Introduction
The Northern mountainous region occupies about 103,000 km2 equivalent to ~ 33% of the total land area in Vietnam, with a population of 12.5 million people, 13% of the total national population (General Statistics Office of Vietnam, 2019). In this region, over 80% of the cultivation area is on sloping land (General Statistics Office of Vietnam, 2019) and long-term cultivation on such steep slopes often leads to soil erosion. Additionally, the widespread use of monocropping systems on steep slopes and the large amounts of chemical fertilizer application, especially nitrogen (N) mineral fertilizers, results in low soil fertility and imbalanced nutrient content. Cassava, an important cash crop for local smallholders, is commonly grown as a monocrop in this region, and we established that during the period between 2016 and 2017, local farmers were applying 100–120 kg N ha−1 year−1, well above the recommended dose from the local agricultural department of 80–100 kg N ha−1 year−1. Excessive application of mineral N fertilizers increases greenhouse gas emission (N2O) (Nyoki and Ndakidemi, Reference Nyoki and Ndakidemi2016) and also affects soil and water by causing soil acidification and toxification, resulting in water contamination, negatively impacting fish and aquatic animals (Bashir et al., Reference Bashir, Ali, Ghauri, Adris and Harun2013; Boman et al., Reference Boman, Wilson and Ontermaa2002; Compton et al., Reference Compton, Harrison, Dennis, Greaver, Hill, Jordan, Walker and Campbell2011).
Agroecological practices, especially intercropping, can improve resilience and sustainable productivity to rural smallholders by mitigating the decline in soil fertility in upland areas (De Schutter, Reference De Schutter2010; Tittonell, Reference Tittonell2014). Numerous studies have indicated that intercropped systems provide advantages such as better use of land and labor, food security, increasing soil nutrients and moisture, decreasing soil erosion, natural pest control, and income benefits for smallholders (Agegnehu et al., Reference Agegnehu, Ghizaw and Sinebo2008; Duchene et al., Reference Duchene, Vian and Celette2017; Dwivedi et al., Reference Dwivedi, Dev, Kumar, Yadav, Yadav, Gupta, Singh and Tomar2015; Knörzer et al., Reference Knörzer, Graeff-Hönninger, Guo, Wang and Claupein2009; Weerarathne et al., Reference Weerarathne, Marambe and Chauhan2017). According to Bedoussac et al. (Reference Bedoussac, Journet, Hauggaard-Nielsen, Naudin, Corre-Hellou, Jensen and Justes2017), intercropping systems, especially with legume crops, help to cover the soil surface, reducing soil erosion, improve soil moisture and soil nutrients. In a study on cassava–peanut intercropping system, the amount of eroded soil was significantly reduced compared to the traditional monocropping (Trung et al., Reference Trung, Nakasathien and Vichukit2013).
One of the most important benefits of legume-based intercropping systems is their unique role of fixing atmospheric N through the process of Biological Nitrogen Fixation (BNF), in symbiosis with soil bacteria known as rhizobia (Nyfeler et al., Reference Nyfeler, Huguenin-Elie, Suter, Frossard, Connolly and Lüscher2009). Studies have reported the N contribution of legume crops in intercropping systems to be equivalent to about 55–96 kg of N fertilizer ha−1 season−1 (Cong et al., Reference Cong, Hoffland, Li, Six, Sun, Bao, Zhang and Van Der Werf2015; Mandimba, Reference Mandimba1995). According to Herridge et al. (Reference Herridge, Peoples and Boddey2008), symbiotically fixed N2 in legumes ranged from 100 to 380 kg N ha−1 year−1, but other studies also reported exceptionally large amounts of more than 500 kg N ha−1 year−1. Herridge (Reference Herridge2002) revealed that a combination of Rhizobium inoculants and N fertilizer at doses of 30–40 kg ha−1 resulted in similar groundnut yield compared to N fertilizer doses of 60–90 kg ha−1. In comparison with chemical fertilizer application, inoculation of soybean with rhizobia products translated to significantly higher economic benefits of about US$ 135.5 ha−1 (Boonkerd, Reference Boonkerd2002). Cowpea (Vigna unguiculata var. cylindrica) is one of the most important, widely cultivated legumes that can fix atmospheric N ranging from 9 to 120 kg N ha−1 (Awonaike et al., Reference Awonaike, Kumarasinghe and Danso1990; Boddey et al., Reference Boddey, Urquiaga, Neves and Peres1990; Toomsan et al., Reference Toomsan, McDonagh, Limpinuntana and Giller1995). Cowpea also shows high tolerance to drought and high temperatures, and can thrive in infertile acidic soils (Watanabe et al., Reference Watanabe, Hakoyama, Terao and Singh1997). While cowpea needs phosphorus (P) and can partly self-support its N requirements through BNF, cassava requires high amounts of potassium (K) for storage root formation and N for leaf production (Howeler, Reference Howeler1991), showing the advantages in nutrient demands of the two crops in an intercropping system. Cowpea is also highly suitable for cassava in terms of growth patterns and canopy development (Howeler and Hershey, Reference Howeler and Hershey2002). Notably, in the Northern mountainous areas of Vietnam, cowpea can effectively increase smallholders’ income due to its high sale price at local markets.
The local agricultural department in Yen Bai province has been encouraging cassava–cowpea intercropping system through farmer associations to mitigate soil degradation and improve soil health. Despite this, we found from our preliminary investigation that the natural nodulation of cowpea is very low (< 10 nodules plant−1 on average) across farms in this province (Supplementary Figure S1). Factors such as the absence of compatible native rhizobia, low population of rhizobia, or ineffective/low effective native rhizobia, may inhibit the symbiosis and BNF in cowpea (Date, Reference Date2000; Ojo et al., Reference Ojo, Dare, Fagbola and Babalola2015; Vanlauwe and Giller, Reference Vanlauwe and Giller2006). This situation could be improved by inoculating cowpea seeds with effective rhizobia strains (Bala et al., Reference Bala, Abaidoo and Woomer2010; Koskey et al., Reference Koskey, Mburu, Njeru, Kimiti, Ombori and Maingi2017). Several authors indicated the importance of utilizing effective native rhizobia strains in increasing cowpea production (Mathu et al., Reference Mathu, Herrmann, Pypers, Matiru, Mwirichia and Lesueur2012; Ngeno, Reference Ngeno2018; Yohane, Reference Yohane2016). Ampomah et al. (Reference Ampomah, Ofori-Ayeh, Solheim and Svenning2008) isolated five native cowpea rhizobia, assessed their symbiotic effectiveness and competitiveness, and reported that the strain All-5-2 was the most effective inoculant for improving cowpea yield in N-deficient regions of Ghana.
However, currently, no available rhizobia products for cowpea are available in the Vietnam market. Promotion of cowpea needs to be combined with identifying, formulating, and scaling up effective rhizobia inoculants to enhance and sustain BNF. Thus, the aims of this study were: (1) to screen the native rhizobia nodulating cowpea and identify potential elite inoculum strains under greenhouse conditions for further field experiments and (2) to evaluate the effective isolates under intercropped field condition and scale-up cowpea intercropping through farmer associations.
Materials and Methods
Greenhouse screening experiment
Cowpea nodules were collected from 12 different farms in Yen Bai province, North Vietnam (5 farms in Mau Dong commune, Van Yen district, and 7 farms in Cat Thinh commune, Van Chan district) where only 1 local cowpea variety Dau Den Xanh Long is cultivated. During the mid-flowering stage, at each farm, we determined the sampling lines based on the water flows and the number of plant samples in each line (a distance of 1 m between plant samples). From each cowpea plant, all the effective nodules were removed from the roots and surface sterilized in 70% ethanol. The nodules were then stored in labeled McCartney bottles containing 10 ml of 40% glycerol. All the bottles were kept in a cool box with ice and transferred to the laboratory for further analyses.
The isolation of native rhizobia from cowpea nodules was performed at the Common Microbial Biotechnology Platform (CMBP) at The Alliance of Bioversity and CIAT, Asia Hub, Hanoi, Vietnam. The rhizobia isolates were purified by phenotypic characterization (colony morphology) and by gram staining. Purified cultures of isolated colonies were sent to the Institute of Genome Research (Hanoi, Vietnam) for DNA extraction, PCR, and 16S rRNA sequencing. The sequence data was then submitted for comparison with the National Center for Biotechnology Information (NCBI) database using Basic Local Alignment Search Tool (BLAST) (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Three native rhizobia strains (CMBP037, CMBP054, and CMBP065) sequenced from cowpea nodules are shown in Table 1. To screen the performance of three native rhizobia strains isolated from cowpea nodules, a pot experiment was established in a greenhouse at the Vietnam National University of Agriculture (VNUA), Hanoi, Vietnam. The five treatments were: uninoculated plant without N application (negative control), uninoculated plant with applied N using KNO3 at a rate of 480 mg N pot−1 (positive control or N+), and separate inoculation treatments with strains CMBP037, CMBP054, and CMBP065. The experiment was arranged in a completely randomized design (CRD) with five replicates.
Table 1. Description of the treatments in the greenhouse experiment

% ID: Sequence similarity (%) of 16S rRNA gene with identical sequences identified by using the National Center for Biotechnology Information (NCBI) database.
CT, Cat Thinh; MD, Mau Dong; VC, Van Chan; VY, Van Yen.
After culturing on Yeast Extract Mannitol Agar (YEMA – 0.5 g l−1 KH2PO4, 0.2 g l−1 MgSO4, 0.1 g l−1 NaCl, 1 g l−1 Yeast Extract, 10 g l−1 Mannitol, 15 g l−1 Agar) plates, single purified colonies of the individual strains were selected to prepare rhizobia inoculant cultures. Each colony was transferred into 50 ml YEM broth in 200 ml Erlenmeyer flasks and incubated at 28 °C on a rotary shaker at 200 rpm, for 2 days for Rhizobium species and 4 days for Bradyrhizobium species. Before applying to the pots, direct cell count for each inoculum was done using the spread plate method (SOP-MI10 LH-V01) to ensure that at least 106 rhizobia cells ml−1 was applied per plant.
Cowpea seeds were surface sterilized by soaking in 3.3% NaOCl solution for 5 min and rinsed thoroughly several times with sterile distilled water. Surface-sterilized seeds were immersed in water for 1 h to imbibe, transferred to Petri dishes with moistened sterile cotton wool, and then placed in a growth chamber at 28°C to germinate in the dark for 24 h. Three preselected healthy seeds of uniform size were chosen and sown in plastic pots (12 cm diameter and 16 cm in length). Each pot was sterilized with 70% ethanol, filled with 1.3 kg of sterilized sand, and irrigated with 150 ml of distilled water in preparation for sowing. Five drainage holes were made at the bottom of each pot.
Four days after sowing (DAS), 3 ml of each inoculant were added to the base of seedlings (1 ml per seedling) in the pots. Plants were thinned to two healthy plants per pot at 7 DAS. Essential nutrients with the exception of N were added to each pot every 2 days, as nutrient solution [K2SO4 0.5 M, KOH, KH2PO4 1 M, CaCl2 2 M, MgSO4 0.5 M, MnSO4 0.002 M, ZnSO4 0.001 M, CuSO4 0.0004 M, CoSO4 0.0002 M, H3BO4 0.004 M, NaMoO4 0.0002 M, FeSO4 0.08 M, and EDTA (C10H16N2O8) 0.08 M] modified from Broughton and Dilworth (Reference Broughton and Dilworth1971). The plants were alternatively watered with 150 ml of distilled water or with 150 ml of the nutrient solution.
Cowpea plants were harvested at the flowering stage (6 weeks after sowing). Chlorophyll content of the youngest fully developed cowpea leaves was measured using a SPAD-502 chlorophyll meter (Minolta corporation, Ltd., Osaka, Japan). Shoots were cut at 1 cm above the surface using a clean, sharp knife. The roots were gently washed and the nodules were separated from the roots. Number of nodules per plant, shoot fresh weight, and root fresh weight were recorded. Shoots, roots, and nodules were oven-dried at 60 °C for 2 days before measuring dry weights. Total N content (%) of the oven-dried shoot was determined by Kjeldahl method (Bremner, Reference Bremner1996). Symbiotic efficiency (SEF) was also calculated using the formulae by Lalande et al. (Reference Lalande, Bigwaneza and Antoun1990):
SEF (%) = (shoot dry weight of inoculated plant/shoot dry weight of positive control plant) * 100.
SEF was classified as: very effective (> 80%); effective (51–80%); less effective (35–50%) or ineffective (<35%).
Field inoculation experiment
In March 2018, based on the results from the greenhouse experiment, an on-farm experiment was conducted to evaluate the effectiveness of native strains inoculated with cowpea in an intercropping system with cassava under field conditions in Mau Dong commune, Van Yen district, Yen Bai province. The field location is shown in Supplementary Figure S2. In order to assess the interaction effect of different native rhizobia from the same location on cowpea production, CMBP037 and CMBP054 were mixed together before inoculation. This experiment was composed of nine treatments resulting from a factorial combination of three inoculation treatments and three field slope categories (steep, moderate, and gentle) commonly encountered in the area. The slope was classified as gentle slope (< 5 °C); moderate slope (5–15 °C); steep slope (> 15 °C) (Jahn et al., Reference Jahn, Blume, Asio, Spaargaren and Schad2006). Supplementary Table S1 shows the soil characteristics of the field site from each slope category. Inoculation treatments included (i) non-inoculated control (Non_I), (ii) a mixed inoculant containing Rhizobium strains CMBP037 and CMBP054 isolated from Mau Dong commune, in a ratio of 1:1 in volume (CMBP (037+054), and (iii) Rhizobium strain CMBP065 isolated from Cat Thinh commune. The treatments were each applied to 6 randomly selected representative farmers’ fields, but the total number was reduced to 24 farms after removing mismanaged farms from the study.
Inoculant preparation and seed sterilization were done as described above for the greenhouse experiment. Inoculants were transported to the field in cooler boxes and applied at a rate of 50 ml kg−1 of seeds. Seed inoculation was done just before sowing in the shade to maintain the viability of bacterial cells. Seeds were allowed to air dry for about 30 min before sowing and were immediately covered by soil after sowing. Cowpea was intercropped with cassava whereby one row of cowpea was planted between two rows of cassava at a density of 10,000 cowpea plants ha−1.
At the mid-flowering stage (7 weeks after sowing), 10 cowpea plants positioned on 2 diagonal lines in each farm were harvested. Each plant sample was considered as one replicate. Shoots were cut at 1 cm above the soil surface using a clean, sharp knife. Roots were gently washed and nodules were separated from the roots and the number of nodules per plant was recorded. Shoots and roots were oven-dried at 60 °C for 2 days to measure dry weights. Oven-dried shoots were analyzed for total N content (%) as described above. At the maturity stage (9 weeks after sowing), three random areas of 5 m2 from each farm were harvested and cowpea seed yield (kg ha−1) was calculated.
Investigation in the expansion of cassava–cowpea intercropping system in 2017–2018 in Van Yen district, Yen Bai province
Muoi village in Mau Dong commune is one of the most productive cassava areas of Van Yen district, Yen Bai province, and the smallholder farmers in this village have participated in this conservation agricultural practice since the beginning of the project started in 2016. Muoi village has a total land area of over 200 ha and 95 households, with an average household owning 0.5 ha of land. In order to identify the adoption and expansion of such a cropping system, we assessed the cassava cropping systems in the area during the period between 2017 and 2018. From all the farms in Muoi village, we surveyed the total number and area of cassava farms and the number and area of cassava–cowpea intercropping farms, as well as their correlative percentages.
Statistical analysis
A two-way analysis of variance (ANOVA) was performed using R version 3.4.2 to assess the effects of slope category, inoculation treatments, and their interaction on nodulation, shoot and root biomasses, shoot N, and yield of cowpea. Significance of difference was evaluated at p < 0.05. Data screening was performed to test whether ANOVA’s assumptions of homoscedasticity and normality were violated. Cowpea nodulation and root biomass have skewed distributions; thus, natural log transformation was applied. Adjusted LS means of treatments were calculated and Tukey’s test was used for multiple comparisons. Simple correlation analysis was used to determine the association between nodule dry weight and shoot dry weight of cowpea.
Results
Response of cowpea to native rhizobia inoculation under greenhouse screening experiment
As shown in Table 2, inoculation with strain CMBP037 recorded the lowest number of nodules (7.7 nodules plant−1). The highest rate of nodulation was found in CMBP054 and CMBP065 inoculants (46.4 and 60.7 nodules per plant, respectively). CMBP065 also recorded the highest nodule dry weight (0.17 g plant−1) while there was no significant difference in nodule dry weight between CMBP037 and CMBP054 treatments.
Table 2. Response of cowpea to native rhizobia inoculation in the greenhouse screening experiment

Means followed by different letters within the same column are significantly different at p < 0.05 according to Tukey’s HSD test.
SEF, symbiotic efficiency; SPAD value, index value displayed by Konica Minolta Chlorophyll meters and having a correlation to chlorophyll density.
Rhizobia inoculation significantly affected cowpea biomass (Table 2). The highest shoot and root dry biomasses were recorded in the positive control N+. There was no significant difference in shoot and root dry biomasses among CMBP037, CMBP054, and CMBP065. As shown in Figure 1, there was a significant positive correlation between nodule dry weight and shoot dry weight of cowpea.
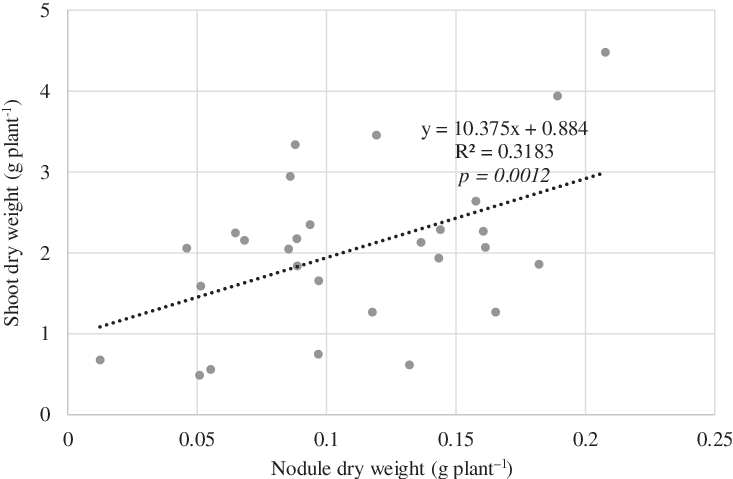
Figure 1. Correlation analysis between nodule dry weight and shoot dry weight in the greenhouse experiment.
All the inoculated treatments produced nodules, therefore, they were considered for SEF determination. There was a significant difference in SEF among native rhizobia isolates (Table 2). CMBP037 had the lowest SEF of 19.27%. The SEF values did not differ significantly between CMBP054 (54.56%) and CMBP065 (55.73%).
Regarding total shoot N analysis, the highest shoot N content was reported in CMBP054 inoculation (6.53%), but this was not significantly different from CMBP037 and CMBP065 inoculation (Table 2). The negative control had the lowest shoot N content (2.18%).
SPAD value determines the relative amount of chlorophyll, which will increase in proportion to the amount of N in a leaf. Therefore, a high SPAD value shows a healthy plant. The highest and lowest SPAD values were recorded in CMBP065 inoculation and control (38.4 and 15.6), respectively, (Table 2). SPAD values differed significantly between rhizobia inoculation and control, but not between rhizobia inoculation and N+ treatment.
Response of cowpea to native rhizobia inoculation under field condition
Table 3 shows responses of cowpea nodulation, shoot, and root dry weight to different rhizobia inoculants and slope categories. Rhizobia inoculation and the interaction effect of inoculation-slope significantly affected cowpea nodulation, shoot dry weight, total shoot N, and yield, while slope significantly affected nodulation, total shoot N, and yield of cowpea. On moderate and steep slopes, the mixture of CMBP (037+054) had the highest nodulation (32.2 and 23.7 nodules plant−1, respectively), while there was no significant difference between the uninoculated control and CMBP065. On gentle slopes, there was no significant difference in the number of nodules per plant for all treatments.
Table 3. Response of cowpea nodulation, shoot, and root dry weight to native rhizobia inoculation and slope in the field experiment

Means followed by different letters within the same column are significantly different at P<0.05 according to Tukey’s HSD test.
There were no significant differences in shoot dry weight among all treatments on gentle slope. On moderate and steep slopes, the highest shoot dry weight was found in CMBP (037+054) (28.07 and 20.09 g plant−1, respectively), while there was no significant difference between the uninoculated control and CMBP065. Root dry weight of cowpea ranged from 1.4 to 2.2 g plant−1, and showed no significant differences among rhizobia inoculants or slope categories in the field experiment (Table 3).
Total shoot N content and yield of cowpea affected by different rhizobia inoculants are shown in Table 4. On gentle slope, total shoot N was lowest in CMBP065 (2.51%) and there was no significant difference between the uninoculated control and CMBP (037+054) (2.88 and 2.99%, respectively). On moderate slope, total shoot N of cowpea was the same for CMBP (037+054) and CMBP065, while the uninoculated control recorded the lowest shoot N content (2.80%). On steep slope, total shoot N was highest in CMBP (037+054) (3.12%), followed by uninoculated (2.92%), and was lowest in CMBP065 treatment (2.77%). On gentle slope, there was no significant difference in total shoot N among all treatments. Regarding cowpea yield, on moderate and steep slopes, the mixture CMBP (037+054) recorded the highest yield (436.4 and 428.6 kg ha−1, respectively) (Table 4). There was no significant difference between the yield of uninoculated control and CMBP065 on moderate slope, while cowpea yield in CMBP065 was higher (403.8 kg ha−1) than the uninoculated control (384.3 kg ha−1) on steep slope.
Table 4. Response of shoot total N content and yield of cowpea to native rhizobia inoculation and slope in the field experiment

Means followed by different letters within the same column are significantly different at P<0.05 according to Tukey’s HSD test.
The expansion of cassava–cowpea intercropping system in 2017–2018 in Van Yen district, Yen Bai province
The adoption level of agricultural practices can be assessed by the expansion rate of such practices through the number of farms using it and also the surface of land dedicated to cassava monocropping versus cassava intercropping with cowpea (Figure 2). The number of farmers practicing cassava–cowpea intercropping in 2018 (52 farmers, or 54.74% of the total cassava farms) had more than tripled since 2017 (only 16 farmers, or 16.84%). Similarly, the area of intercropped fields in 2018 (18.0 ha, or 40 % of the total area) was 4.8 times higher than in 2017 (3.7 ha, or 8.22% of the total area).

Figure 2. Number of farms and areas practicing cassava–cowpea intercropping system at Mau Dong commune, Van Yen district, Yen Bai province, Vietnam during 2017–2018.
Discussion
The results from the greenhouse experiment showed that rhizobia inoculation with CMBP054 and CMBP065 strains significantly increased cowpea nodulation and shoot N accumulation as compared to the non-inoculation treatments (Table 2). The superior performance obtained from these inoculants can be attributed to their ability to infect, form nodules, and fix N with cowpea. These results concur with previous studies (Ampomah et al., Reference Ampomah, Ofori-Ayeh, Solheim and Svenning2008; Gómez Padilla et al., Reference Gómez Padilla, Ruiz-Díez, Fernández-Pascual, López Sánchez, Bloem and Eichler-Löbermann2016; Yohane, Reference Yohane2016), which showed the competitive potential of native isolates nodulating cowpea when compared to the non-inoculated treatment. Gómez Padilla et al. (Reference Gómez Padilla, Ruiz-Díez, Fernández-Pascual, López Sánchez, Bloem and Eichler-Löbermann2016) reported that the isolated strain VIBA-1 (Bradyrhizobium liaoningense) highly competed against other native strains in the soil. Yohane (Reference Yohane2016) concluded that the native rhizobia strains isolated from the fields had higher symbiotic effectiveness than the strain (MG5013) used in inoculant products. The result from our greenhouse experiment also showed a significant correlation between shoot dry weight and nodule dry weight of cowpea (Figure 1), which is consistent with previous studies (Kawaka et al., Reference Kawaka, Dida, Opala, Ombori, Maingi, Osoro, Muthini, Amoding, Mukaminega and Muoma2014; Koskey et al., Reference Koskey, Mburu, Njeru, Kimiti, Ombori and Maingi2017). As N-fixing capacity can be assessed by shoot dry weight of legumes (Beck et al., Reference Beck, Materon and Afandi1993; Gibson, Reference Gibson1987), this finding revealed that inoculation with native rhizobia strains enhanced nodulation of cowpea, which consequently improved shoot biomass and symbiotic N fixation.
SEF plays an important role in evaluating the response of legumes to inoculation and choosing effective isolates for inoculant production (Fening and Danso, Reference Fening and Danso2002). It is well known that diverse rhizobia strains show wide variation in their SEF on host plants and in this study, there were significant differences with respect to SEF among the inoculation treatments in the greenhouse (Table 2). Based on the SEF classification by Lalande et al. (Reference Lalande, Bigwaneza and Antoun1990), CMBP037 was rated as ineffective, as this strain could not deliver any functional advantage as compared to the negative controls. Whereas, CMBP054 and CMBP065 were rated as effective isolates (> 50%), and thus potential native strains for enhancing cowpea N fixation needs to be evaluated under further intercropped field condition. Of these two effective strains, CMBP065 showed significantly higher nodule dry matter under greenhouse conditions. According to Beck et al. (Reference Beck, Materon and Afandi1993), high nodule dry weight can lead to higher efficiency in BNF and higher shoot biomass.
In the field experiment, inoculation with the treatment CMBP (037+054) significantly increased cowpea nodulation, shoot dry weight, shoot total N, and yield on moderate and steep slopes, showing that this mixture is the most effective inoculant for moderate and steep sloping fields at this location. These findings are supported by numerous studies, which showed that inoculation with Bradyrhizobium strains resulted in a significant increase in cowpea nodulation and yield (Nyoki and Ndakidemi, Reference Nyoki and Ndakidemi2013; Ulzen et al., Reference Ulzen, Abaidoo, Mensah, Masso and AbdelGadir2016; Yoseph et al., Reference Yoseph, Baraso and Ayalew2017).
The superior performance of the combination of native rhizobia isolates from Mau Dong commune may be attributed to the ability to outcompete other native strains in the soil, nodule infection competitiveness, as well as SEF. Numerous studies have shown the positive effects of native rhizobia strains nodulating cowpea in comparison to the uninoculated controls under field conditions (Danso and Owiredu, Reference Danso and Owiredu1988; Gómez Padilla et al., Reference Gómez Padilla, Ruiz-Díez, Fernández-Pascual, López Sánchez, Bloem and Eichler-Löbermann2016; Yoseph et al., Reference Yoseph, Baraso and Ayalew2017).
Although strain CMBP065 was more effective that strain CMBP054 in the greenhouse experiment, CMBP065 was less effective than the mixture CMBP (037+054) in the field conditions. This suggests that strain CMBP037, which had the lowest SEF in the greenhouse experiment, may enhance the effectiveness of CMBP054 strain when co-inoculated. The two strains CMBP037 and CMBP054 were isolated from Mau Dong commune and the advantage of using both strains could be attributed to their better adaption to the local soil and climatic conditions, as well as the relationship with other rhizospheric microorganisms (Koskey et al., Reference Koskey, Mburu, Njeru, Kimiti, Ombori and Maingi2017; Meghvansi et al., Reference Meghvansi, Prasad and Mahna2010; Svenning et al., Reference Svenning, Gudmundsson, Fagerli and Leinonen2001). One other possible explanation of this is the synergism between these two native rhizobia strains. This finding is on the contrary to several reports, which showed that inoculation with multiple strains is less effective as compared to the single rhizobia strain (Danso and Owiredu, Reference Danso and Owiredu1988; Martinez-Romero, Reference Martinez-Romero2003; Nkot et al., Reference Nkot, Fankem, Adamou, Ngakou, Nwaga and Etoa2015), or different strains may compete with each other in the same inoculant (Raposeiras et al., Reference Raposeiras, Marriel, Muzzi, Paiva, Pereira Filho, Carvalhais, Passos, Pinto and Sá2006). Synergistic interaction between rhizobia and plant growth-promoting rhizobacteria (PGPR) or phosphate-solubilizing bacteria (PSB) has been shown (Korir et al., Reference Korir, Mungai, Thuita, Hamba and Masso2017; Veena and Poonam, Reference Veena and Poonam2011), but little is known of the synergism between different rhizobia strains.
Degree of slope, one of the important geographical factors, could affect the soil characteristics, plant physiological processes, and soil microorganisms. The combination of CMBP (037+054) significantly improved cowpea nodulation, shoot dry weight, total shoot N, and yield on moderate and steep slopes, whereas, there was no significant effect of rhizobia inoculations on cowpea on gentle slope. This finding indicates the significant interaction between rhizobia inoculation and the physical slope factor. The influence on local climate, erosion process, soil characteristics, and plant communities, soil slope has been shown to indirectly or directly affect bacterial diversity, composition, and activities (Haiyan et al., Reference Haiyan, Xiang, Jian, Adams, Zhang, Yuntao and Yu2016; Orwin et al., Reference Orwin, Wardle and Greenfield2006). It is still unclear how sloping land affects rhizobia BNF efficiency, particularly in the Northern mountainous areas of Vietnam. Therefore, further studies should be conducted in order to identify the mechanism of the interaction between slope and rhizobia inoculation.
The results from our investigation about cassava–cowpea intercropping expansion revealed the high adoption level of local farmers with the inclusion of cassava–cowpea intercropping system. However, due to the low natural nodulation of cowpea (as shown in Figure S1), resulting in low BNF in cowpea, it showed the urgent need to improve cowpea yield in such intercropping system by inoculation with effective native rhizobia.
Conclusion
This study revealed the potential of the mixture of native rhizobia strains from Mau Dong [CMBP (037+054)] as an effective inoculant for improving cowpea N fixation and yield, especially on moderate and steep slopes. While the native isolate CMBP065 showed the highest SEF under the greenhouse conditions, the mixture of native isolates CMBP (037+054) displayed superior performance and adaptability under the intercropped field conditions. The results suggest that it would make sense to isolate and screen more native rhizobia in order to get more effective and competitive strains. Further studies should also be conducted to better characterize the populations of rhizobia for cowpea at the experimental sites. We have shown that it is possible to promote the utilization of native rhizobia strains and provide cheap and effective inoculants for cowpea to local smallholders. By the inclusion of cassava–cowpea intercropping system, farmers can significantly reduce the utilization of mineral fertilizers, sustain the production, and consequently increase economic benefits.
Acknowledgements
This work was financially supported by the Southeast Asian Regional Center for Graduate Study and Research in Agriculture (SEARCA), and the Alliance of Bioversity and CIAT, and by the ACTAE project funded by the French Agency of Development (AFD).
The authors are indebted to Dr Lambert Bräu for English language editing.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0014479720000344.









