Introduction
The sea urchin embryo cleaves after fertilization, and eventually forms the 64-cell hollow blastula. Shortly thereafter, at about 20–24 h post-fertilization, gastrulation begins by invagination at the vegetal region of the embryo. The experiments described in the current paper were begun at this stage.
Gastrulation in the sea urchin is a model for identifying mechanisms of morphogenesis across the animal phyla and has been designated by the National Institutes of Health as a model to help understand the physiology of human health and disease (Davidson & Cameron, Reference Davidson and Cameron2002; Davidson, Reference Davidson2006). The simplicity and transparency of the embryos have helped to identify specific mechanisms such as cellular migration, differential adhesiveness and contractility that control morphogenetic events (Oppenheimer & Carroll, Reference Oppenheimer and Carroll2004). The molecular basis of archenteron elongation and adhesion to the blastocoel roof is not well understood but it is a fundamental component of the gastrulation process (Herbst, Reference Herbst1900; Ernst, Reference Ernst1997).
The identification of polyglucans has been identified recently as involved in mediation of the adhesive interaction between the tip of the archenteron and the blastocoel roof (Singh et al., Reference Singh, Karabidian, Kandel, Metzenberg, Carroll and Oppenheimer2013). In the present study, the sugar l(–)-rhamnose is identified as a putative component in the control of archenteron elongation and attachment to the blastocoel roof. The aim of the current study was to improve our understanding of the molecular basis of gastrulation, particularly the factors responsible for archenteron elongation and attachment to the blastocoel roof.
Materials and methods
Solutions
Artificial seawater (ASW; 423 mM NaCl, 9 mM KCl, 9.3 mM CaCl2, 22.9 mM MgCl2, 25.5 mM MgSO4, 2.1 mM NaHCO3, pH 8.0), was prepared in distilled water using the Marine Biological Laboratory (Wood Hole, Massachusetts, USA) formula. Low-calcium artificial seawater (LCASW) was prepared by reducing the calcium concentration to 1.5 mM (Bidwell & Spotte, Reference Bidwell and Spotte1985). All reagents were obtained from Sigma-Aldrich Chemical Co., St. Louis, Missouri, USA.
Gametes and fertilization
Gametes were extracted from Lytechinus pictus sea urchins obtained from Marinus, Inc., Garden Grove, California, USA by injecting them with 0.55 M KCl in distilled water as described previously by Razinia et al. (Reference Razinia, Carroll and Oppenheimer2007). Sperm were kept undiluted on a Petri dish on ice and eggs were washed three times with ASW (Razinia et al., Reference Razinia, Carroll and Oppenheimer2007). One ml of freshly diluted sperm (10-fold dilution in ASW) was added to the washed diluted eggs in a small glass casserole dish. Eggs were fertilized and checked for the presence of fertilization membranes to assure that fertilization occurred (Razinia et al., Reference Razinia, Carroll and Oppenheimer2007). Fertilized eggs were washed three times in ASW to remove excess sperm. Embryos were maintained in pH 8.0 ASW in large glass casserole-type dishes and at 15°C.
Carbohydrate treatments
Six carbohydrates were used in the current study: (i) alpha-cyclodextrin (Sigma-Aldrich cat. no. C4642), (ii) melibiose (Sigma-Aldrich cat. no. M5500), (iii) trehalose (Sigma-Aldrich cat. no. T5251), (iv) d(+)xylose (Sigma-Aldrich cat. no. X3877), (v) l(–)-xylose (Sigma-Aldrich cat. no. X1625), and (vi) l(–)-rhamnose (Sigma-Aldrich cat. no. R3875). Each carbohydrate was tested at five different concentrations (0.03 M, 0.015 M, 0.003 M, 0.0015 M or 0.0009 M), prepared in pH 8.0 LCASW, used here because it allows easy access of reagents into the interior of the embryos (Latham et al., Reference Latham, Martinez, Cazares, Hamburger, Tully and Oppenheimer1998; Itza & Mozingo, Reference Itza and Mozingo2005). Embryos in LCASW continued to swim normally up to 48 h and after. The sugars were selected in a comprehensive programme of testing, which to date have shown little activity in the sea urchin system. The sugar concentrations were chosen based on previous unpublished results that identified the range of concentrations that can affect the embryos. The sugars were added to embryos at 24 and 30 h post-fertilization in 96-well microplates in mixtures that totalled 50 μl or 75 μl per well and either contained sugar or were sugar-free controls. The 24 h time point represents the early gastrula and the 30 h time point is the mid gastrula with some archenteron advancement. These two time points allow for assessment of the effects before the archenteron develops and after it has partially elongated. In total, 39,369 embryos were examined at 48 h post-fertilization after fixation by the addition of 10 μl 10% formaldehyde to each well. The 48 h time point represents the late gastrula, in which the archenteron is attached to the blastocoel roof in controls. As archenteron elongation and attachment were being examined, the 48 h time point was chosen at which to stop the experiment. Embryos in each well were counted and classified as possessing one of the following morphologies: (i) completely attached archenteron (CA), (ii) unattached archenteron (UA), (iii) exogastrulation (EXO), (iv) non-viable (NV), or (v) non-invaginated (NINVG).
Statistics
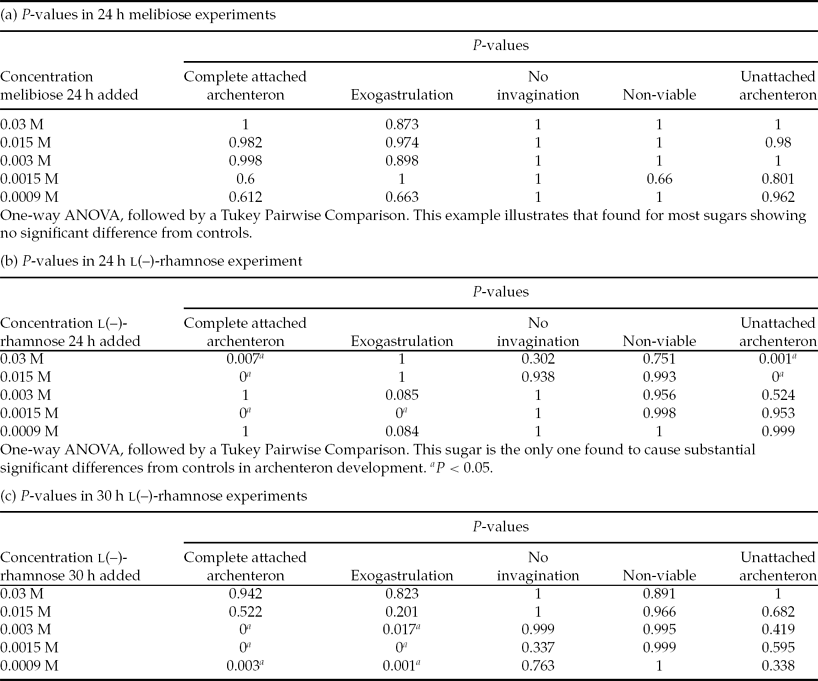
Each morphology for each sugar concentration and controls was tallied and combined to give a total population size. Percentages of the resulting morphologies for each sugar concentration and controls were calculated and grouped with standard error of the mean. The results that compared control and sugar at each sugar concentration for each morphology were analysed using one-way analysis of variance (ANOVA) followed by a Tukey Pairwise comparison test and calculated using SYSTAT 13 software. Significant differences between each morphology in control and experimental populations at each sugar concentration were indicated by a P-value <0.05.
Results
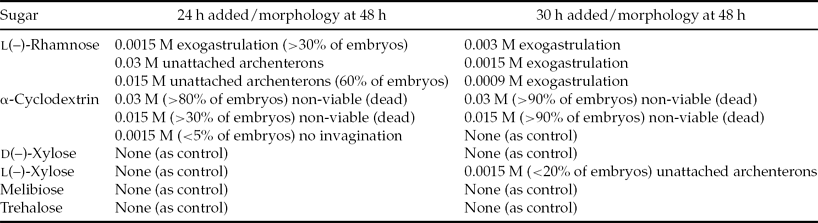
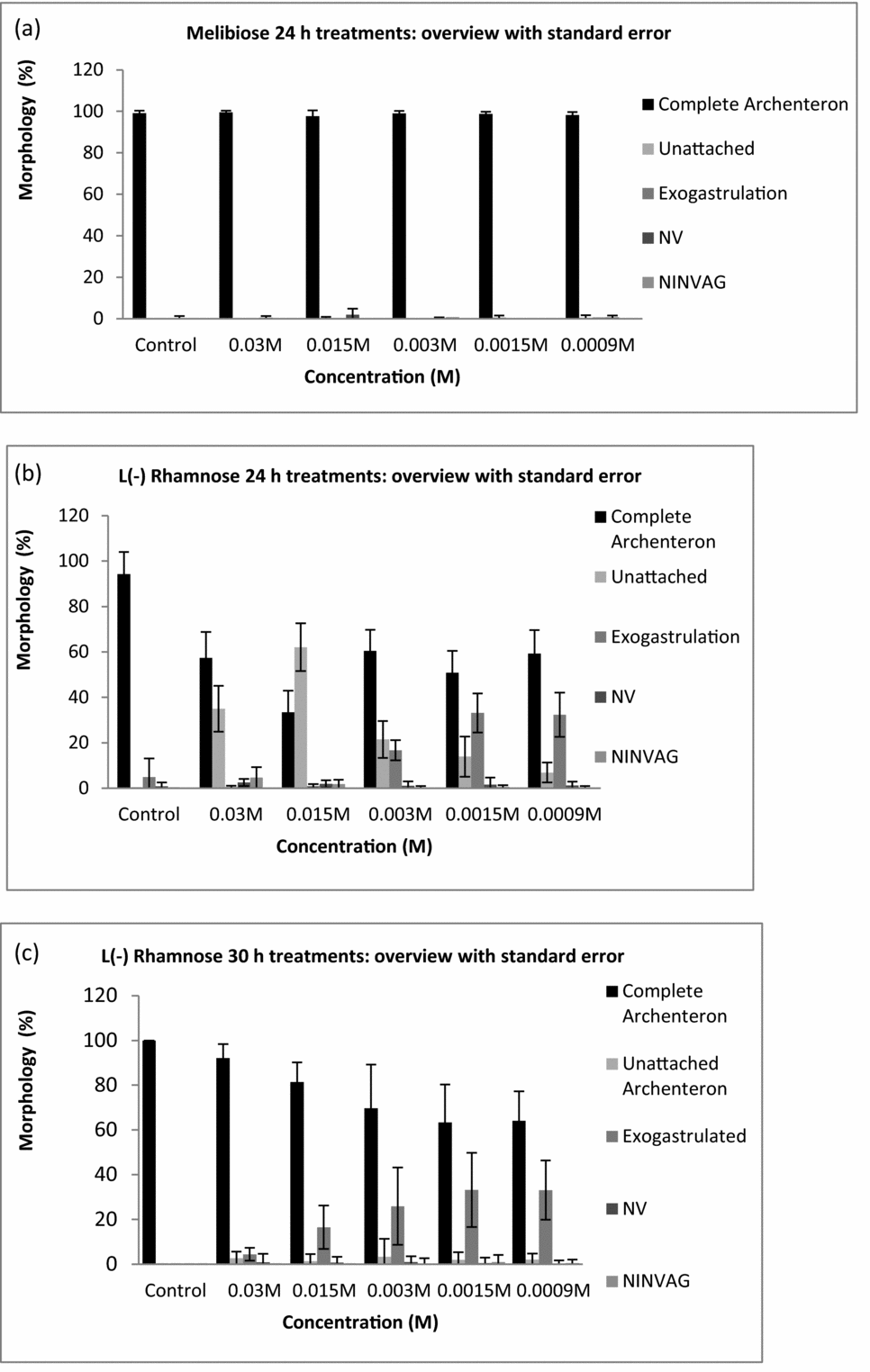
When viewed post-fertilization, no statistically significant differences were observed for any of the morphologies compared with the controls at any of the concentrations of melibiose, trehalose and d(–)-xylose added at either 24 or 30 h post-fertilization (Figs. 1 and 2, Tables 1 and 2). All P-values were >0.05. The results for l(–)-xylose showed a small statistically significant increase in percentages of UAs at 0.0015 M only and only at 30 h post-reagent addition.
Table 1 Morphologies at 48 h for reagent effects that were significantly different from controls (P < 0.05)

None indicates no significant difference from controls; 39,369 embryos were examined.
Table 2 P-values of examples of results in sugar experiments

One-way ANOVA, followed by a Tukey Pairwise Comparison. This sugar is the only one found to cause substantial significant differences from controls in archenteron development. aP < 0.05.

Figure 1 Morphologies at 48 h post-fertilization of Lytechinus pictus embryos at various sugar concentrations. (a) Example of sugar that had no effect on embryos, which is typical of most sugars and controls. Melibiose added at 24 h post-fertilization; completely attached archenteron (CA), unattached archenteron (UA), exogastrulation (EXO), non-viable (NV), and no invagination (NINVG). (b) l(–)-rhamnose added at 24 h post-fertilization. (c) l(–)-rhamnose added at 30 h post-fertilization. Error bars indicate standard error of the mean.

Figure 2 Photographs of sugar effects on Lytechinus pictus embryos at 48 h post-fertilization. (a) 0.03 M trehalose treated at 24 h post-fertilization. An example of a typical observation for most ineffective sugars and controls. Arrows show attached archenterons. (b) 0.0015 M l(–)-rhamnose treated at 30 h post-fertilization. Arrows show exogastrulated (EXO) and unattached archenterons (UA). Scale bar = 100 μm.
The addition of α-cyclodextrin at 24 h caused statistically significant increases in percentages of non-viable (dead) and non-invaginated embryos at 0.03 and 0.015 M when compared with the controls. At 30 h post-fertilization, the addition of 0.03 or 0.015 M α-cyclodextrin caused statistically significant increases in the percentages of non-viable (dead) embryos compared with the controls (Table 1).
When viewed at 48 h post-fertilization, the addition of l(–)-rhamnose at 0.03 or 0.015 M caused statistically significant (P < 0.05) increases in percentages of swimming embryos with UAs and exogastrulation (0.0015 M) when added at 24 h post-fertilization, compared with controls (Figs. 1 and 2, Tables 1 and 2). Statistically significant (P < 0.05) increases in the percentages of exogastrulated embryos compared with the controls were observed when 0.003, 0.0015 or 0.0009 M l(–)-rhamnose was added at 30 h post-fertilization (Figs. 1 and 2, Tables 1 and 2). By 30 h, much of the gastrulation had been completed, so it is reasonable to expect that when l(–)-rhamnose was added at 30 h it only caused exogastrulation because none of the other events occurred after 30 h.
Discussion
While it is not known exactly how l(–)-rhamnose exerts its effects on gastrulation in the sea urchin embryo, the current quantitative approach has shown that l(–)-rhamnose is remarkably specific in its statistically significant action on the developing archenteron in living, swimming embryos compared with the other sugars tested. We know of no past work that implicates this sugar in sea urchin gastrulation events. l(–)-rhamnose-binding lectins, however, have been identified in the sea urchin (Ozeki et al., Reference Ozeki, Matsui, Suzuki and Titani1991) and in many other organisms (Tateno et al., Reference Tateno, Saneyoshi, Ogawa, Muramota, Kamiya and Saneyoshi1998; Hosono et al., Reference Hosono, Ishikawa, Mineki, Murayama, Numata, Ogawa, Takayanagi and Nitta1999, Reference Hosono, Sugawara, Ogawa, Kohno, Takayanagi and Nitta2005; Terada et al., Reference Terada, Wantanabe, Tateno, Naganuma, Ogawa, Muramoto and Kamiya2007; Watanabe et al., Reference Watanabe, Shiina, Shinozaki, Yokoyama, Kominami, Nakamura-Tsuruta, Hirabayashi, Sugahara, Kamiya, Matsubara, Ogawa and Muramoto2008, Reference Watanabe, Abolhassani, Tojo, Suda, Miyazawa, Igarahsi, Sakuma, Ogawa and Muramoto2009; Shirai et al., Reference Shirai, Watanabe, Lee, Ogawa and Muramoto2009; Kim et al., Reference Kim, Binnington, Sakac, Fernandes, Shi, Lingwood and Branch2011). l(–)-rhamnose-binding lectins can generally also bind d(–)-galactose, although to a lesser extent (Shrivastava et al., Reference Shrivastava, Rhodes, Pochiraju, Nakane and McBride2012), as the same hydroxyl group orientation at C2 and C4 of the pyranose ring is possessed by l(–)-rhamnose and d(–)-galactose (Tateno et al., Reference Tateno, Saneyoshi, Ogawa, Muramota, Kamiya and Saneyoshi1998). The sea urchin l(–)-rhamnose-binding lectin is a disulphide-linked two subunit homodimer (Ozeki et al., Reference Ozeki, Matsui, Suzuki and Titani1991).
The work presented here, in addition to the past work on identifying l(–)-rhamnose-binding lectin in sea urchins, suggests for the first time that l(–)-rhamnose and l(–)-rhamnose-binding receptors play a role in a process that has interested investigators for over 100 years – archenteron elongation and adhesion to the blastocoel roof (Herbst, Reference Herbst1900; Ernst, Reference Ernst1997). In at least one system (Flavobacterium johnsoniae) a rhamnose-binding lectin appears to function as an adhesin (Shrivastava et al., Reference Shrivastava, Rhodes, Pochiraju, Nakane and McBride2012) and rhamnose-binding lectins have been implicated in various immune functions that often involve adhesion (Schwarz et al., Reference Schwarz, Hodes-Villamar, Fitzpatrick, Fain, Hughes and Cadavid2007; Watanabe et al., Reference Watanabe, Abolhassani, Tojo, Suda, Miyazawa, Igarahsi, Sakuma, Ogawa and Muramoto2009; Franchi et al., Reference Franchi, Schiavon, Carletto, Gasparini, Bertoloni, Tosatto and Ballarin2011; Lopez et al., Reference Lopez, Fain and Cadavid2011) and in pathogenesis (Beck et al., Reference Beck, Farmer, Straus, Li and Peatman2012). Whether LCASW has a synergistic relationship with l(–)-rhamnose in causing the observed effects is unknown, but LCASW did not interfere with development in most of the other sugar solutions or in the controls without reagents. About 100 per cent of the control embryos and those embryos in most of the sugars exhibited complete attached archenteron development in normal swimming embryos throughout the time course of the experiments.
If a sugar interferes with a gastrulation event, this sugar may bind to a receptor on the embryo cell surface as molecules enter the embryo interior without microinjection (Itza & Mozingo, Reference Itza and Mozingo2005; Latham et al., Reference Latham, Martinez, Cazares, Hamburger, Tully and Oppenheimer1998). In the embryo, it is likely that a sugar such as l(–)-rhamnose, present on cell surfaces, binds to l(–)-rhamnose-binding receptors (e.g. lectins) that mediate cellular interactions. As noted earlier, a rhamnose-binding lectin has been found to be a likely adhesin (Shrivastava et al., Reference Shrivastava, Rhodes, Pochiraju, Nakane and McBride2012). We suggest that when exogenous l(–)-rhamnose is added, it binds to the l(–)-rhamnose-binding receptors (lectins) in the embryo, blocking the binding of cell surface l(–)-rhamnose-containing ligand(s) involved in the cellular interaction to the l(–)-rhamnose-binding receptor(s). This idea is a hapten inhibition concept widely used in many fields of biology (Shrivastava et al., Reference Shrivastava, Rhodes, Pochiraju, Nakane and McBride2012). The most important finding is that the current assay has quantitatively been able to identify a specific sugar of interest with great statistical reliability in thousands of living, swimming embryos. The next series of experiments will involve use of α-l-rhamnosidase in similar quantitative assays and also using micro-dissected archenterons and blastocoel roofs (Singh et al., Reference Singh, Karabidian, Kandel, Metzenberg, Carroll and Oppenheimer2013) to determine if l(–)-rhamnose is directly involved in adhesion between the archenteron and blastocoel roof. This series will help to pinpoint l(–)-rhamnose's mechanism of action. The micro-dissection approach has been used recently to identify a polyglucan involved in the adhesion between the archenteron tip and blastocoel roof (Singh et al., Reference Singh, Karabidian, Kandel, Metzenberg, Carroll and Oppenheimer2013). In this approach the archenterons and the blastocoel roofs were dissected out of the embryos and tested for their adhesiveness to each other in the presence and absence of specific reagents. The dissection approach directly measures the adhesion between the archenteron and the blastocoel roof in small numbers of samples. The method used in the present study has the advantage of assessing archenteron elongation and attachment in thousands of living embryos at a time.
The use of the quantitative microplate assay described in this report is ideal to assess the effects of environmental agents such as pollutants on the model sea urchin embryo system.
Acknowledgements
Supported by the National Institutes of Health (NIH) Score (506 48680), RISE, MARC, Joseph Drown Foundation, Sydney Stern Memorial Trust and NSF Presidential Award (0731633).