INTRODUCTION
Large mammalian herbivore species coexist by partitioning the key niche dimensions of diet and habitat (Chase & Leibold Reference CHASE and LEIBOLD2003, Schoener Reference SCHOENER1974). In areas where resources vary seasonally, the diet and habitat selection by large herbivores vary both temporally and spatially (Kleynhans et al. Reference KLEYNHANS, JOLLES, BOS and OLFF2011, O'Kane et al. Reference O'KANE, DUFFY, PAGE and MACDONALD2011). In tropical areas with wet–dry cyclic weather patterns, plant quality varies seasonally (Hopkins Reference HOPKINS and Hopkins2000, Prins & Loth Reference PRINS and LOTH1988, Styles & Skinner Reference STYLES and SKINNER1997): the wet season, when plants have low fibre and high nutrient concentrations, is the season with the highest quality forage for herbivores; the dry season, when plants invest more in structural carbohydrates (fibre) and have their highest carbon : nitrogen ratios, is the season with lowest quality forage for herbivores. Therefore, in tropical areas where the year is divided into wet and dry seasons, large herbivores are challenged to satisfy their nutritional needs more in the dry season rather than in the wet season.
The role of body mass has been a cornerstone in understanding resource partitioning by large-herbivore species ever since the Bell–Jarman principle proposed that larger-bodied herbivores are better equipped to satisfy their metabolic requirements on low-quality forage than are smaller-bodied herbivores (Bell Reference BELL1971, Jarman Reference JARMAN1974, Yoshihara et al. Reference YOSHIHARA, ITO, LHAGVASUREN and TAKATSUKI2008). Large-bodied species, however, have higher absolute metabolic requirements per unit time, and in order to find resources to satisfy their large absolute metabolic requirements, they range over larger areas when compared with smaller-bodied species (Jetz et al. Reference JETZ, CARBONE, FULFORD and BROWN2004, Lindstedt et al. Reference LINDSTEDT, MILLER and BUSKIRK1986, McNab Reference McNAB1963).
The majority of studies that have investigated large-herbivore community ecology have emerged from Africa, Europe and North America. Asia, the continent with the largest land area and therefore the highest number of large-herbivore species (> 5 kg; Groves & Grubb Reference GROVES and GRUBB2011), has contributed disproportionately little to our understanding of large-herbivore ecology. One reason for this is because individual large-herbivore assemblages in Asia have low species richness that provide low sample sizes and statistical power to validate community ecology theory; the highest species richness of a large herbivore assemblage in Asia is 10 (Ahrestani et al. Reference AHRESTANI, HEITKÖNIG, LANGEVELDE, SRINIVAS, MADHUSUDAN and PRINS2011a), while Africa has assemblages with more than 30 species (Prins & Olff Reference PRINS, OLFF, Newbery, Prins and Brown1998). Also, most of the species-rich large-herbivore assemblages in Asia inhabit forested habitats, which have made it difficult to study these animals in Asia. Therefore, there exists a significant gap, which needs to be filled, in understanding large-herbivore forage and habitat preferences and their community ecology in Asia.
To this end, we studied the diets and the habitat overlap amongst an assemblage of four large-herbivore species – 60-kg chital Axis axis Erxleben, 200-kg sambar Cervus unicolor Kerr, 700-kg gaur Bos gaurus Smith and the 3100-kg Asian elephant Elephas maximus Linnaeus – in a South Indian seasonal tropical forest. We focused on understanding what impact the seasonal variation in forage quality would have on the browse : graze ratio of these species’ diets and the overlap of their habitat-niche breadths. Based on knowledge from African assemblages, we predicted that, (1) chital, being the smallest ruminant, and therefore the most dependent on high-quality forage, would increase the proportion of browse in its diet during the dry season when grass quality is at its lowest, (2) sambar, suspected to be a browser (Johnsingh Reference JOHNSINGH1991, Prater Reference PRATER1993, Schaller Reference SCHALLER1967), would retain a browse diet throughout the year, (3) gaur, similar to other large Bovini species, would primarily be a grazer, and (4) the elephant, because of its large metabolic requirements is known to be a non-selective bulk feeder that ranges over large areas (Sukumar Reference SUKUMAR1990, Sukumar & Ramesh Reference SUKUMAR and RAMESH1992), would have the highest habitat-niche breadth, which would overlap to a high degree with that of the other species.
MATERIALS AND METHODS
Study area
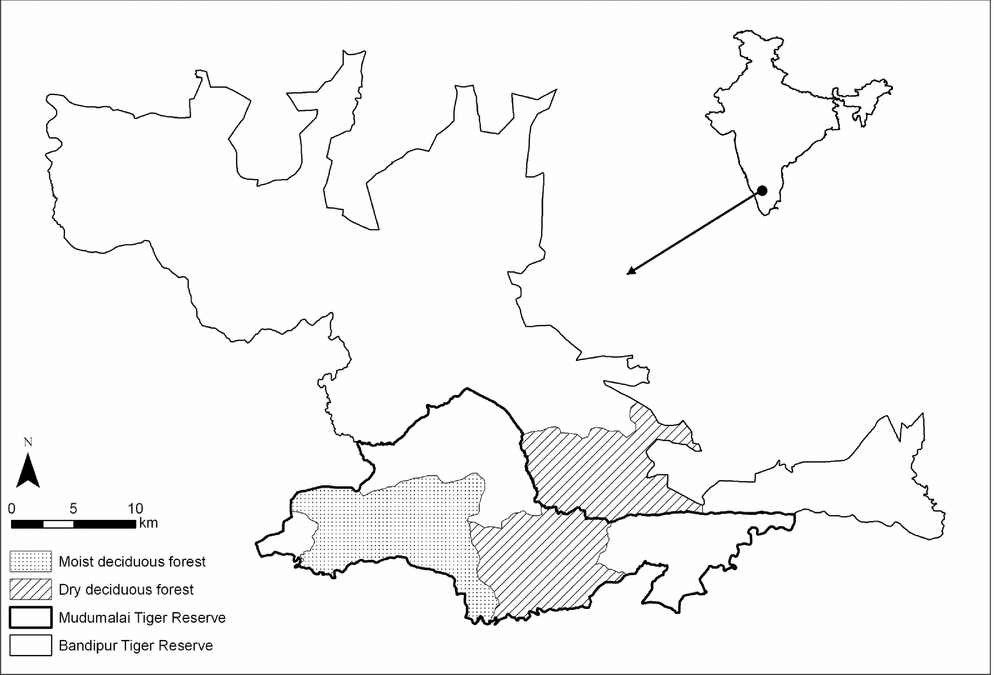
The study area (400 km2; 11° 32′–41′N, 76° 22′–41′ E) was divided between the contiguous Mudumalai and Bandipur Tiger Reserves (Figure 1), which are located on the lower elevations of the Western Ghats, South India, and have undulating (250–400 m) terrain. Rainfall in the area is monsoon driven (annual average = 1050 mm) and there are three primary seasons: the South-West Monsoon wet season (May–July: 60% of rainfall), the North-East Monsoon wet season (August–November: 40% of rainfall), and the dry season (December–April). The study area has an east–west moisture gradient with the east being dominated by dry deciduous (the extreme east is thorn-scrub forests) and the west by moist deciduous forests (the extreme west is semi-evergreen). A conspicuous feature of the study area, particularly in the dry deciduous region, was that the understorey had become dominated by non-native invasive species – primarily Lantana camara and Chromolaena odorata, and to a lesser extent Parthenium hysterophorus – that are not preferred as forage by large-herbivore species.

Figure 1. Map of the study area (400 km2; 11° 32′–41′N, 76° 22′–41′ E) divided into moist deciduous forest and dry deciduous forest regions. The study area was spread over the contiguous Bandipur and Mudumalai Tiger Reserves, South India.
Data collection and analyses
To measure the seasonal variation of the forage quality, the aboveground graminoid layer was sampled seasonally (late wet, early dry, late dry, early wet, middle wet) between October 2006 and July 2007 in the dry deciduous region. On each sampling occasion an average of 15 randomly selected 0.5 × 0.5-m quadrats were clipped from different areas where the study species were observed grazing. The clipped graminoid samples were separated into green leaf, dry leaf, green stem and dry stem. The separated samples were dried in the sun until constant dry mass and then weighed using an electronic balance, providing measurements of the seasonal variation in leaf and stem components of graminoid production. Finally, arbitrarily selecting three to five green-leaf samples from each season's sampling, we measured the seasonal nitrogen concentration of green leaves using a dry-combustion automatic nitrogen analyser at the National Institute of Animal Nutrition and Physiology, Bengaluru, India. To test the hypotheses on forage quality with respect to large herbivores, we report green leaf quality in terms of crude protein concentration (i.e. nitrogen concentration × 6.25) in the results.
As it was difficult to observe species foraging, we collected and analysed faeces to determine the ratio of grass to browse in the diet of the species. Faecal samples (n = 5–13) from distinct and fresh faecal piles were collected opportunistically from the dry deciduous region every month between May 2006 and July 2007, and we pooled these to make monthly composite samples. We determined the ratio of the browse to grass in the species diets by carbon isotope analysis of subsamples of the monthly composite faecal samples (Cerling & Harris Reference CERLING and HARRIS1999, Codron et al. Reference CODRON, CODRON, LEE-THORP, SPONHEIMER, DE RUITER, SEALY, GRANT and FOURIE2007, Sponheimer et al. Reference SPONHEIMER, LEE-THORP, DERUITER, SMITH, VAN DER MERWE, REED, GRANT, AYLIFFE, ROBINSON, HEIDELBERGER and MARCUS2003). Since C3 and C4 plants fractionate against the stable heavy carbon isotope 13C differently, the measure of δ13C in the faeces is therefore determined by the ratio of C3 to C4 plants consumed by the herbivore (Tieszen et al. Reference TIESZEN, HEIN, QVORTRUP, TROUGHTON and IMBAMBA1979, Vogel Reference VOGEL1978). Although we did not conduct carbon isotope analysis of the vegetation, we are confident that the carbon isotopic measurements reflected the ratio of grasses (C4) to browse (C3) in the herbivore diets because: (1) all the dominant grass species (Bothriochloa pertusa, Heteropogon contortus, Eragrostis atropurpurea, Digitaria sp., Sporobolus indicus and Themeda tremula) found in a field-based experimental study (Ahrestani et al. Reference AHRESTANI, HEITKÖNIG and PRINS2011b) conducted on grassland contiguous with the boundary of this study area at the same time of this study were dominant in this study area and were all C4; (2) all additional dominant grass species in the study area, namely Cynodon dactylon, Chrysopogon zizanioides, Apluda mutica, Themeda triandra, Setaria pumila and Echinochloa colona, are also all C4 species; and (3) the tree species in the study area (Saldanha & Nicholson Reference SALDANHA and NICHOLSON1976, Sharma et al. Reference SHARMA, SHETTY, VIVEKANANDAN and RATHAKRISHNAN1978) were all C3 species. As we were analysing composite samples we chose to analyse only two subsamples from each composite to check for laboratory errors; we report their mean since measures of variance have little meaning with this procedure. The spectrometry analyses of the faeces were done at the Isotope Laboratory, Agricultural University, Bengaluru, India.
To measure the species habitat-niche breadth and overlap the study area was surveyed on a grid basis twice during the study period, once during the dry season (March 2007) and once during the wet season (July 2007). The study area was divided into 250 1 × 1-km square grid cells. Every alternate grid cell (n = 123) was sampled using a transect 0.5 km long × 2 m wide that ran diagonally across and centred at the midpoint of each sampled grid cell. The principal investigator and two assistants walked all the transects (n = 123) and counted the number of faecal piles of the four species along the entire length of each transect. It is nearly impossible to confuse elephant faecal piles with those of any other species in the study area, and although gaur faecal piles can potentially be confused with those of cattle, cattle were found in < 1% of the sampled grid cells. Chital and sambar faecal pellets are similarly shaped, but sambar pellets are significantly bigger; only piles that could be accurately identified as that of chital or sambar were counted. During the transect sampling we also measured habitat parameters (Table 1) within each sampled grid cell using five circular sampling plots (each 5 m in radius) that were equidistantly spaced at 100-m intervals along each transect. We quantified habitat parameters in each sampled grid cell by the arithmetic mean of the measurements from the five circular habitat-sampling plots within each cell.
Table 1. Comparison of rainfall and habitat variables found in the two major habitat types in study area. Habitat variables were measured in 5-m-radius circular plots along strip sampling transects within grid cells of the study area. The cover of Lantana camara, Chromolaena odorata, Tall grass and short grass were estimated using a scale of 0–4: 0 = absent; 1 = 1–25%; 2 = 26–50%; 3 = 51–75%; 4 = 76–100%.

Using the faecal pile counts from the grid-based survey, we quantified the habitat-niche breadth (BA; Hulbert Reference HULBERT1978, Levins Reference LEVINS1968) of the four species,
Pj = proportion of individuals (based on faecal pile counts) found in a grid cell j; n = total number of available grid cells. This estimate of habitat-niche breadth, while not disclosing much about the constituents of the niche, allows us to compare the relative use of the available habitat by the different species. In addition, we also calculated the habitat-niche overlap between pairs of species in both seasons using a symmetrical measure of niche overlap Ojk (Pianka Reference PIANKA1973)
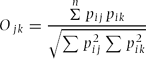
Pij = frequency of utilization (based on faecal pile counts) of grid cell i by species j and Pik = frequency of utilization (based on faecal pile counts) of grid cell i by species k.
The number of faecal piles found during sampling may have been impacted by the environmental heterogeneity in the habitats, the variation in the defecation and mobility rates of the herbivores, and the decay rates of the faecal piles (Putman Reference PUTMAN1984). Since there is near complete lack of data on key variables like defecation and decay rates of all species, we assumed that the variation in the different factors would have affected the counts of each species equally and therefore would not undermine the usefulness of faecal counts. This was a reasonable assumption to make also because we used the counts only for interspecific comparisons and not to accurately count species’ populations (using faecal counts to determine species’ populations is fraught with issues; Putman Reference PUTMAN1984).
We analysed the grid-based data using linear models to determine which habitat variables helped explain the seasonal presence of the species. Since the faecal count (the response variable) data for all species were zero-inflated, i.e. there were many zero faecal counts, determining the distribution models that best fit the data was difficult, i.e. tests of the Poisson, Gaussian and Weibull distributions failed. However, we found that residuals from a linear regression of the square root of faecal count were approximately normal (based on qq plots), and therefore we analysed these data using an ordinary least squares linear multiple regression model of the square root of faecal counts in relation to the habitat variables. As an additional test, we also fitted a Poisson multiple regression model modified to take into account zero-inflated data (the ‘zeroinfl’ model in the R package ‘pscl’), which provided similar, but more conservative results (i.e. the significance of relevant explanatory variables were of lower magnitudes). These analyses were done in the R statistical programming environment (R Development Core Team, Vienna, Austria).
RESULTS
Forage quality of the dry deciduous area
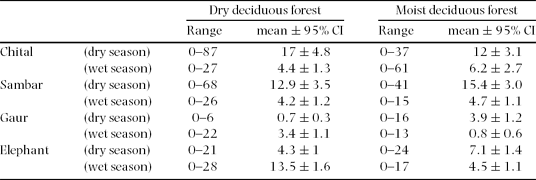
Analysis of the graminoid quality showed that the crude protein concentration of green grass leaves varied 6.5–18.2% (1–2.8% nitrogen), was highest in the wet season, decreased as the wet season progressed, and was lowest in the dry season (Figure 2a). Measurements of the separated dry and green components of the leaves and stems showed that the leaf component (50–100%), particularly that of the green leaf component (0–100%), in standing graminoid biomass was high in the wet season and low in the dry season (Figure 2b), similar to the seasonal variation of green leaf protein concentration.

Figure 2. Mean (± 95% CI) monthly measurements (October 2006–July 2007) of the leaf crude protein concentration (a) and the leaf % and its green component (b) of the standing biomass of herbaceous layer found in the dry deciduous habitat in Bandipur Tiger Reserve, South India.
Ratio of browse to grass in diet
The low δ13C values found for sambar indicate that the sambar consumed the greatest proportion of browse among all the species throughout the year. Except for the low δ13C value of −25.4‰ in June, the remaining elevated δ13C values for gaur indicated that the gaur consumed the highest proportion of grasses among the four species during the year (Figure 3). Consumption of grasses by chital was the highest in the early wet season, decreased in the dry season, and then increased in the following wet season (Figure 3). The proportion of grasses in elephant diet was relatively high over the entire year, but a little less than the proportion of grasses in gaur diet (Figure 3).

Figure 3. δ13C found in faeces of four large-herbivore species in Bandipur and Mudumalai Tiger Reserves, South India (May 2006–July 2007). A low value of δ13C suggests a browse-based diet, and a high value of δ13C suggests a graze-based diet.
Habitat comparison
The moist deciduous region received 800 mm more rainfall than the dry deciduous region during the study period (Table 1), while the moist deciduous region had perennial flowing streams during the dry season and the dry deciduous region did not (pers. obs.). Although the invasive shrub Chromolaena odorata was found equally in the two habitats, the invasive shrub Lantana camara was three times more abundant in the dry than the moist deciduous region (Table 1). The ‘tall grass’ component was taller and more abundant in the moist than the dry deciduous region, but the ‘short grass’ height and availability were similar in the two regions (Table 1). True to the structural nature of their respective tree communities, we found a greater number of larger trees and a lesser number of smaller trees in the moist deciduous forests when compared with the dry deciduous forests.
The results of the multiple regression analysis (Table 2) revealed that collectively the habitat variables explained species distribution rather weakly, the highest adjusted R2 was 0.32 for chital in the dry season and the lowest adjusted R2 was 0.06 for elephant in the wet season. None of the habitat variables were significant in explaining the use of the study area by elephant (Table 2), a result that supports the relatively large habitat-niche breadth for elephant in the entire study area in both seasons (Table 4). Habitat type was significant only for gaur (Table 2), in both seasons, a result that supports the seasonal differences in the habitat preference by gaur (Table 4). Although the number of large trees was (significantly) negatively correlated to chital in both seasons and sambar in the dry season, the coefficients of these relations, and that of all the other significant relations (Table 2) were too small to permit any meaningful interpretation of these relations.
Table 2. The significant coefficients of the explanatory habitat variables in the ordinary least-squares multiple linear regression models that were used to fit the square root of the species faecal counts recorded during the grid-based survey of the study area. Habitat type = Dry deciduous or Moist deciduous. None of the coefficients of the model variables used to fit elephant data was significant, which is why elephant is not presented in the table.

Habitat-niche breadth and overlap
The faecal counts from the grid-based sampling (Table 3) showed that with respect to the overall study area the habitat-niche breadths of all the species were smaller in the wet season compared with the dry season, and gaur niche breadth was the smallest in both seasons followed by chital, sambar and elephant (Table 4). With respect to the dry deciduous forest, the habitat-niche breadths of the species did not vary much between seasons; gaur and elephant had higher values in the wet season while chital and sambar had higher values in the dry season (Table 4). With respect to the moist deciduous forest, the niche breadth of all species was higher in the dry season than in the wet season: four times higher for gaur and nearly twice as high for chital (Table 4).
Table 3. Comparison of faecal-pile counts of four large-herbivore species found in the two major habitat types in study area.
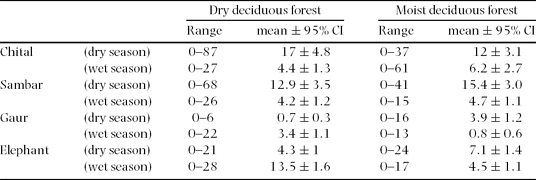
Table 4. Habitat-niche breadth of four large-herbivore species in the dry and wet seasons in Bandipur and Mudumalai Tiger Reserves, South India (2006–2007). The values presented were calculated using a niche-breadth measure (Hulbert Reference HULBERT1978, Levins Reference LEVINS1968). Niche-breadth range = 0–1, higher values indicating larger niche breadth.

The gaur was found in high densities in the moist deciduous region and was absent from the eastern half of the dry deciduous region (the driest region of the study area) in the dry season. In the wet season, however, gaur was found in high densities and nearly exclusively in the dry deciduous region. Elephant, similar to the gaur, was also absent from the eastern region of the dry deciduous forest in the dry season, but was present in this region in the wet season. The habitat-niche breadth overlap of gaur and elephant was high in both seasons, in particular the 94% overlap in the moist deciduous region in the wet season (Table 5). Elephant had a high overlap with both chital and sambar in both seasons (Table 5).
Table 5. The overlap of habitat-niche between different pairs of four large-herbivore species in the dry and wet seasons in Bandipur and Mudumalai Tiger Reserves, South India (2006–2007). Range overlaps between species were calculated using a symmetrical measure of niche overlap (Pianka Reference PIANKA1973), overlap of 1 indicates complete overlap and overlap of 0 indicates no overlap.

The habitat-niche breadth of sambar remained relatively unchanged across the two seasons in both habitats (Table 4). Considering the entire study area, sambar habitat-niche breadth overlapped with that of chital the most, gaur the least, and was relatively high and unchanged with elephant in the two seasons (Table 5). Chital habitat-niche breadth was lower than that of sambar in both seasons (Table 4), and chital was found to avoid the extreme western evergreen region of the study area in both seasons, the only species to do so.
DISCUSSION
Chital increased the proportion of browse in its diet as the wet season changed to the dry, lending support to prediction 1. Chital being the smallest ruminant in the assemblage would have been the most affected by the decreasing forage quality in the dry season (Bell–Jarman principle: Bell Reference BELL1971, Jarman Reference JARMAN1974). Other studies too have found the chital to show seasonal diet preferences; in the Sub-Himalayan terai region, chital increased its consumption of grasses in recently burnt habitats during the late dry/early wet season (Mishra Reference MISHRA1982, Moe & Wegge Reference MOE and WEGGE1994, Reference MOE and WEGGE1997). We lacked data on forage quality from the evergreen region, which prevents us from relating the absence of chital from the evergreen region (extreme western region) to the theoretical understanding that small-bodied herbivores would avoid habitats with high moisture, and therefore lower-quality forage (Olff et al. Reference OLFF, RITCHIE and PRINS2002, Prins & Olff Reference PRINS, OLFF, Newbery, Prins and Brown1998). However, the absence of chital from the evergreen region, where browse quality would have been low (Scogings et al. Reference SCOGINGS, DZIBA and GORDON2004), was consistent with the fact that in general chital are absent from evergreen habitat over their entire geographic range (Prater Reference PRATER1993).
The sambar retaining a browse habit throughout the year confirmed prediction 2. Sambar and chital having a high spatial overlap for much of the year, but at the same time having different diets, is similar to what Bagchi et al. (Reference BAGCHI, GOYAL and SANKAR2003) found for chital and sambar in Ranthambhore Tiger Reserve, i.e. a high spatial overlap but a differentiation in diet. Sambar habitat-niche breadth was found to be as high as that of elephant and their extensive use of the study area hardly changed between the seasons, suggesting that the sambar is capable of satisfying its metabolic requirements from a variety of habitats (Schaller Reference SCHALLER1967).
Our results showed that grasses made up the bulk of the gaur diet throughout the year, which lent support for prediction 3. This preference to graze by gaur is consistent with the classification of large Bovini species as grazers (Hofmann Reference HOFMANN1989); in general, other Bovini species like the American bison Bison bison (Knapp et al. Reference KNAPP, BLAIR, BRIGGS, COLLINS, HARTNETT, JOHNSON and TOWNE1999) and African buffalo Syncerus caffer (Prins Reference PRINS1996, Sinclair Reference SINCLAIR1977) are also grazers. Although contemporary studies have found the European bison Bison bonasus to be a mixed feeder, this is understood to be a result of European bison being forced to adapt to forested habitat over the last few centuries because of persecution (Krasinska & Krasinski Reference KRASINSKA and KRASINSKI2007). Like the European buffalo, the gaur too survives mainly in hilly-forested areas, having been displaced from the grasslands of the plains, but it is primarily a grazer. This does not mean that the gaur does not browse; Chetri (Reference CHETRI2003), Schaller (Reference SCHALLER1967) and Shukla & Khare (Reference SHUKLA and KHARE1998) found gaur to forage on multiple browse species, but since none of them quantified the browse : graze ratio it was not possible to compare this study's results with theirs.
Probably the most interesting result was finding gaur habitat-niche breadth to be the narrowest among the four species. Although gaur niche breadth with respect to the entire study area did not vary in size between seasons, the gaur clearly selected different habitats in different seasons. Speaking to local forest officials it appears that the near absence of gaur, except for a few individual old males and a herd or two, from the eastern (driest) part of the study area during the dry season is an annual phenomenon. We submit, therefore, that the gaur in the study area follow a local cyclic migration synchronized with the cyclic rainfall pattern – grazing in the dry deciduous region during the wet season and then switching to the moist deciduous region in the dry season.
The results lend support for prediction 4, which was that elephant niche breadth would be the highest among the species and that elephant would have a high overlap with that of the other species in both seasons (Damuth Reference DAMUTH1981). Although the δ13C values for elephant were not as low as that for sambar, the results suggested that the elephant was a mixed-feeder. This was consistent with earlier results from the study area (Sukumar Reference SUKUMAR1990) and another study from Nepal that also found Asian elephant to be a mixed-feeder (Pradhan et al. Reference PRADHAN, WEGGE, MOE and SHRESTHA2008). The African elephant Loxodonta africana is not that different and is often a mixed feeder, increasing its intake of grasses in open habitats and increasing its browse intake in closed-canopy habitats (Beekman & Prins Reference BEEKMAN and PRINS1989, Cerling et al. Reference CERLING, OMONDI and MACHARIA2007, Codron et al. Reference CODRON, CODRON, LEE-THORP, SPONHEIMER, KIRKMAN, DUFFY and SEALY2011).
Although the grid-based survey did not detect many relevant habitat correlates of species presence, it revealed that in areas blanketed by the invasive Lantana camara (like in the dry deciduous tourism zone of Bandipur) all three ruminant species were near completely absent and that elephant used these areas only marginally. Lantana camara-blanketed areas offer few palatable understorey resources besides Lantana camara itself (Prasad Reference PRASAD2010). In general large herbivores avoid Lantana camara – lantadenes, the chemical toxins found in Lantana camara leaves, are hepatotoxic and cause photosensitization, severe digestive problems and ultimately death (Sharma et al. Reference SHARMA, SHARMA, PATTABHI, MAHATO and SHARMA2007) – and therefore the further expansion of this invasive has the potential to restrict resource availability and possibly change the foraging ecology of large herbivores in areas that they invade (Bhatt et al. Reference BHATT, RAWAT and SINGH1994, Murali & Shetty Reference MURALI and SHETTY2001, Raghubanshi et al. Reference RAGHUBANSHI, RAI, GAUR and SINGH2005).
In conclusion, our results shed light on how large herbivore species vary their habitat and diet selection in a seasonal South India region. The gaur was found to be primarily a grazer with a habitat-niche breadth that was the narrowest among the four species. This study provides the first evidence for chital increasing its intake of browse during the dry season when grass quality is annually at its lowest. Sambar occupying multiple habitats confirmed results of other studies, and the evidence of its penchant for browse supports a long-held notion of its diet preference. Not surprisingly the elephant consistently had a high spatial overlap with the other species in both habitats and seasons and was found to be a mixed-feeder. This study's results are important as no other study has investigated interspecific differences of the browse : graze ratio in the diets or the habitat-niche overlap within a South Indian large herbivore assemblage. Also, the study area is located in the Nilgiri Biosphere, one of the largest contiguous protected areas in India with probably the highest large herbivore biomass and population sizes in Asia, which means that these results could be important if the future management of Asia's largest large herbivore assemblage needs to address issues that relate to the diet and habitat use of these species.
ACKNOWLEDGEMENTS
We thank the forest departments of both Tamil Nadu and Karnataka for giving us permission to collect data for this study and helping with logistics during the study. We thank Manba and Murali for their hard work in assisting with data collection – without their help this study would not have been possible. We thank Raman Sukumar and his colleagues for providing data on Mudumalai, Henjo de Knegt and Srinivas Vaidyanathan for their GIS expertise and M. S. Sheshshayee and his lab for conducting the spectrometry analyses. This study was supported by the Foundation for the Advancement of Tropical Research, the Netherlands, grant WB 84–588.