hirose, Honda, Fulka, Tamura-Nakano, Matoba, Tomishima, Mochida, Hasegawa, Nagashima, Inoue, Ohtsuka, Baba, Yanagimachi and Ogura2020), although there is some controversy on this point, as it has been observed that the physiological acrosomal reaction in mice occurs before the spermatozoon reaches the oocyte (Ded et al., Reference Ded, Hwang, Miki, Shi and Chung2020), thus acrosin would be released before the contact with the ZP. In any case, it appears that when the sperm enters the oocyte, it does so in the absence of the acrosome. This is of particular relevance in species with larger volume acrosomes such as hamsters and bovines (Morozumi and Yanagimachi, Reference Morozumi and Yanagimachi2005). In fact, injecting more than one intact mouse sperm head or more than three intact human spermatozoa into mouse oocytes, severally affected not only the blastocyst rate, but also oocyte deformation and lysis was observed (Morozumi and Yanagimachi, Reference Morozumi and Yanagimachi2005). Oocytes also suffered drastic deformations when one or more hamster sperm heads, or one or more bovine or porcine spermatozoa with intact acrosome, were injected (Morozumi and Yanagimachi, Reference Morozumi and Yanagimachi2005). Interestingly, this damage was not observed when the acrosome was removed prior to ICSI, supporting the idea that the presence of the acrosome inside the oocyte is detrimental to its viability and further embryo development (Morozumi and Yanagimachi, Reference Morozumi and Yanagimachi2005).
Accordingly, different methods have been evaluated to remove or destabilize both the plasma and acrosome membranes in bovine spermatozoa prior to ICSI. For example, incubation of bovine spermatozoa in alkalinized NaOH medium showed 90% of spermatozoa with damage in both membranes, while maintaining DNA integrity. However, this treatment generated only a moderate improvement in the development to blastocyst stage (17%) in combination with Io + CHX activation treatment after ICSI (Arias et al., Reference Arias, Sánchez, Risopatrón, Pérez and Felmer2014).
Other compounds assessed are lysolecithin (LL), also known as lysophosphatidylcholine, which can interact with a membrane composed of lecithin–cholesterol releasing lipid fragments from it (Bangham and Horne, Reference Bangham and Horne1964), and the detergent Triton X-100 (TX-100), capable of solubilizing membranes by releasing cholesterol, phospholipids and proteins (Jakop et al., Reference Jakop, Fuchs, Süß, Wibbelt, Braun, Müller and Schiller2009). Injection of mouse oocytes with mouse, human, bovine and porcine spermatozoa previously treated with LL or TX-100 allowed earlier oocyte activation, according to the extrusion of the 2nd PB, than injecting isolated sperm heads by piezoelectric pulses or intact spermatozoa (Morozumi et al., Reference Morozumi, Shikano, Miyazaki and Yanagimachi2006). Individual treatment of murine spermatozoa with 0.02% LL immediately before injection resulted in 72% normal live offspring, which was higher than TX-100 treatment performed with the same procedure (59%) (Morozumi et al., Reference Morozumi, Shikano, Miyazaki and Yanagimachi2006).
In cattle, sperm pretreatment with LL or TX-100 at concentrations of more than 0.02% generated 100% plasma membrane damage, whereas 0.05% was required to achieved 100% acrosomal damage. In this case, oocyte injection with LL and TX-100 pretreated sperm generated a blastocyst rate of 27% and 29%, respectively, being both higher than the control without treatment (21%) (Zambrano et al., Reference Zambrano, Águila, Arias, Sánchez and Felmer2016). However, in a different study, the combination of LL and TX-100 with GSH to promote sperm head decondensation did not improve embryonic development, despite the fact that these treatments induced plasma membrane disruption, promoted nuclear decondensation, and improved pronuclear formation, which could be attributed to the need for a longer incubation time with GSH (Zambrano et al., Reference Zambrano, Águila, Arias, Sánchez and Felmer2017).
Sperm selection methods
Another important factor to consider is the sperm selection method used, as these methods can damage the membranes of the sperm. Some of the most used methods to select sperm in ICSI protocols are swim-up and Percoll gradient selection. Studies in bovine and ram supported the idea that sperm selection using Percoll gradient increased the proportion of live sperm with intact acrosome compared with swim-up (Somfai et al., Reference Somfai, Bodó, Nagy, Papp, Iváncsics, Baranyai, Gócza and Kovács2002; Arias et al., Reference Arias, Andara, Briones and Felmer2017; Olivares et al., Reference Olivares, Souza-Fabjan, Fonseca, Balaro, Freitas, Oliveira and Brandão2017). However, other studies showed that swim-up selection resulted in a higher proportion of sperm with intact plasma membrane than for the Percoll gradient, without differences in the percentage of acrosome reaction (Mehmood et al., Reference Mehmood, Anwar and Naqvi2009). A significant proportion of acrosomal loss after sperm selection by Percoll gradient has also been described compared with swim-up (Cesari et al., Reference Cesari, Kaiser, Mucci, Mutto, Vincenti, Fornés and Alberio2006). These discrepancies could be associated with different incubation times in the swim-up protocols (Mehmood et al., Reference Mehmood, Anwar and Naqvi2009), or different speeds and centrifugation times (Arias et al., Reference Arias, Andara, Briones and Felmer2017). It should also be mentioned that, although the Percoll selection generated higher sperm concentration (Somfai et al., Reference Somfai, Bodó, Nagy, Papp, Iváncsics, Baranyai, Gócza and Kovács2002), it also increased the ROS levels (Arias et al., Reference Arias, Andara, Briones and Felmer2017). However, although there are some studies that evaluate the effect of different sperm selection methods on the viability and quality of bovine sperm, there have been few studies comparing the efficiency of these methods on embryonic development in bovine ICSI.
Sperm chromatin decondensation
Protamines found in mammals are classified into types 1 and 2 (P1 and P2). It has been observed that the higher number of cysteine residues present in P1 tended to result in a greater stability of sperm DNA, due to the formation of intermolecular and intramolecular disulfide bonds (Balhorn, Reference Balhorn1982; Kosower et al., Reference Kosower, Katayose and Yanagimachi1992). P2, conversely, has less cysteine and more histidine, therefore it is expected to form fewer disulfide bonds (Perreault et al., Reference Perreault, Barbee, Elstein, Zucker and Keefer1988b; Balhorn, Reference Balhorn and Adolph1989). Nevertheless, unlike some mammals such as humans and mice, whose DNA compaction is carried out by P1 and P2, in bovine this process is performed only by P1 (Corzett et al., Reference Corzett, Mazrimas and Balhorn2002), which could explain the difficulty in the decondensation of bovine sperm DNA after ICSI (Perreault et al., Reference Perreault, Barbee, Elstein, Zucker and Keefer1988b). This idea is supported by the fact that when hamster oocytes were injected with bovine spermatozoa, no chromatin decondensation was observed unless the sperm was pretreated with DTT (Perreault et al., Reference Perreault, Barbee, Elstein, Zucker and Keefer1988b). It is also worth mentioning that a comparative meta-analysis between fertile and infertile patients showed a significant increase in the P1/P2 ratio in patients with fertility problems, suggesting that the cause of this condition may be an excess in P1 content (Ni et al., Reference Ni, Spiess, Schuppe and Steger2016). Because of this, different disulfide bond reducing agents have been used before or after ICSI in cattle. In this regard, Galli et al. (Reference Galli, Vassiliev, Lagutina, Galli and Lazzari2003) determined that the treatment of sperm with DTT prior to piezoelectric ICSI in combination with oocyte activation with a treatment similar to Io + CHX increased the embryonic development at day 7 (22%) with respect to the control (5%), although the total blastocysts recorded at day 8 did not differ (24% and 22%). In addition, in the same study, the treatment of oocytes with DTT after piezoelectric injection with untreated sperm, resulted in increased rates of blastocysts at days 7 (from 7% to 19%) and 8 (from 8% to 20%) without exogenous activation and accelerated development to blastocysts at day 7 (4% vs. 21%) upon exogenous activation with the same activation treatment (Galli et al., Reference Galli, Vassiliev, Lagutina, Galli and Lazzari2003). However, the negative effects of DTT sperm treatment have also been observed on the developmental potential of bovine embryos generated by ICSI with conventional injection (Suttner et al., Reference Suttner, Zakhartchenko, Stojkovic, Müller, Alberio, Medjugorac, Brem, Wolf and Stojkovic2000; Sekhavati et al., Reference Sekhavati, Shadanloo, Hosseini, Tahmoorespur, Nasiri, Hajian and Nasr-Esfahani2012; Arias et al., Reference Arias, Sánchez, Risopatrón, Pérez and Felmer2014). In addition, it has been shown that prolonged exposure of spermatozoa to this compound (∼1 h) generated high percentages of morphological alterations, DNA fragmentation, and decreased expression of genes important for embryonic development in bovines after ICSI (Ock et al., Reference Ock, Bhak, Balasubramanian, Lee, Choe and Rho2003; Sekhavati et al., Reference Sekhavati, Shadanloo, Hosseini, Tahmoorespur, Nasiri, Hajian and Nasr-Esfahani2012).
These data suggest the need to identify a new disulfide bond reducing agent for sperm treatment. In this regard, Sekhavati et al. (Reference Sekhavati, Shadanloo, Hosseini, Tahmoorespur, Nasiri, Hajian and Nasr-Esfahani2012) reported that sperm pretreatment with Hep + GSH 7 h, combined with exogenous oocyte activation, is a more efficient method in terms of sperm head decondensation than DTT, in addition to generating a significant increase in the rate of blastocyst (20%) compared with the control (10%) (Sekhavati et al., Reference Sekhavati, Shadanloo, Hosseini, Tahmoorespur, Nasiri, Hajian and Nasr-Esfahani2012). These results are in agreement with those obtained by Canel et al. (Reference Canel, Bevacqua, Hiriart, Rabelo, de Almeida Camargo, Romanato, de Calvo and Salamone2017) who treated spermatozoa with Hep + GSH 20 h prior to ICSI and later activated the oocytes, obtaining a significant increase in cleavage (61%) and blastocyst (19%) rates compared with the control (35% and 5%, respectively) (Canel et al., Reference Canel, Bevacqua, Hiriart, Rabelo, de Almeida Camargo, Romanato, de Calvo and Salamone2017). Furthermore, treating bovine sperm with GSH for 120 min prior to piezo-ICSI and activating oocytes with Io × 3 + 3 h + Et, allowed a 28% blastocyst rate, significantly higher than pretreating sperm with DTT (18%) or GSH followed by LL (17%) (Lee et al., Reference Lee, Chang, Wu, Liu, Wang, Chu and Shen2015). Conversely, when bovine spermatozoa were treated with GSH, a significant decrease in the number of disulfide bonds was obtained with respect to the control, highlighting that in this study the oocyte activation treatment was not necessary to obtain a blastocyst rate of 31% after piezo-directed ICSI with GSH-treated sperm (Oikawa et al., Reference Oikawa, Itahashi, Yajima and Numabe2018). In a different study, the addition of 1 mM GSH to the culture medium of bovine oocytes subjected to ICSI increased the rate of blastocyst formation (30%) and decreased ROS levels (24), compared with the control without GSH treatment (18% and 70%, respectively) (Ashibe et al., Reference Ashibe, Miyamoto, Kato and Nagao2019).
Interestingly, Suttirojpattana et al. (Reference Suttirojpattana, Somfai, Matoba, Nagai, Parnpai and Geshi2016) recently described the pretreatment of bovine spermatozoa with dithiobutylamine (DTBA), a new compound that reduces disulfide bonds faster and more efficiently than DTT under the same pH conditions (Lukesh et al., Reference Lukesh, Palte and Raines2012). The results not only confirmed that DTBA reduced disulfide bonds in bovine spermatozoa, but also higher embryo development to the blastocyst stage (26% and 27%, at days 8 and 9, respectively) compared with DTT (19% at days 8 and 9) was observed, without affecting embryo ploidy (Suttirojpattana et al., Reference Suttirojpattana, Somfai, Matoba, Nagai, Parnpai and Geshi2016).
Technical factors
Injection technique
A variant of conventional ICSI is piezo-directed injection, in which electrical pulses are applied to the injection pipette to pierce both the ZP and the plasma membrane of the oocyte, instead of using mechanical means (Kimura and Yanagimachi, Reference Kimura and Yanagimachi1995). This variant of ICSI is beginning to be used in different species (Yanagida et al., Reference Yanagida, Katayose, Yazawa, Kimura, Konnai and Sato1999; Wang et al., Reference Wang, Baldassarre, Pierson, Cote, Rao and Karatzas2003; Furuhashi et al., Reference Furuhashi, Saeki, Enatsu, Iwasaki, Ito, Mizusawa, Matsumoto, Kokeguchi and Shiotani2019; Tsujimoto et al., Reference Tsujimoto, Fujiki, Alam, Tsukamoto, Azuma, Kanegi, Anzai, Inaba, Sugiura and Hatoya2019; Ressaissi et al., Reference Ressaissi, Anzalone, Palazzese, Czernik and Loi2021), as Kimura and Yanagimachi (Reference Kimura and Yanagimachi1995) obtained successful results by applying this technique to mice. In this species, the oolemma is more elastic than in other mammals, therefore, when pushing a conventional injection pipette against the murine oolemma it does not break easily, and sometimes it is necessary to puncture it more than once, causing greater damage to the oocyte (Kimura and Yanagimachi, Reference Kimura and Yanagimachi1995). By using a piezo-operated micropipette, the injection is smother and less traumatic, causing minimal damage to the oocyte (Kimura and Yanagimachi, Reference Kimura and Yanagimachi1995). Thus, in the preliminary studies with this system, piezo-directed microinjection increased murine oocyte survival (80%) and blastocyst rates (68%) importantly, compared with conventional injection (16% and 33%, respectively) also allowing the generation of live offspring (Kimura and Yanagimachi, Reference Kimura and Yanagimachi1995).
In bovine oocytes, which are also difficult to inject by conventional ICSI, piezo-ICSI either with or without activation with CaI for 10 min, generated a 72% fertilization rate, by which the authors concluded that exogenous activation is not necessary for the oocytes to reach this stage (Katayose et al., Reference Katayose, Yanagida, Shinoki, Kawahara, Horiuchi and Sato1999). However, these authors did not analyze development to the blastocyst stage. A few years later, Wei and Fukui (Reference Wei and Fukui2002) achieved high rates of correctly fertilized oocytes (86%), cleavage (72%), development to blastocyst (23%), pregnancy of four recipient females, and births of healthy offspring from piezo-ICSI without oocyte activation. Conversely, in the study by Horiuch et al. (Reference Horiuch, Emuta, Yamauchi, Oikawa, Numabe and Yanagimachi2002), the blastocyst rate obtained by piezo-ICSI was significantly higher with exogenous oocyte activation with Et at 4 h after ICSI (20%) compared with the group without activation (12%), highlighting the controversy that still remains regarding the need to exogenously activate oocytes after the piezoelectric microinjection to obtain high rates of pronuclear formation and preimplantation embryonic development (Horiuch et al., Reference Horiuch, Emuta, Yamauchi, Oikawa, Numabe and Yanagimachi2002).
In general, studies that have compared conventional and piezoelectric injection methods are scarce and, although some have shown improved oocyte survival, 2PN formation or blastocyst rates using piezo-ICSI (Kimura and Yanagimachi, Reference Kimura and Yanagimachi1995; Shahverdi et al., Reference Shahverdi, Movahedin, Rezazadeh Valojerdi and Kazemi Ashtiani2007; Furuhashi et al., Reference Furuhashi, Saeki, Enatsu, Iwasaki, Ito, Mizusawa, Matsumoto, Kokeguchi and Shiotani2019; Zander-Fox et al., Reference Zander-Fox, Lam, Pacella-Ince, Tully, Hamilton, Hiraoka, McPherson and Tremellen2021), contradictory results have also been reported. For example, a recent study in equines comparing both injection methods evidenced no differences in the cleavage and embryo development rates to blastocyst, although a higher number of nuclei and less nuclear fragmentation were reported in embryos generated by piezo-ICSI (Salgado et al., Reference Salgado, Brom-de-Luna, Resende, Canesin and Hinrichs2018).
For bovines, studies that compared both injection methods are even scarcer and none has analyzed the development and quality of bovine embryos generated with these techniques at a molecular level. Table 3 summarizes the main results obtained using piezo-ICSI in cattle.
Table 3. Main results obtained by piezo-directed ICSI in cattle

Sperm immobilization medium
Polyvinylpyrrolidone (PVP) is a polymer with a molecular weight of 360,000 Da that is used in ICSI procedures to increase the viscosity of the sperm solution, which reduces the sperm motility and adhesion to the injection pipette (Kato and Nagao, Reference Kato and Nagao2012; Parmar et al., Reference Parmar, Pant, Karuppanas, Mili, Upadhyay and Kant2013). Therefore, during ICSI procedures, a small volume of medium containing PVP is inevitably injected with the sperm into the oocytes (Kato and Nagao, Reference Kato and Nagao2012). In this sense, it has been observed that, when incubating human sperm for 10 min in PVP 10%, there is a significant increase in DNA fragmentation and a significant decrease in viability compared with the control (Nabi et al., Reference Nabi, Entezari, Miresmaeili, Vahidi, Lorian, Anbari and Motamedzadeh2021). However, another human study reported that 5% PVP increased the development of embryos generated by ICSI compared with higher concentrations (Ding et al., Reference Ding, Wang, Li, Chen, Zou, Ji and Zhang2020).
In bovine IVF, injection of embryos with 10% PVP decreased the cleavage (52%) and blastocysts (7%) rates compared with the control (81% and 24%, respectively), without affecting chromosomal integrity. Furthermore, the presence of PVP was detected in 41% of IVF embryos (Kato and Nagao, Reference Kato and Nagao2009). Although PVP is still used in ICSI protocols, more studies are required to evaluate its possible harmful effects in bovine ICSI (Parmar et al., Reference Parmar, Pant, Karuppanas, Mili, Upadhyay and Kant2013).
Alternative approaches to improve the efficiency of ICSI in cattle
Despite the different methods that have been used to improve the efficiency of ICSI in cattle, such as those discussed above, a protocol that substantially improves the results has not been achieved. Therefore, it is necessary to assess new strategies that can significantly increase the rates of oocyte activation, blastocyst formation, and the generation of live offspring in cattle by ICSI. It is known that extracellular vesicles (EVs) such as prostasomes, have a high Ca2+ content (Kumar et al., Reference Kumar, Pandita, Ganguly, Soren and Pagrut2018), essential during oocyte activation, as explained above. However, there have been no studies evaluating the interaction of prostasomes with oocytes. In bovines, similar vesicles such as the folliculosomes have been shown to deliver their contents to cumulus cells, which ultimately transfer different molecules (such as RNA, lipids or proteins) to the oocyte through cytoplasmic extensions called transzonal projections (da Silveira et al., Reference da Silveira, Andrade, Del Collado, Sampaio, Sangalli, Silva, Pinaffi, Jardim, Cesar, Nogueira, Cesar, Coutinho, Pereira, Perecin and Meirelles2017), a mechanism by which prostasomes could deliver Ca2+ to the oocyte, if co-incubated with cumulus–oocyte complexes (COCs), to subsequently modulate activation-related processes.
MPF inhibition, another important process during oocyte activation, could also be modulated using EVs, as bovine oviductosomes have been reported to contain miRNAs such as miR-92a-3p and miR-429 that specifically inhibit CDK1 and cyclin B expression (Almiñana et al., Reference Almiñana, Tsikis, Labas, Uzbekov, da Silveira, Bauersachs and Mermillod2018). Therefore, co-incubation of COCs with prostasomes and oviductosomes could represent an alternative strategy to chemical or physical oocyte activation, with low cytotoxicity and high specificity.
In a recent study it was observed that the incubation of frozen–thawed bovine spermatozoa with estrous oviductal fluid (EOF) from heifers induced tyrosine phosphorylation and acrosomal reaction at a higher proportion compared with incubation with oviductal fluid from animals in the luteal (LOF) phase (Kumaresan et al., Reference Kumaresan, Johannisson, Humblot and Bergqvist2019). Therefore, incubation with EOF could be considered as a sperm treatment prior to ICSI, which would allow injecting spermatozoa biochemically and physiologically more similar to those that fertilize an oocyte in vivo, as they would have experienced the same processes induced by a substance present in the natural fertilization environment.
During in vitro maturation (IVM) at atmospheric oxygen levels, a higher ROS content is generated in the medium, decreasing the developmental capacity of bovine oocytes (Hashimoto et al., Reference Hashimoto, Minami, Takakura, Yamada, Imai and Kashima2000), which could be due to an increased ‘expenditure’ of GSH to protect against oxidative stress damage. Therefore, optimization of the IVM medium, to maintain higher levels of GSH in the ooplasm, would allow oocytes destined for ICSI to retain sufficient GSH to aid in the decondensation of the sperm nucleus.
It has recently been reported that H2O2-exposed granulosa cells release EVs with a high content of mRNAs, encoding for components of the defence system against oxidative stress such as the transcription factor NRF2 and enzymes like catalase and thioredoxin, with the expression of these enzymes being regulated under NRF2 (Dreger et al., Reference Dreger, Westphal, Weller, Baumann, Stangl, Meiners and Stangl2009; Saeed-Zidane et al., Reference Saeed-Zidane, Linden, Salilew-Wondim, Held, Neuhoff, Tholen, Hoelker, Schellander and Tesfaye2017; Luo et al., Reference Luo, Shang, Brooks, Jiagge, Zhu, Buschhaus, Conley, Fath, Davis, Gheordunescu, Wang, Harouaka, Lozier, Triner, McDermott, Merajver, Luker, Spitz and Wicha2018). Therefore, another interesting approach to reduce GSH loss due to excess ROS in bovine oocytes destined for ICSI could be the supplementation of the medium with these EVs during IVM. In fact, different antioxidants have been used during IVM of bovine oocytes, such as melatonin, vitamin C and cysteamine, which have been shown to protect oocytes from oxidative stress in vitro, either by increasing GSH levels, reducing ROS levels or both, which subsequently have allowed higher rates of embryo development by IVF (Sovernigo et al., Reference Sovernigo, Adona, Monzani, Guemra, Barros, Lopes, Leal, Lopes and Leal2017; Pang et al., Reference Pang, Zhao, Sun, Jiang, Hao, Du and Zhu2018; Zhao et al., Reference Zhao, Wang, Hao, Li, Zhao, Yan, Wang, Du, Wang, Liu, Pang and Zhu2018). Some other antioxidants have also been assessed in the IVM medium, such as lycopene, quercetin, α-lipoic acid and anethole, with promising results in protecting oocytes from oxidative stress in vitro and significantly improving the developmental potential of IVF-generated embryos (Chowdhury et al., Reference Chowdhury, Choi, Khan, Lee, Mesalam, Song, Xu, Joo, Afrin and Kong2017; Hassan et al., Reference Hassan, Fang, Roy, Shin and Cho2017; Sovernigo et al., Reference Sovernigo, Adona, Monzani, Guemra, Barros, Lopes, Leal, Lopes and Leal2017; Sá et al., Reference Sá, Vieira, Ferreira, Cadenas, Bruno, Maside, Sousa, Cibin, Alves, Rodrigues, Leal-Cardoso, Gastal and Figueiredo2020). Moreover, supplementation with lipid metabolism regulators, such as carnitine during IVM of bovine oocytes, has also shown interesting results by increasing GSH levels and IVF-derived blastocyst rates (Sovernigo et al., Reference Sovernigo, Adona, Monzani, Guemra, Barros, Lopes, Leal, Lopes and Leal2017) and by enhancing embryonic developmental potential from meiotically less competent oocytes (Knitlova et al., Reference Knitlova, Hulinska, Jeseta, Hanzalova, Kempisty and Machatkova2017), which suggests evaluating these strategies in bovine embryos generated by ICSI.
Finally, it should be mentioned that hyaluronate (HA) has been suggested as a possible replacement for PVP in the immobilization medium of sperm as, unlike PVP, this non-sulphated anionic glycosaminoglycan it is degraded to sugar molecules that can be easily metabolized by lysosomes (Moreira et al., Reference Moreira, De la Fuente, Palasz and Gutiérrez-Adán2005; Kato and Nagao, Reference Kato and Nagao2012). In this way, when comparing the use of both compounds in human ICSI protocols, similar fertilization and pregnancy rates were obtained (Kato and Nagao, Reference Kato and Nagao2012) and in murine ICSI protocols, similar embryonic development rates (2-cell, 4–8-cell, blastocysts) and number of fetuses were obtained, although HA showed adherence of the sperm to the injection pipette (Moreira et al., Reference Moreira, De la Fuente, Palasz and Gutiérrez-Adán2005). Therefore, if this disadvantage is overcome, the use of this compound in the sperm immobilization medium could be tested in bovine ICSI protocols. Table 4 summarizes these alternative approaches proposed to improve the efficiency of bovine ICSI and their possible mechanisms of action.
Table 4. Alternative approaches to improve the efficiency of ICSI in cattle and possible mechanisms of action

COCs: cumulus–oocyte complexes; cAMP: cyclic AMP; IBMX: 3-isobutyl-1-methylxanthine; EVs: extracellular vesicles; HA: hyaluronate; PVP: polyvinylpyrrolidone.; ROS: reactive oxygen species.
Conclusion
It is important to emphasize that some of the differences and discrepancies observed in the studies reviewed could also be related to differences in the protocols employed including IVM, embryo culture and the ICSI technique, among others. The ICSI procedure involves a series of steps and the use of different media prepared with different compounds or with different concentrations of these compounds, therefore small variations generated in each laboratory could contribute to the lack of consistency observed in some studies. Furthermore, there has been no consensus on which oocytes to consider as activated or fertilized or whether blastocyst rates should be evaluated with respect to the total injected, surviving or cleaved oocytes, which makes it difficult to compare results between different research groups, when this information is not available in the manuscript.
Although Io + 3 h + DMAP activation treatment after piezo-ICSI generated a high percentage of bovine blastocysts, it has been documented that DMAP generates a large proportion of parthenogenetic embryos. Conversely, activation with Io + ANY after ICSI has also generated high blastocyst rates and does not show the disadvantages of DMAP, although the demonstration of live offspring is still pending with this treatment.
Piezo-ICSI has allowed the generation of offspring with or without exogenous activation, although there is still controversy around the requirement of oocyte activation with this technique. However, special attention should be paid to the fact that the birth of only one calf has been described from oocytes activated with DMAP, whereas no reports have been described with CHX so far, which markedly contrast with the birth of a superior number of calves generated by piezo-ICSI or conventional injection, all activated with Et, suggesting the need for a more in-depth study on the activation mechanism of these compounds.
Finally, the use of EVs revealed many applications in different areas of research. Some alternative approaches to improve the efficiency of ICSI in cattle could include the use of EVs such as prostasomes and oviductosomes as ‘biological activation methods’. Other alternative approaches could include the incubation of sperm with EOF for a more physiological capacitation before ICSI, the incorporation of antioxidants and EVs containing mRNAs involved in the defence of oxidative stress in in vitro oocyte maturation medium, and the replacement of PVP by HA in sperm immobilization medium.
Acknowledgements
The authors gratefully acknowledge funding support from FONDECYT 1201166, ANID, Chile, Doctoral scholarship ANID 21191408, Chile and the Office of Research, Universidad de La Frontera, Temuco, Chile.
Author contributions
FF wrote the initial manuscript and created the figures and tables. EM collaborated in the writing of the section on ‘Alternative approaches to improve the efficiency of ICSI in cattle’ and in reviewing the manuscript. MC collaborated in the writing of the section on ‘Sperm capacitation’. MA collaborated in the revision of the manuscript. RF collaborated in the writing and revision of the manuscript.
Financial support
This work was supported by the Chilean National Agency for Research and Development (ANID), through project FONDECYT 1201166 and Doctoral scholarship ANID 21191408, Chile.
Conflict of interest
None of the author has any conflict of interest to declare.
Ethical standards
With reference to this type of article (review), no animals were involved.









