Introduction
Parasitoids have been widely studied as model organisms to test ecological and evolutionary theory (e.g. Godfray, Reference Godfray1994; Godfray & Shimada, Reference Godfray and Shimada1999; Wajnberg et al., Reference Wajnberg, Bernstein and van Alphen2008). Host-parasitoid interactions tend to be extremely strong because the host represents the only resource available for parasitoid development (Godfray, Reference Godfray1994). Host quality due to different host species, host stage and size at parasitisation determines most of the overall fitness of parasitoid progeny (Godfray, Reference Godfray1994; Brodeur & Boivin, Reference Brodeur and Boivin2004).
Host quality is also influenced by previous parasitisation events. Superparasitism results from multiple parasitisation of a host by the same parasitoid species (van Alphen & Visser, Reference van Alphen and Visser1990; Godfray, Reference Godfray1994). Competition for resources occurs within hosts following superparasitism. In solitary parasitoids, only one offspring survives to adulthood; superparasitism, therefore, implies the death of all except one offspring (Godfray, Reference Godfray1994). In gregarious parasitoids, superparasitism implies a reduction in the resources available per parasitoid (van Alphen & Visser, Reference van Alphen and Visser1990; Dorn & Beckage, Reference Dorn and Beckage2007).
The effect of superparasitism on parasitoid fitness is somewhat idiosyncratic. In many cases, progeny from superparasitised hosts are smaller and/or weigh less (van Alphen & Visser, Reference van Alphen and Visser1990; Potting et al., Reference Potting, Shellen and Vet1997; Harvey et al., Reference Harvey, Vet, Jiang and Gols1998; Elzinga et al., Reference Elzinga, Harvey and Biere2003; Gu et al., Reference Gu, Wang and Dorn2003; Santolamazza-Carbone & Rivera, Reference Santolamazza-Carbone and Rivera2003; Keasar et al., Reference Keasar, Segoli, Barak, Steinberg, Giron, Strand, Bouskila and Harari2006). Superparasitism has also been shown to both shorten (Potting et al., Reference Potting, Shellen and Vet1997) and lengthen (Gu et al., Reference Gu, Wang and Dorn2003) development time. Superparasitism can also influence the proportion of successful offspring production (White & Andow, Reference White and Andow2008).
Since there are disadvantages to superparasitism, it would be advantageous for females to be able to detect previous parasitisation. There is strong evidence that hymenopteran parasitoids can distinguish hosts that have been already parasitised (Speirs et al., Reference Speirs, Sherratt and Hubbard1991; Godfray, Reference Godfray1994; Darrouzet et al., Reference Darrouzet, Boivin and Chevrier2008; Tena et al., Reference Tena, Kapranas, Garcia-Mari and Luck2008). Most studies addressing host-parasitoid interactions have been carried out on Hymenoptera (Potting et al., Reference Potting, Shellen and Vet1997; Harvey et al., Reference Harvey, Vet, Jiang and Gols1998; Cloutier et al., Reference Cloutier, Duperon, Tertuliano and McNeil2000; Fidgen et al., Reference Fidgen, Eveleigh and Quiring2000; Mackauer & Chau, Reference Mackauer and Chau2001; Elzinga et al., Reference Elzinga, Harvey and Biere2003; Gu et al., Reference Gu, Wang and Dorn2003; Santolamazza-Carbone & Rivera, Reference Santolamazza-Carbone and Rivera2003; Harvey et al., Reference Harvey, Bezemer, Elzinga and Strand2004; Keasar et al., Reference Keasar, Segoli, Barak, Steinberg, Giron, Strand, Bouskila and Harari2006; Darrouzet et al., Reference Darrouzet, Boivin and Chevrier2008; Tena et al., Reference Tena, Kapranas, Garcia-Mari and Luck2008; White & Andow, Reference White and Andow2008) rather than Diptera (reviewed by Feener & Brown, Reference Feener and Brown1997). All members of the dipteran family, the Tachinidae, are parasitoids and display various parasitisation strategies (Belshaw, Reference Belshaw, Hawkins and Sheehan1994). Polyphagy (parasitizing more than one species) is more frequent (Belshaw, Reference Belshaw, Hawkins and Sheehan1994; Feener & Brown, Reference Feener and Brown1997; Stireman & Singer, Reference Stireman and Singer2003), and host discrimination is less highly evolved in tachinids than in hymenopteran parasitoids. For example, no evidence for discrimination of already parasitised host has been found in tachinids (Belshaw, Reference Belshaw, Hawkins and Sheehan1994).
This study examines the effects of host attributes and superparasitism on the success of a tachinid parasitoid, Compsilura concinnata Meigen. The aim was to assess host discrimination and host quality as a function of host age and superparasitism by C. concinnata attacking Trichoplusia ni Hübner (Lepidoptera: Noctuidae). We hypothesized that superparasitism would reduce fly fitness-related traits, due to intra host competition between larvae.
Material and methods
Study organisms
Trichoplusia ni
Trichoplusia ni, commonly known as the cabbage looper, is native to North America. It is highly polyphagous and is an important economic pest of field and greenhouse crops (Sutherland & Greene, Reference Sutherland, Greene, Lingren and Green1984; Janmaat & Myers, Reference Janmaat and Myers2003). Several generations occur during summer. Females can lay more than 1000 eggs (Mitchell & Chalfant, Reference Mitchell, Chalfant, Lingren and Green1984). Larvae go through five to seven instars depending on conditions, with overwintering occurring at the pupal stage (Shorey et al., Reference Shorey, Andres and Hale1962).
Compsilura concinnata
Compsilura concinnata
Meigen (Diptera: Tachinidae) was introduced to North America from Europe between 1906 and 1986 to control 13 different lepidopteran host species, especially the gypsy moth (Lymantria dispar) (reviewed by Boettner et al., Reference Boettner, Elkinton and Boettner2000). The fly is widely distributed in eastern North America but also occurs on the west coast, where L. dispar is rarely present and is subject to strict control using microbial pesticides. Compsilura concinnata is a highly polyphagous parasitoid and has over 160 different hosts in North America (Arnaud, Reference Arnaud1978; Strazanac et al., Reference Strazanac, Plaugher, Pertrice and Butler2001), mostly Lepidoptera. Compsilura concinnata larviposits directly into the larval stage of the host with a sickle-shaped larvipositor formed by the seventh segment of its abdomen. The larva remains inside the host, attached to the tracheoles between the midgut wall and the peritrophic membrane where it remains until the prepupation stage of the host, when rapid larval development begins. The fly larva exits during prepupation and pupation of the host and forms a puparium (Culver, Reference Culver1919; Ichiki & Shima, Reference Ichiki and Shima2003). Under laboratory conditions, C. concinnata readily superparasitised T. ni larvae (Caron et al., Reference Caron, Janmaat, Ericsson and Myers2008a), while, under field conditions, superparasitism was uncommon when population sizes of T. ni were high (Caron, Reference Caron2005). Superparasitism by C. concinnata was found to be common in field populations of other hosts (Eichhorn, Reference Eichhorn1996; Kellogg et al., Reference Kellogg, Fink and Brower2003).
Insect rearing
Laboratory populations were established to investigate the interaction between T. ni and C. concinnata. The T. ni colony had been reared for more than 15 years under laboratory conditions, while the colony of C. concinnata originated from parasitoids emerging from T. ni larvae collected in organic broccoli fields in Delta, British Columbia. Rearing was achieved following protocols described by Caron et al. (Reference Caron, Janmaat, Ericsson and Myers2008a, Reference Caron, Myers and Gillespieb). Briefly, Trichoplusia ni were reared in groups of 15 larvae on wheat germ-based artificial diet (Ignoffo, Reference Ignoffo1963) at a temperature of 25°C and light:dark photoperiod of 16:8 h. Adults were kept in mesh wire cages and paper towels were used to provide oviposition sites. Adults C. concinnata were kept in a 0.25 m3 cage at room temperature (20–25°C) and fed 10% sugar solution in groups of no more than 50 individuals. To maintain colony numbers, 15 third or fourth instar T. ni were put in the fly cage. After four hours, T. ni larvae were retrieved and held at 25°C until the emergence of the parasitoid.
Impact of host instar on parasitisation rate
To assess the effect of the host larval stage on host acceptance by adult parasitoids, second, third and fourth instar T. ni from the laboratory populations were exposed separately to flies from the rearing cages. Groups of 15 larvae were reared in 175 ml Styrofoam cups, which were placed one cup at a time in the fly cage at the required instar and retrieved after four hours. Each larva represented one experimental unit (i.e. larvae in cups were at the same stage, size and are unlikely to interact with each other). Only one instar was presented during a 24-h period, with the instar chosen randomly. Trichoplusia ni larvae were kept in the cup until prepupation, when they were moved singly to 20-ml plastic cups. Cups were checked every day for parasitoid emergence. Fly puparia were put singly in Petri dishes until fly emergence. The proportion of hosts parasitised was calculated for each host instar.
Effects of host instar and superparasitism on fly fitness
To assess the effect of host instar and superparasitism on the fitness of parasitoid offspring, as in the previous experiment, T. ni of three different ages were presented separately to groups of flies for a period of four hours. For this experiment, the second instar was not tested as only one host was found to be parasitised at that instar in the first experiment. Instead, we chose third instar larvae that were close to moulting referred to as instar 3.5. Larvae were kept in groups of 15 until host prepupation, when they were moved singly to cups until parasitoid emergence. The level of superparasitism was determined by the number of fly puparia and could only be assessed at parasitoid emergence. Since larvae were exposed to several flies for a substantial amount of time, superparasitism could be the product of one or more females. The date when a fly puparium was found, puparium weight, fly emergence and gender were recorded.
Statistical analyses
All analyses were performed in JMP IN 4.0.3 (SAS Institute Inc., 2000). To assess the proportion of T. ni that were parasitised and superparasitised, as well as the relationship between superparasitism and sex of offspring, Chi-Square analyses were used. To assess the effect of the host instar at parasitisation and superparasitism on parasitoid fitness, host instar, number of puparia per host and fly gender were used as factors in a three-way ANOVA comparing parasitoid larval development time and parasitoid puparium weight separately. Superparasitism greater than four flies per host was excluded from analyses due to small sample size. The correlation between puparium weight and parasitoid mortality was assessed separately using a logistic regression followed by a Wald test.
Results
Parasitisation and superparasitism rates
Trichoplusia ni larval stage had a significant effect on the rate of parasitisation by C. concinnata (df=2, χ2=46.65, P<0.001). Compsilura concinnata can parasitise all three instars tested. However, for the three instar levels tested, older instars were strongly favoured (fig. 1). Only one second instar individual was parasitised, whereas 46.7% of fourth instar individuals were parasitised over four hours. Observations suggested that they could possibly parasitize the last instar of the host, but host aggression may reduce the success rate of these encounters. Female parasitoids suffered substantial damage from fifth instar T. ni, which fiercely bit the attacker (Caron, personal observation).

Fig. 1. Effect of host instar on parasitisation level of second, third and fourth instar of T. ni. The percentage of exposed hosts parasitised (black) and superparasitised (white) are shown with sample sizes above each bar.
The maximum number of flies found per host was eight. The number of superparasitised hosts varied significantly with host developmental stage. The fourth instar was more heavily superparasitised than the other stages (df=2, χ2=17.34, P<0.001) (fig. 1). The stage of the T. ni hosts at parasitoid emergence was significantly influenced by superparasitism (df=3, Pearson χ2=93.955, P<0.001), with a higher proportion of superparasitised hosts being at the prepupal stage when the fly larvae emerged (fig. 2). Superparasitism did not influence the proportion of puparia that produced adult flies (N=841, df=3, Pearson χ2=1.831, P=0.608).

Fig. 2. Trichoplusia ni development stage (pupa: grey; prepupa: black) at emergence of parasitoid larvae as a function of superparasitism.
Effects of host instar and superparasitism on fly fitness
Compsilura concinnata sex ratios were influenced by superparasitism. More females emerged from singly parasitised hosts (female:male=1.26) and more males from superparasitised hosts (female:male=0.84) (N=770, df=1, χ2=8.052, P=0.005). Development time of C. concinnata larvae was strongly affected by host larval stage at parasitisation; the mean fly larval development times within hosts parasitised at instar 3.5 and instar 4 were significantly shorter than those parasitised at the third instar, suggesting that longer exposure to the host results in an accumulating cost in terms of development time (tables 1 and 2). Fly larval development time was also influenced by superparasitism (table 2). Development time was shorter when two or more parasitoid larvae were present in one host (fig. 3). Fly gender had no effect on larval development time either singly or via interactions with other factors (table 2).

Fig. 3. Larval development time of C. concinnata as a function of T. ni instar at parasitisation and the number of flies found per host.
Table 1. Larval development time and puparium weight (±standard errors) as a function of T. ni instar at parasitisation.
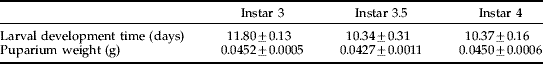
Table 2. Results of analysis of variance from the effects of host instar at parasitisation, superparasitism and parasitoid offspring gender on larval development time and puparium weight.
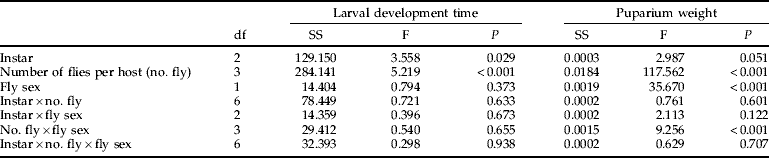
Compsilura concinnata puparium weight was greater for females (table 2 and fig. 4). Although instar of Trichoplusia ni at parasitisation had no statistically significant effect on puparium weight (P=0.051), there was a trend towards lower puparium weight of flies from instar 3.5. Superparasitism reduced puparium weight (fig. 4). There was an interaction between superparasitism and fly gender, with females losing more weight than males when superparasitised (table 2 and fig. 4). Mortality at the pupal stage was higher for C. concinnata with lower puparium weight (N=904, df=1, Wald χ2=35.44, P<0.001).

Fig. 4. Puparium weight of C. concinnata as a function of gender (female: grey; male: white) and the number of flies found per host.
Discussion
The relationship between host quality and fitness in C. concinnata illustrates some of the potential costs of a lack of host discrimination. For parasitoid larvae, the host is the only resource available; consequently, host quality is critical (Godfray, Reference Godfray1994; Brodeur & Boivin, Reference Brodeur and Boivin2004). Koinobiont parasitoids do not kill the host at parasitisation, but rather grow with the host, and thus the initial host size is not as important (Godfray, Reference Godfray1994). This study shows that T. ni stage at parasitisation has no significant effect on the parasitoid puparium weight, indicating that a younger or smaller host at parasitisation has similar resource potential as an older or bigger host. However, there may be evidence of a weak trend towards reduced puparium weight from flies emerging from T. ni parasitised at the intermediate instar. Some aspect of the transition from third to fourth instar could either reduce resources or impair the ability of the parasitoid to capture those resources.
Despite the non-significant effect of host instar on parasitoid pupal weight, larval development time of the parasitoid was shorter in later host instars. This result is consistent with development times for C. concinnata in Lymantria dispar (Weseloh, Reference Weseloh1982, Reference Weseloh1984) and Bombyx mori (Ichiki & Shima, Reference Ichiki and Shima2003). Compsilura concinnata larvae grow very slowly at first by remaining between the host peritrophic membrane and midgut until the host reaches prepupation, and then they develop rapidly (Culver, Reference Culver1919; Ichiki & Shima, Reference Ichiki and Shima2003; Caron et al., Reference Caron, Janmaat, Ericsson and Myers2008a). The larval stage of the host represents a lag time in parasitoid development. By parasitizing an older instar, less time is required until the host reaches prepupation; and, therefore, parasitoid larvae have a shorter development time. Considering this, one would predict that it might be better for a fly to parasitize an older instar since the amount of time that the parasitoid larva is exposed to predation risk via consumption of its host is reduced. The observation that flies attacking the oldest (fifth) instar are frequently injured suggests that there should be a trade-off between host defence and host quality in this system. Similar results were found with an aphid parasitoid, Aphelinus asychis; oviposition is strongly influenced by instar-specific host defence strategies (Gerling et al., Reference Gerling, Roitberg and Mackauer1990).
Superparasitism was frequent in T. ni, consistent with other studies on T. ni (Caron et al., Reference Caron, Janmaat, Ericsson and Myers2008a) and other hosts of C. concinnata (Eichhorn, Reference Eichhorn1996; Kellogg et al., Reference Kellogg, Fink and Brower2003). In this study, superparasitism was more frequent in fourth instar hosts. Theory predicts that the number of fly larvae per host should be a function of the fitness return per number of flies (van Alphen & Visser, Reference van Alphen and Visser1990; Dorn & Beckage, Reference Dorn and Beckage2007). Therefore, if larger hosts provide more resources and can sustain a higher number of parasitoid larvae, this may explain the higher superparasitism found in older hosts. In highly superparasitised T. ni, puparium weight was lower, indicating potentially lower fecundity in parasitoid adults emerging from those puparia, puparium weight being correlated to fecundity (Bourchier, Reference Bourchier1991). On the other hand, larval development time was faster in heavily superparasitised hosts. In superparasitised hosts, it is likely that resource availability is lower per individual, which may result in larvae being resource limited and forced to mature faster. This would reduce both the puparium weight and larval development time. This is supported by the host stage at emergence of the parasitoid; a higher proportion of parasitoids emerged from prepupae than pupae under higher superparasitism. The cost of lower puparium weight, however, could be compensated for by the faster development time under superparasitism, reducing chances of predation.
The sex ratio of C. concinnata offspring was altered in superparasitised hosts. Female parasitoids more commonly emerged from singly-parasitised hosts. Females are usually heavier than males and, therefore, would require more resources to develop, meaning that females could potentially be outcompeted by males inside the host. Alternatively, females might preferably larviposit males in hosts already parasitised. Santolamazza-Carbone & Rivera (Reference Santolamazza-Carbone and Rivera2003) showed that superparasitism of the snout beetle (Gonipterus scutellatus) by a parasitoid wasp, Anaphes nitens, had more impact on females than males: sex ratio was skewed towards females at low rates of parasitisation, and more males were produced from superparasitised host. Similarly, the wasp Eupelmus vuilleti was shown to produce different offspring sex ratio depending on the host parasitisation status (Darrouzet et al., Reference Darrouzet, Boivin and Chevrier2008). Therefore, there is the potential for superparasitism to skew sex ratios; fitness losses from production of male offspring should not be sufficient to select for flies that avoid superparasitism.
In some instance, the fitness costs due to superparasitism could be outweighed by other positive effects. For example, in gregarious parasitoids, higher superparasitism may overcome the host immune system (van Alphen & Visser, Reference van Alphen and Visser1990). However, due to the life history of C. concinnata, which avoids the host immune system by hiding in specific tissue, this seems unlikely (Caron et al., Reference Caron, Janmaat, Ericsson and Myers2008a). Since fitness seems to be reduced by high rates of superparasitism, it should be an advantage for females to detect if the host is already parasitised (Brodeur & Boivin, Reference Brodeur and Boivin2004). Superparasitism is very common in dipteran parasitoids and can reach high levels, suggesting that they cannot discriminate if the host is already parasitised (Feener & Brown, Reference Feener and Brown1997). Superparasitism in gregarious parasitoids is disadvantageous for parasites arriving later due to competition (Godfray, Reference Godfray1994; Feener & Brown, Reference Feener and Brown1997). In the case of C. concinnata, intra-host competition may not provide selection pressure for host discrimination of parasitised hosts because larval development is delayed until the host reaches the prepupal stage. Therefore, parasitoid larvae being laid later in time are not disadvantaged and have the same potential to consume the available resource as the first-laid parasitoid.
If host species are aggressive, it is not advantageous for female parasitoids to spend time assessing host quality (Feener & Brown, Reference Feener and Brown1997). This seems to be the case for the host-parasitoid system studied here. Parasitisation encounters by C. concinnata are extremely fast (less than a second (Weseloh, Reference Weseloh1980)), indicating that no physical assessment of the host seems to take place during attack, though C. concinnata is thought to examine the host from a distance before striking (Culver, Reference Culver1919; Weseloh, Reference Weseloh1980). Females did not attack second instars extensively. They were frequently injured attacking fifth instar caterpillars and also avoided attacking this stage. Parasitisation was found to be triggered by movement of the host (Weseloh, Reference Weseloh1980; Caron, personal observation); therefore, some level of host recognition was operating. Although superparasitism and host age at parasitism had fitness impacts on weight, sex ratio or development time, this information does not seem to be used for mid-size instars. It appears likely that females are assessing host quality prior to attack, through host movement and size.
Conclusions
This study has shown that the dynamics of the interaction between T. ni and C. concinnata are influenced by host stage at parasitisation and intra-specific competition within the host. In the laboratory, C. concinnata parasitised older and larger T. ni more frequently. This relationship could have evolved because older instars allow faster development of the offspring, and thus reduce the time parasitoid larvae risk predation. Superparasitism was shown to be frequent under laboratory conditions and reduced the progeny fitness most likely due to competition within the host. Superparasitism influenced the sex ratio of the fly progeny and females may have been out-competed due to their slower development. Host discrimination mechanisms seem poorly developed in C. concinnata. Considering the wide host range of C. concinnata, developing host discrimination mechanism to one host species may not be critical, and avoiding potential superparasitism could naturally occur by not specializing on one host type.
Acknowledgements
We would like to thank Alida F. Janmaat, Veronica Cervantes, Tamara Richardson, Patricia Duffels and Ross M. Thompson. Comments from two anonymous reviewers substantially improved this manuscript. This research was funded by the Canadian NSERC Biocontrol Network and the British Columbian Greenhouse Growers' Association.








