Surgical site infections (SSIs) are dangerous for patients; they are associated with significant morbidity and mortality as well as increased healthcare costs.Reference de Lissovoy, Fraeman, Hutchins, Murphy, Song and Vaughn 1 Guidelines exist to mitigate the risk for SSI.Reference Durando, Bassetti and Orengo 2 However, infections occur in the operating room (OR), even with best practices.Reference Andersson, Bergh, Karlsson, Eriksson and Nilsson 3 , Reference Benenson, Moses and Cohen 4 It is difficult to directly trace an individual SSI to an infection source or variable, so prior studies have measured microbial loads in the OR and/or have used readmissions data for postsurgical infection estimates.Reference Anthony, Peterson, Polgreen, Sewell and Polgreen 5
One potential contributing factor to increased risk of SSI is microbial contamination in the OR, which previous studies have suggested could exceed the recommended 10 CFU/m3 more than 50% of the time.Reference Andersson, Bergh, Karlsson, Eriksson and Nilsson 3 The OR team is a major potential source of contaminationReference Birgand, Saliou and Lucet 6 , Reference Friberg, Friberg and Burman 7 and their movement in the OR has a statistically significant correlation to the microbial load in the OR. However, findings that correlate the number of people in the OR to microbial load are mixedReference Andersson, Bergh, Karlsson, Eriksson and Nilsson 3 . Door openings in the OR have also been investigated to determine how they affect microbial load, and these studies have produced mixed results for orthopedic procedures.Reference Durando, Bassetti and Orengo 2 , Reference Tjade and Gabor 8 – Reference Yinnon, Wiener-Well and Jerassy 11 However, studies of OR door openings during cardiac, vascular, and general surgeries have been positively correlated with microbial load.Reference Borer, Gilad and Meydan 12 – Reference Van der Slegt, van der Laan, Veen, Hendriks, Romme and Kluytmans 14
Prior studies have demonstrated that behavioral and safety protocols (eg, maintaining surgical asepsis) and good OR environmental discipline practices (eg, maintaining air pressure, temperature, and humidity) reduce the patient risk for SSI.Reference Birgand, Saliou and Lucet 6 , Reference Beldi, Bisch-Knaden, Banz, Mühlemann and Candinas 15 Also, surgical practices,Reference Durando, Bassetti and Orengo 2 scrub attire,Reference Tammelin and Blomfeldt 16 and reduced OR trafficReference Andersson, Bergh, Karlsson, Eriksson and Nilsson 3 reduce microbial load. The role of external risk factors to the development of SSI has been identified in past researchReference Mangram, Horan, Pearson, Silver and Jarvis 17 – Reference Alfonso-Sanchez, Martinez and Martin-Moreno 20 ; however, the relationship between SSI and microbial load levels is not well understood.Reference Wan, Chung and Tang 21 – Reference Armellino 23 A recent review of patient infection rates in the OR found that only 24 of 2,086 studies published prior to 2013 provided evidence-based findings to connect components of an OR procedure to contamination.Reference Birgand, Saliou and Lucet 6
In this study, we sought to understand how the movement of the patient, equipment, materials, staff and OR door openings affect OR microbial loads at various locations. We aimed to develop evidence-based guidelines for OR workflow design. Although the general assumption is that these flows occur to support tasks required for the surgical procedures, the traffic may also transfer bacteria and fungi within the OR.Reference Loison, Troughton and Raymond 24
METHODS
In this study, we used hierarchical regression to identify factors influencing microbial load in the OR. Factors considered were the OR itself, sampling time of year, OR temperature and humidity, number of door openings, number of people in the OR, and amount of traffic in the OR. A total of 27 procedures were recorded, beginning with the previous case patient leaving the room and ending with the current case patient leaving the room. The procedures selected ensured a range of OR practices for orthopedic and pediatric surgeries, but were recorded only if the prior consent of the entire surgical team had been obtained. All observed procedures occurred in 4 different ORs in a large academic hospital (>600 beds) in the United States.
To ensure coding validity, 4 researchers independently coded all 4 camera views of a representative procedure, using a standardized coding schema in Observer XT software (Noldus Information Technology, Wageningen, Netherlands). All 27 recorded procedures had similar traffic flows. Observations had 100% interrater reliability of door openings and number of people in the OR. However, coding differences existed regarding the activity of staff and the activity duration. These were addressed by revising the standardized coding schema through multiple group discussions, and the revised coding schema was then used to code door openings and all movement at locations for the remaining 26 videos. Each microbial-load sampling location was classified as either high or low traffic, based on (1) the number of transitions past the location, and (2) the proximity to a doorway. Notably, the proximity to a doorway automatically resulted in assignment to the high-traffic classification.
To measure the microbial load, 2 types of air samplers (Sartorious Stedim MD8 Airscan and AirChek XR5000 Sample Pump) and 9-cm settle plates were used. The 2 air samplers had flow rates of 2.0 m3/hour and 0.24 m3/hour, respectively, and use 3-μm gelatin and 0.8-μm polycarbonate filters, respectively. Post sampling, the gelatin filters were dissolved in basal salts medium to obtain 4 mL liquid, of which 500 μL was plated on the media, while the polycarbonate filters were extracted with 2 mL of basal salts liquid and 200 μL was plated on various media. Colony-forming units (CFU) were counted, and measurements were adjusted to report data as CFU per cubic meter.
The settle plates were incubated at 35°C for 48 hours for bacterial counts and 26°C for 5–7 days for fungal counts. Resulting CFUs were counted, and measurements were adjusted to report data as CFU/m2/hour. The measurement techniques for both the air sampler and settle plates were made using best practices.Reference Pasquarella, Pitzurra and Savino 25 Similar procedures were used to sample twice during the same year, the first set in March 2016 and the second set in September 2016. Both pediatric and orthopedic procedures in 4 different ORs were sampled for a total of 21 procedures (12 orthopedic and 9 pediatric procedures); however, 2 procedures were not included in the analysis due to technical difficulties. Observation of the procedures showed that the surgical teams were compliant with surgical attire requirements.
All data were then analyzed using hierarchical regression, with separate models for bacteria and fungi for both air sampler and settle-plate data. The dependent variable was the CFU rate measured from the sampling devices, with a log transformation to reduce the influence of outliers.Reference Wooldridge 26 The final control variables contained in the 6 models presented in the Methods section are as follows:
Model 1: Sampling Period (March or September)
Model 2: Temperature and Humidity + Model 1
Model 3: Operating Room + Model 2
Model 4: Number of Door Openings + Model 3
Model 5: Number of People in the Room + Model 4
Model 6: Amount of Traffic + Model 5
RESULTS
A spaghetti diagram of all staff movements from patient-in-room to patient-out-of-room during 4 sample procedures in a single OR is shown in Figure 1. Similar diagrams exist for each observed procedure. Figure 1A shows only the movements of the circulating nurse, and Figure 1B shows all staff movements. Table 1 presents the average movement (in terms of transitions) near a location in the coded videos, the location proximity to the door, and the resulting designation as a high or low traffic location, the final independent variable tested in the hierarchical regression analysis.
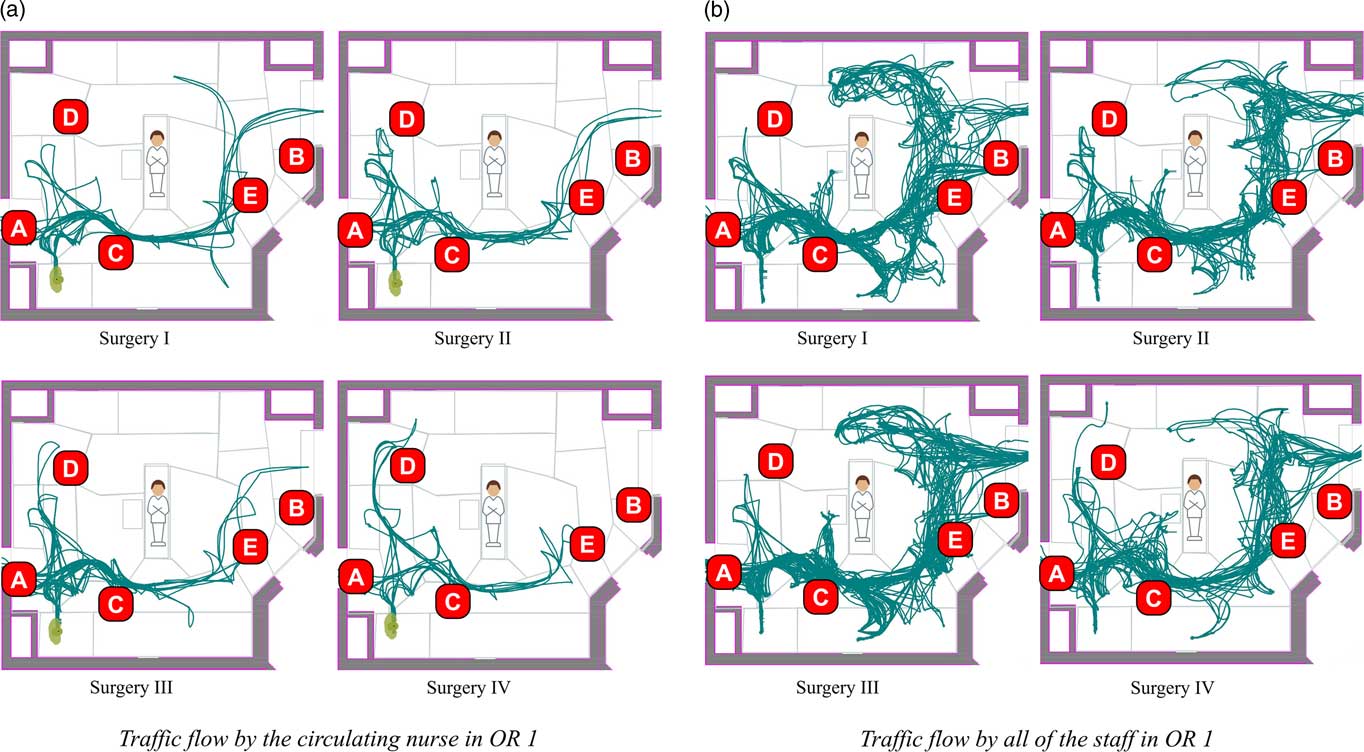
FIGURE 1 Traffic flow and sampling locations (operating room 1). A: near door 1, high traffic area; B: near door 2, high traffic area; C: transition location, high traffic area; D: low traffic area; E: near door 2, high traffic area.
TABLE 1 Traffic Classifications (High/Low) for Each Sampling Location and Operating Room

NOTE. OR, operating room; A: near door 1, high traffic area; B: near door 2, high traffic area; C: transition location, high traffic area; D: low traffic area; E: near door 2, high traffic area.
Table 2 reports the average microbial load with bacteria and fungi measures identified by the time of year when they were taken (March and September) for each sampling method. A previous studyReference Anthony, Peterson, Polgreen, Sewell and Polgreen 5 indicated a seasonality effect on bacteria levels when sampling. Although we did not test for seasonality, all bacteria samples are higher in September than in March. The effect for fungi is not as clear, with some measurements being higher in March than in September. The data for the orthopedic and pediatric procedures were separated due to their distinct nature. It is standard practice for total joint arthroplasty surgical teams to wear surgical attire specific for those procedures, which includes gowns made from higher barrier protection material and head gear with a battery-operated fan that provides greater contamination protection. These teams also restrict traffic from the outer-corridor OR door during the intraoperative phase. As expected, the average microbial load for the air sampler measures was lower for the orthopedic procedures. However, contrary to expectations, the average settle-plate measures for orthopedic procedures exceeded that of the pediatric procedures during both the March and September samples.
TABLE 2 Average by Sampling Time, Sampling Method, and Procedure Type

NOTE. CFU, colony-forming units; SD, standard deviation; Min, minimum; Max, maximum.
Tables 3 and 4 present the results of the hierarchical regression analysis using air sampling data and settle-plate data, respectively. Notably, all orthopedic procedures took place in the same OR, and the pediatric cases took place in the other 3 observed ORs. This aspect of our study was captured in model 3 using 3 categorical variables for the pediatric procedures where the OR used for orthopedic procedures was coded as 0.
TABLE 3 Analysis of Bacterial Load Using Air SamplersFootnote a

NOTE. CFU, colony-forming units; OR, operating room.
a All values are shown the log of CFU/m3.
b Month: 0, March; 1, September.
c P<.10, where P is the probability of observation when null hypothesis of no difference is true.
d OR: 0, orthopedic; 1, pediatric.
e Traffic level at location: low, 0; high, 1.
f P<.01, where P is the probability of observation when null hypothesis of no difference is true.
g F test for the overall significance of the specified model.
TABLE 4 Analysis of Bacterial Load Using Settle PlatesFootnote a

NOTE. OR, operating room; CFU, colony-forming units.
a All values are shown in log of CFU/m2/hour.
b Month: 0, March; 1, September.
c P<.01, where P is the probability of observation when null hypothesis of no difference is true.
d P<.05, where P is the probability of observation when null hypothesis of no difference is true.
e OR: 0, orthopedic; 1, pediatric.
f P<0.10, where P is the probability of observation when null hypothesis of no difference is true.
g Traffic level at location: low, 0; high, 1.
h F test for the overall significance of the specified model.
The 6 regression models using air sampling data all have nonsignificant adjusted R2 values (Table 3). All 6 models using the settle-plate data yielded statistically significant results, and at least 1 control variable per model is significant (Table 4). In Table 4, model 1 shows an increase in bacteria in September (P<.001). The amount of traffic (model 6) at a location also significantly affects the microbial load (P<.05). Temperature, humidity, operating room, and number of door openings were nonsignificant in all models. The number of people in the room (model 5) also did not produce a statistically significant result with a P value between .05 and .10.
DISCUSSION
Analysis of the air sampling data did not demonstrate differences by location in the bacterial load. Although the level of traffic was significant in model 6 (Table 3), the model itself did not yield statistically significant results. The lack of significant differences in the air sampling data may have several explanations. The first is that we used 2 types of air samplers and that we had fewer air samplers than we did settle plates, so the sample size was much smaller for air samples. Also, living particles may become inactive through contact with the air sampler,Reference Pasquarella, Pitzurra and Savino 25 which offsets the air sampling device advantage (compared to settle plates) of exposing a known volume of air to the sampling medium.
The major findings of this study are that higher-traffic areas in the OR have a higher microbial load than lower-traffic areas (see model 6 in Tables 3 and 4) and that the number of door openings did not have a significant impact on microbial load. While door openings were not identified as a significant factor on its own, proximity to a door still influenced bacterial count, which was embedded into the classification of “high traffic versus low traffic” (Table 1). Importantly, in both model 5 and model 6, the number of people in the room may have increased the bacterial count (Table 4). All hierarchical regression models of the settle-plate CFU identified the sampling period as significant (Table 4), which supported prior researchReference Anthony, Peterson, Polgreen, Sewell and Polgreen 5 that SSI rates vary according to the season.
The finding that high-traffic areas have higher microbial loads as measured by the settle plates indicates that it is important to control the traffic in the OR and move it away from the surgical field. This finding supports prior research that the measurements of microbial load near the wound are the most important.Reference Napoli, Tafuri and Montenegro 27 , Reference Napoli, Marcotrigiano and Montagna 28 It also suggests that intraoperative asepsis methods that would keep traffic away from the surgical field would contribute to reducing microbial load near the wound and consequently to fewer SSI. The a priori video analysis used to create Figure 1 identified that certain areas of the OR attract traffic, such as areas near the OR doors, telephone, computer work stations, and cabinet storage. Extensive traffic was also observed between the anesthesia area and the door, as well as circulating nurse movements around the room and through the door. Therefore, to the extent possible, these high traffic areas should be situated near each other and away from the surgical field.
High-traffic areas by role in the OR could be related to the type of movements that staff engage in within the OR. For example, it would be important to enable the circulating nurse, whose role leads to high foot traffic, to have a workstation area situated away from the surgical field and to have the phone and storage cabinets adjacent to the workstation. For the anesthesia team, who may enter and leave during the procedure, it would be helpful to have a pathway that does not cross the surgical field and, instead, remains behind the head of the patient. This adjustment could include having a door dedicated to the anesthesia team. Each of these suggestions requires further research into understanding how the OR design parameters interact with staff responsibilities to affect staff movement.
It is also important to consider the causes for movement, possibly sorting necessary task movements from controllable movements caused by lack of communication or lack of materials and supplies, which are both causes of increased traffic flow in the OR.Reference Uçkay, Harbarth, Peter, Lew, Hoffmeyer and Pittet 18 To this effect, the location of supplies can greatly influence traffic levels because the location of the supply cabinets, workstations and functional areas (eg, back table) are all attractors of movement at different points in the procedure. Our results suggest that OR and workflow design could reduce contamination by limiting the spread of microbes to the surgical field.
Other drivers of OR traffic movement are visual and auditory constraints. Staff may have to move to observe activity in and around the surgical field. This need to move could also be true for students and other observers, so better views could possibly be provided by strategically mounted cameras and projection systems. To hear voice prompts, staff may need to approach the surgical field more often when there is excess noise in the OR. Improving OR acoustics would reduce the need for such movement.
While the number of people in the OR did not contribute significantly to microbial load in the model, further research should be conducted. It would be useful to investigate which areas in the OR will allow observation without interfering with the procedure and without increasing the amount of traffic in the OR. Nevertheless, limiting the movement of people present in the OR should be prioritized over limiting the number of people in the OR. There results of our study showed no significant difference in the bacteria count between the ORs. Because orthopedic surgeons take special precautions to prevent microbial contamination, it was expected that microbial load in the orthopedic procedures would be lower. Lastly, the nonsignificance of the number of door openings also suggests that the amount of traffic is more important than door openings. To further address these factors, future research could investigate correlation between traffic level, number of people in the room, and the number of door openings.
The significance of the sampling period was also not expected. Although the outside bacterial counts are expected to increase during the summer and fall because of humidity and temperature, it was expected that the environmental controls inside the OR would mitigate the effects of weather. A post hoc analysis showed no difference in the temperature during the 2 sampling periods, but a significant difference in the range of the humidity measurements were detected, with the September humidity measurements being much more extreme than the March measurements.
This study included only a limited number of sampling locations in the OR. A sampling plan that took more samples and ensured that samples were taken from every part of the OR would provide more information about the distribution of microbes throughout the OR. This improvement would allow better estimates of how door placement and cabinet placement affect the microbial load.
Also, it is not possible to determine the specific contribution to microbial load in the OR from colonized OR staff in our study, and this factor is beyond the scope of our experiments. However, several studies have demonstrated that OR staff can be linked to contamination of the environment with airborne bacteria due to skin shedding and that this has led to SSIs. A recent comprehensive review of this topic was undertaken by the Association of periOperative Registered Nurses.Reference Spruce 29
While OR sampling was limited to orthopedic and pediatric procedures, conclusions drawn from this research are applicable to other types of operative procedures. By understanding the level and reasons for intraoperative movement (regardless of procedure type), informed workflow design could potentially reduce the amount of movement, which would ultimately reduce microbial loads, and thereby lessen SSI risk.
ACKNOWLEDGMENTS
The authors thank the RIPCHD.OR Study Group for their contributions in supporting the work in this study.
Financial support: This work was supported the Agency for Healthcare Research and Quality (grant no. P30HS0O24380).
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.







