Introduction
The reproductive systems of arthropod hosts are often manipulated by endosymbiotic bacteria, such as Spiroplasma, Rickettsia, Wolbachia, Arsenophonus and Cardinium (O'Neill et al., Reference O'Neill, Hoffmann and Werren1997; Bourtzis & Miller, Reference Bourtzis and Miller2003, Reference Bourtzis and Miller2006). Among these, Wolbachia are particularly focused upon due to their high prevalence in arthropod hosts and the various types of reproductive aberrations they induce (Werren et al., Reference Werren, Windsor and Guo1995; Jeyaprakash & Hoy, Reference Jeyaprakash and Hoy2000; Werren & Windsor, Reference Werren and Windsor2000). Wolbachia strains can extensively manipulate host reproduction by inducing parthenogenesis, feminizing genetic males, killing male embryos or causing cytoplasmic incompatibility between gametes (O'Neill et al., Reference O'Neill, Hoffmann and Werren1997; Hiroki et al., Reference Hiroki, Kato, Kamito and Miura2002, Reference Hiroki, Tagami, Miura and Kato2004; Bourtzis & Miller, Reference Bourtzis and Miller2003; Narita et al., Reference Narita, Kageyama, Nomura and Fukatsu2007a). The most common type of Wolbachia-induced reproductive manipulation is cytoplasmic incompatibility. Cytoplasmic incompatibility results in embryonic mortality for mating between insects of the same species with differing Wolbachia infection statuses (Bourtzis et al., Reference Bourtzis, Dobson, Braig and O'Neill1998; Bourtzis & Miller, Reference Bourtzis and Miller2003) and can be either unidirectional or bidirectional. Unidirectional cytoplasmic incompatibility is typically expressed when an infected male mates with an uninfected female. The reciprocal mating is fully compatible, as are matings between infected individuals. Bidirectional cytoplasmic incompatibility usually occurs in matings between infected individuals harboring different strains of Wolbachia (Bourtzis & Miller, Reference Bourtzis and Miller2003).
The pale clouded yellow butterfly Colias erate poliographus Motschulsky (Lepidoptera: Pieridae) is distributed in Far East Russia, Sakhalin, the Korean Peninsula, China and Japan. A previous survey of Wolbachia infection among lepidopteran insects in Japanese populations revealed that ten out of 11 C. erate poliographus individuals examined were infected with Wolbachia (Tagami & Miura, Reference Tagami and Miura2004). However, the biological effects of Wolbachia infection on C. erate poliographus, such as reproductive manipulations or fitness effects, remained to be examined.
In the present study, we examined (i) the infection frequencies of other Japanese populations of C. erate poliographus, (ii) the type of Wolbachia-inducing reproductive manipulation in C. erate poliographus, (iii) the vertical transmission efficiency of the Wolbachia strain in its host C. erate poliographus and (iv) the fitness effects of Wolbachia infection on the survival rate and development period of C. erate poliographus. Furthermore, a multilocus sequencing typing (MLST) analysis (Baldo et al., Reference Baldo, Dunning, Jolley, Bordenstein, Biber, Choudhury, Hayashi, Maiden, Tettelin and Werren2006) and phylogenetic analysis were employed to characterize the Wolbachia strain.
Materials and methods
Field sampling
In 2007, adult individuals of C. erate poliographus were collected at seven geographic locations in Japan, namely Morioka (MO1 and MO2), Minamisaku (MS), Tsukuba (TB), Kashiwa (KW), Matsudo (MD) and Nishinoomote (NO) (fig. 1, table 1).

Fig. 1. Collection localities of C. erate poliographus in Japan. MO, Morioka; MS, Minamisaku; TB, Tsukuba; KW, Kashiwa; MD, Matsudo; NO, Nishinoomote.
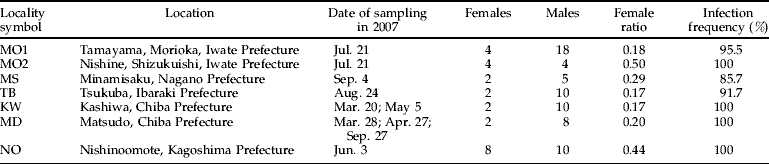
Table 1. Wolbachia infection status of C. erate poliographus collected at seven geographic locations in Japan.
Diagnostic PCR
Leg tissues from each adult butterfly were crushed using plastic pestles in 0.5-ml tubes containing 10 μl of proteinase K (20 mg ml−1). Following addition of 190 μl of STE buffer (10 mM Tris-HCl pH 8.0, 1 mM EDTA pH 8.0, 150 mM NaCl), the samples were sequentially incubated at 55°C for 35 min and 95°C for 5 min. After centrifugation at 13,000 rpm for 1 min, the supernatants were subjected to diagnostic PCR. Compared to other tissues, such as abdomen and thorax, legs are more effectual for the simple preparation of DNA since they contain fewer amounts of substances (e.g. pigments or fat) that can inhibit PCR. Reliability of Wolbachia detection using leg tissues has been proven by Narita et al. (Reference Narita, Nomura and Kageyama2007b), which compared Wolbachia infection status in various tissues (legs, ovaries, testes, Malpighian tubules and fat body) in Wolbachia-infected butterflies.
PCR detection of Wolbachia infection was performed using primers groEfl (5′-TTG TAG CCT GCT ATG GTA TAA CT-3′) and groErl (5′-GAA TAG GTA TGA TTT TCA TGT-3′) for the groE gene (Masui et al., Reference Masui, Sasaki and Ishikawa1997) and primers wsp81F (5′-TGG TCC AAT AAG TGA TGA AGA AAC-3′) and 691R (5′-AAA AAT TAA ACG CTA CTC CA-3′) for the wsp gene (Zhou et al., Reference Zhou, Rousset and O'Neill1998).
To confirm that DNA was properly extracted, the host mitochondrial cytoplasmic c oxidase I (COI) gene was amplified using the primer set COI-321F (5′-GAT TTT TTG GAC ATC CTG AAG-3′) and COI-689R (5′-CTA AAA TTA CTC CTG TTA ATC C-3′) (Narita et al., Reference Narita, Nomura and Kageyama2007b) in the same samples. PCR amplifications were conducted under the following temperature profile: 35 cycles of 95°C for 1 min, 55°C for 1 min and 72°C for 1.5 min, followed by incubation at 72°C for 7 min. Samples in which the COI gene failed to be amplified were excluded from the analysis.
Cytoplasmic incompatibility
A total of eight naturally infected female butterflies collected from Morioka (MO1 and MO2) were individually allowed to oviposit on the leaves of the white clover Trifolium repens L. in plastic cups. Hatched larvae derived from each mother were separated into two groups. Individuals in the first group were mass-reared in plastic cases (10–20 individuals per case) on fresh leaves of T. repens in a laboratory at 25°C under a long-day regimen (16 h light:8 h dark). The emerged adults were kept in plastic cups and fed with 10% sucrose solution. These individuals were referred to as untreated individuals (infected individuals) and used for crossing experiments.
Individuals in the second group were individually fed with an artificial diet (Kato & Sakakura, Reference Kato and Sakakura1994) containing 0.05% tetracycline hydrochloride from the 1st larval stage until pupation. The emerged adults were kept in plastic cups and fed with 10% sucrose solution. These individuals were referred to as treated individuals and used in crossing experiments.
Treated and untreated males and females were crossed in all four possible combinations. All crosses were between non-siblings. The hatching rates of the resulting eggs were recorded.
To check whether the females used in crossing were fertilized, the female bursa copulatrix was dissected after oviposition, and the presence or absence of spermatophores was examined.
Survival rate, development period and sex ratio
The larvae derived from each crossing were reared individually on an artificial diet containing cut leaves of the white clover T. repens. Since no cannibalism was possible, this individual rearing allowed us to obtain precise data for the sex ratios at the adult stage, survival rates during the larval stages and pupal stage, and the development times during the larval stages and pupal stage.
Adults were distinguished as males and females by their wing colors and their abdominal tip morphologies.
The development period data were subjected to statistical analyses using the software R ver. 2.4.0 (R Development Core Team, 2005). Since some of the data sets did not exhibit normal and/or homogeneous variance, we adopted a generalized linear model (McCullagh & Nelder, Reference McCullagh and Nelder1989) for Gaussian, inverse Gaussian, gamma and negative binomial distributions, which were selected according to the Akaike information criterion.
MLST analysis of Wolbachia
Thoracic muscles were dissected from the mother of brood C1 and father of brood B1 and stored at −20°C until DNA extraction. DNA was extracted using a DNeasy Tissue Kit™ (QIAGEN). These two infected butterflies were fully characterized by MLST and WSP analyses. The wsp gene and the five MLST genes (coxA, gatB, hcpA, ftsZ and fbpA) were sequenced using standard protocols (Baldo et al., Reference Baldo, Dunning, Jolley, Bordenstein, Biber, Choudhury, Hayashi, Maiden, Tettelin and Werren2006).
The host and strain information have been submitted to the MLST database (http://pubmlst.org/wolbachia/).
The sequence data were aligned with published Wolbachia sequences from other insects. Likelihood-ratio tests were performed using MODELTEST VERSION 3.06 (Posada & Crandall, Reference Posada and Crandall2001) to determine the models of evolution with the best fit for each gene and the concatenated MLST data. Phylogenetic trees were constructed by the maximum likelihood and maximum parsimony methods using PAUP 4.0b10 (Swofford, Reference Swofford2001). Bootstrap support for clades was evaluated using 250 (maximum likelihood method) or 1000 (maximum parsimony method) pseudoreplicates, and the 50% majority rule bootstrap was applied.
Results
Infection frequencies of Wolbachia in seven local populations
To investigate the infection frequencies of Wolbachia in seven local populations (fig. 1), diagnostic PCR was performed on all the collected butterflies (n=79). The infection frequencies in all seven local populations were extremely high. Butterflies collected from Morioka (MO2), Kashiwa (KW), Matsudo (MD) and Nishinoomote (NO) were all infected with Wolbachia. On the other hand, some butterflies collected from Morioka (MO1), Minamisaku (MS) and Tsukuba (TB) were not infected with Wolbachia (table 1).
Strong cytoplasmic incompatibility caused by Wolbachia
To examine whether Wolbachia induced cytoplasmic incompatibility in C. erate poliographus, all four possible crossing combinations were performed between infected and cured parents (table 2). When crossings were performed between cured females and infected males, none of the 656 eggs hatched (broods B1, B2 and B3). In contrast, when the other three crossing combinations were performed, large numbers of the eggs hatched (egg hatching rate: 27.1–77.3%).
Table 2. Hatching rates of eggs produced by four crossing combinations of Wolbachia-infected and cured C. erate poliographus.
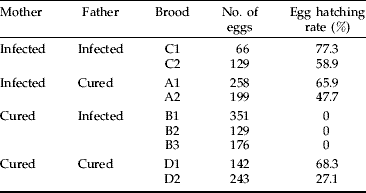
After oviposition, we dissected the females used for crossing and confirmed that they were all fertilized by examining the presence of spermatophores in the bursa copulatrix. These data suggest that Wolbachia causes strong cytoplasmic incompatibility in C. erate poliographus.
Survival rates and sex ratios
We compared the survival rates of the larval stages and pupal stage among the broods. The survival rates of the larval stages in broods D1 and D2 were significantly lower than those in broods A1, A2, C1 and C2 (table 3; P<0.001, Fisher's exact probability test), while the survival rates of the pupal stage did not differ significantly among the broods.
Table 3. Survival rates of offspring produced by four crossing combinations of Wolbachia-infected and cured C. erate poliographus.
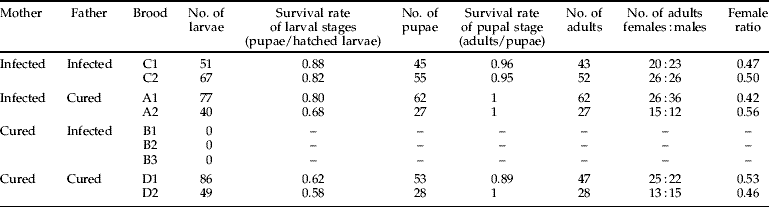
The sex ratios in all six broods (C1, C2, A1, A2, D1 and D2) did not deviate significantly from 1:1 (table 3; P>0.05, Fisher's exact probability test). These results indicate that sex ratio distortions, such as feminization or male killing, do not occur in C. erate poliographus infected with Wolbachia.
Development period
We compared the development periods of the larval stages and pupal stage among the broods. The development periods of the larval stages in broods A1 and A2 were significantly longer than those in broods C1, C2, D1 and D2 (table 4; P<0.001, generalized linear model), while the development periods of the pupal stage did not differ significantly among the broods.
Table 4. Development periods of offspring produced by four crossing combinations of Wolbachia-infected and cured C. erate poliographus.
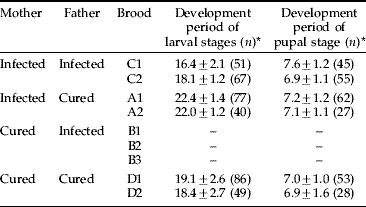
* Mean (days)±standard deviation.
Vertical transmission rates
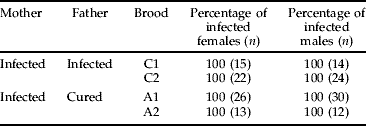
When adult butterflies emerged, we examined the presence or absence of Wolbachia in their legs by diagnostic PCR. All butterflies (n=156) derived from four broods (C1, C2, A1 and A2) were infected with Wolbachia (table 5).
Table 5. Proportions of infected individuals among offspring produced by Wolbachia-infected and cured C. erate poliographus.
Characterization of the Wolbachia strain
Two Wolbachia-infected adults (the mother of brood C1 and father of brood B1) were subjected to PCR amplification of the wsp gene and five MLST genes (coxA, gatB, hcpA, ftsZ and fbpA) and the DNA sequences were determined. The nucleotide sequences of the MLST genes and WSP gene of Wolbachia from C. erate poliographus have been deposited in the DDBJ/EMBL/GenBank databases under accession numbers AB436683-AB436694, respectively. The wsp gene sequences of the Wolbachia strain were identical with those of the Wolbachia strains from C. erate poliographus reported by Tagami & Miura (Reference Tagami and Miura2004).
According to the MLST scheme, the Wolbachia in C. erate poliographus is a novel sequence type (ST141) and belongs to supergroup B. Regarding the phylogeny, this Wolbachia strain formed a monophyletic group with the cytoplasmic-incompatibility-inducing Wolbachia strains found in the pyralid moth Ephestia kuehniella (ST20) and the mosquito Culex pipiens (ST9) and the male-killing Wolbachia strain found in the nymphalid butterfly Hypolimnas bolina (ST125), which was supported by high bootstrap probabilities (100% in both the maximum likelihood and maximum parsimony methods) (fig. 2).

Fig. 2. Maximum likelihood phylogeny using the GTR+I+G model based on the concatenated data set for the five MLST loci of Wolbachia from C. erate poliographus and the 17 other sequence types (STs) belonging to supergroup B (2079 bp). The tree was rooted with Wolbachia in supergroup A (ST13 and ST91). Branches supported by bootstrap probabilities of less than 50% in the maximum likelihood or maximum parsimony method were collapsed. The maximum likelihood (left) and maximum parsimony (right) bootstrap values of >50% are shown for each node.
Discussion
Type of Wolbachia-induced reproductive manipulation in C. erate poliographus
Wolbachia are known to manipulate the reproduction of their hosts in various ways, such as induction of parthenogenesis, feminization, male killing or induction of cytoplasmic incompatibility. We examined the type of Wolbachia-induced reproductive manipulation by crossing experiments and rearing of C. erate poliographus.
Thelytokous parthenogenesis is a phenomenon in which females produce exclusively female offspring without fertilization, and is only known in haplodiploid insect groups, such as Hymenoptera and Thysanoptera (Stouthamer, Reference Stouthamer, O'Neill, Hoffmann and Werren1997; Arakaki et al., Reference Arakaki, Miyoshi and Noda2001). In the present study, male-derived spermatophores were present in both infected and antibiotic-treated mothers of nine broods. Thus, the possibility of parthenogenesis induction by Wolbachia infection is extremely low in C. erate poliographus.
Feminization is a phenomenon in which inherently genetic males are phenotypically changed into females, and is only known in the woodlice Armadillidium and the butterfly Eurema hecabe (Rigaud, Reference Rigaud, O'Neill, Hoffmann and Werren1997; Rigaud et al., Reference Rigaud, Juchault and Mocquard1997; Hiroki et al., Reference Hiroki, Kato, Kamito and Miura2002, Reference Hiroki, Tagami, Miura and Kato2004; Narita et al., Reference Narita, Kageyama, Nomura and Fukatsu2007a). Male killing is a widely occurring phenomenon in insects in which male progeny are selectively killed (Hurst & Jiggins, Reference Hurst and Jiggins2000). If feminization or male killing by Wolbachia infection occurred in C. erate poliographus, the sex ratio of infected broods would be female-biased. In this study, the sex ratios of all broods were 1:1 irrespective of their Wolbachia infection status, thus excluding the possibility of feminization or male killing.
Cytoplasmic incompatibility is typically expressed when an infected male mates with an uninfected female. The underlying mechanism of cytoplasmic incompatibility is considered to be a modification-rescue system. In other words, a Wolbachia strain in males modifies the sperm so as to kill the offspring during embryogenesis. If the same Wolbachia strain is also possessed by females, the offspring will be rescued by removal of the modification (Werren, Reference Werren1997; Bourtzis & Miller, Reference Bourtzis and Miller2003; Poinsot et al., Reference Poinsot, Charlat and Mercot2003). Cytoplasmic incompatibility is the most common type of host manipulation cased by Wolbachia (Bourtzis & Miller, Reference Bourtzis and Miller2003). The hatching rates of eggs produced by such incompatible crossings are low. In this study, we examined all four possible crossing combinations of Wolbachia-infected and cured C. erate poliographus. Among them, complete suppression of egg hatching was only observed in one combination (between cured females and infected males), which is a typical phenomenon of strong cytoplasmic incompatibility.
We further found that the cytoplasmic-incompatibility-inducing Wolbachia strain in C. erate poliographus is a novel sequence type (ST141) by using the MLST scheme. The monophyly of Wolbachia strains in C. erate poliographus (ST141), Ephestia kueniella (ST20), Culex pipiens (ST9) and Hypolimnas bolina (ST125) was supported by high bootstrap probabilities. Unfortunately, these findings provide us with very little information regarding the evolutionary origin of these Wolbachia strains at present. Future discoveries of novel Wolbachia strains in this clade may allow us to infer some historical processes of horizontal transfer of Wolbachia.
Fitness effect of Wolbachia infection on C. erate poliographus
A number of studies have investigated the fitness effects of Wolbachia infection and variously reported a positive effect (Vavre et al., Reference Vavre, Girin and Bouletreau1999; Dobson et al., Reference Dobson, Marsland and Rattanadechakul2002; Fry & Rand, Reference Fry and Rand2002) or a negative or lack of effect (Hoffmann & Turelli, Reference Hoffmann and Turelli1988; Giordano et al., Reference Giordano, O'Neill and Robertson1995; Johanowicz & Hoy, Reference Johanowicz and Hoy1999; Bordenstein & Werren, Reference Bordenstein and Werren2000; Harcombe & Hoffmann, Reference Harcombe and Hoffmann2004). In this study, we investigated the fitness effect of Wolbachia infection on C. erate poliographus from the data for the survival rates and growth rates (development periods). In C. erate poliographus, the survival rates during the larval stages were significantly higher in broods produced by infected mothers than in broods produced by cured mothers, although there was no significant difference in the growth rates. These results imply that the Wolbachia have beneficial effects on their larval hosts. However, we must remain cautious about this finding because the parents were treated with antibiotics, which might have had negative maternal effects on their progeny.
How the high prevalence of Wolbachia infection is accomplished in natural populations of C. erate poliographus
For vertically transmitted endosymbionts like Wolbachia, vertical transmission efficiency is one of the most important factors for successful maintenance in the host populations (Hoffmann et al., Reference Hoffmann, Turelli and Harshman1990, Reference Hoffmann, Hercus and Dagher1998; Turelli & Hoffmann, Reference Turelli and Hoffmann1995; Werren, Reference Werren1997).
We found that the vertical transmission rates of Wolbachia were 100% in C. erate poliographus. Furthermore, this Wolbachia strain was found to cause strong cytoplasmic incompatibility in C. erate poliographus.
It has been reported that, due to the effect of the strong cytoplasmic incompatibility induced by Wolbachia, even small numbers of Wolbachia-infected individuals invading previously uninfected populations led to rapid spreading and fixation of infection in a Californian population of Drosophila simulans (Turelli & Hoffmann, Reference Turelli and Hoffmann1991; Turelli et al., Reference Turelli, Hoffmann and McKechnie1992), a Japanese population of Laodelphax striatellus (Hoshizaki & Shimada, Reference Hoshizaki and Shimada1995) and a Japanese population of Eurema hecabe (Hiroki et al., Reference Hiroki, Ishii and Kato2005). In a similar way, the strong cytoplasmic incompatibility, high vertical transmission rate and possible beneficial effects on their hosts revealed in the present study may explain the extremely high frequencies of Wolbachia infection in Japanese populations of C. erate poliographus.
Acknowledgements
We thank D. Kageyama for helpful comments and suggestions regarding this manuscript. S.N. was supported by a Japan Society for the Promotion of Science (JSPS) fellowship for Young Scientists.









