Introduction
In humans and other animals, new competencies may be learned through active experience or observation of others’ experiences (Bandura, Reference Bandura1977; Petrosini, Reference Petrosini2007; Meltzoff et al. Reference Meltzoff, Kuhl, Movellan and Sejnowski2009). Observing another person performing a complex action accelerates the observer's acquisition of the same action and limits the time-consuming process of learning by trial and error (Bird & Heyes, Reference Bird and Heyes2005; Meltzoff et al. Reference Meltzoff, Kuhl, Movellan and Sejnowski2009). Observational learning not only involves copying an action but also requires that the observer transforms the observation into an action as similar as possible to the model in terms of the goal to be reached and the motor strategies to be applied (Meltzoff & Andrew, Reference Meltzoff and Andrew1995; Gallese & Goldman, Reference Gallese and Goldman1998; Meltzoff & Decety, Reference Meltzoff and Decety2003). It requires the coordination of complex cognitive functions, such as action representation, attention and motivation, and at same time it requires understanding others’ gestures, and making inferences about their behaviours (Bandura, Reference Bandura1977; Meltzoff et al. Reference Meltzoff, Kuhl, Movellan and Sejnowski2009).
Observational learning is already present at birth (Meltzoff & Moore, Reference Meltzoff and Moore1997; Nadel & Butterworth, Reference Nadel and Butterworth1998; Nadel, Reference Nadel, Meltzoff and Prinz2002) and it is crucial for developing complex abilities such as language, social responsiveness and the use of instruments to get things done, thus representing a powerful social learning mechanism (Kokkinakki & Kugiumutzakis, Reference Kokkinakki and Kugiumutzakis2000; Meltzoff & Decety, Reference Meltzoff and Decety2003). Developmental research indicates that the capacity to learn by observation is a slow process in the typical development (Herbert et al. Reference Herbert, Gross and Hayne2006; Esseily et al. Reference Esseily, Nadel and Fagard2010). To learn by observation, infants need to attend to and observe the actor, understand others’ actions and anticipate the effect of the observed action, and after a delay, match some properties of the observed behaviour. Thus, attending, imitating and understanding contingencies are specific skills required to learn by observation. Research has demonstrated that children with autism spectrum disorder (ASD) display deficits in these skills. Indeed, deficits in attending, such as poor or inconsistent eye contact (APA, 1994), inability to follow eye gaze (Leekam et al. Reference Leekam, Hunnisett and Moore1998), not orienting to toys or materials (Donnelly et al. Reference Donnelly, Luyben and Zan2009) and failure to engage in joint attention (Mundy & Crowson, Reference Mundy and Crowson1997), are some of the core diagnostic indicators for autism. In addition, the monitoring of social activities is disrupted early in the developmental progression of autism, limiting the subsequent abilities for observational learning (Shic et al. Reference Shic, Bradshaw, Klin, Scassellati and Chawarska2011). As ASD individuals show deficits in crucial skills to learn by observation (Nadel et al. Reference Nadel, Aouka, Coulon, Gras-Vincendon, Canet, Fagard and Bursztejn2011; Taylor & DeQuinzio, Reference Taylor and DeQuinzio2012), it is important to clarify the features of observational learning in the presence of ASD. To this aim we chose to use an experimental protocol that allowed observational learning to be analysed, and compared it with learning by trial and error. We have used this protocol previously in studies on children with Williams syndrome and dyslexia (Menghini et al. Reference Menghini, Vicari, Mandolesi and Petrosini2011; Foti et al. Reference Foti, Menghini, Mandolesi, Federico, Vicari and Petrosini2013), demonstrating that it was suitable for children and faceting multiple components of learning. Performances of a group of ASD children were compared with those of a chronological age (CA)-, gender- and IQ-matched group of typically developing (TD) children by using such a task of learning by observation or by trial and error of a visuomotor sequence (Fig. 1). With the exception of the imitative competencies, both learning tasks required a good knowledge and anticipatory expectations of effects related to actions, goal-directed actions and motor imagery, allowing recombination of novel actions with novel effects.
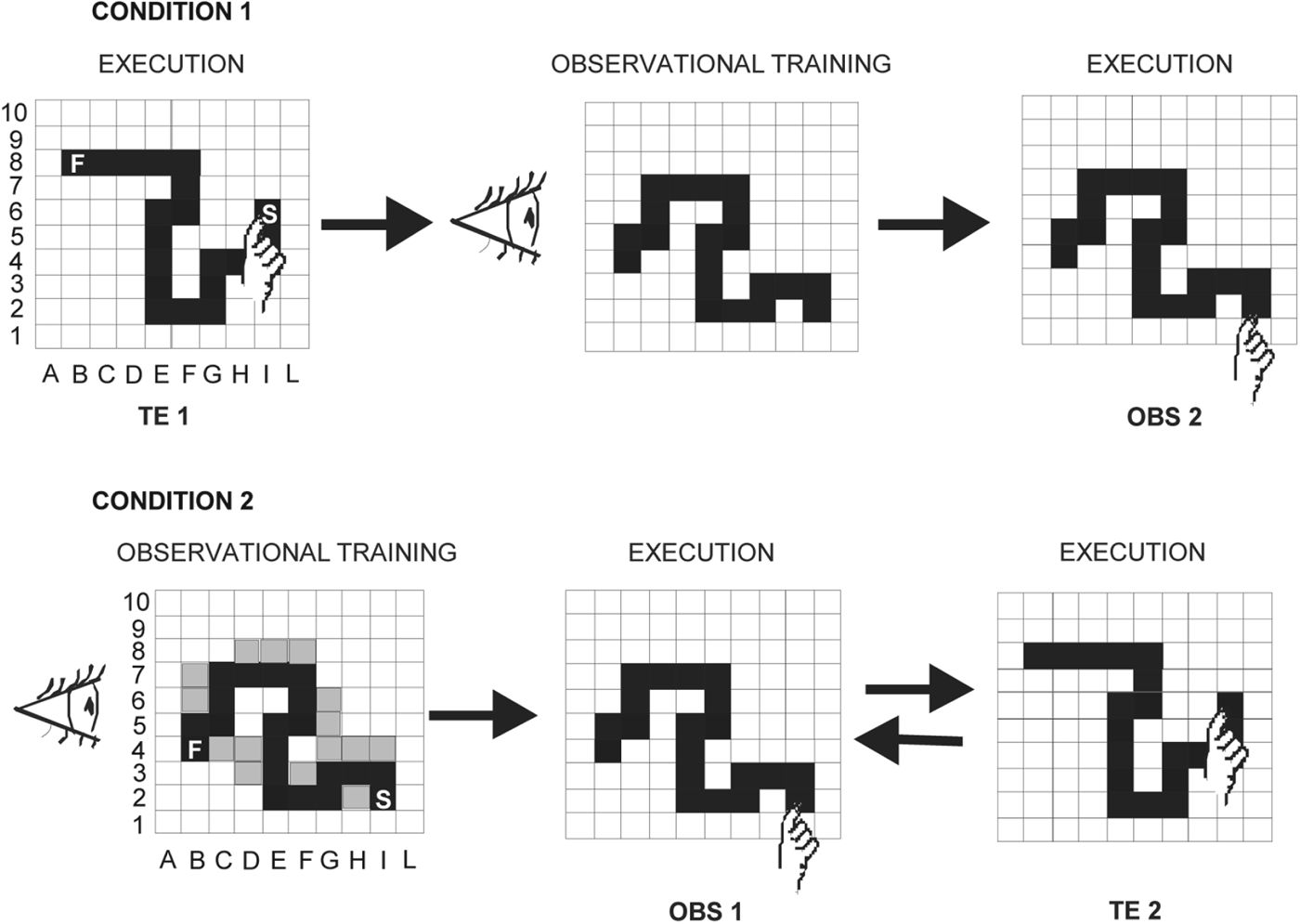
Fig 1. Schematic diagrams of the two experimental conditions. Condition 1 (learning by trial and error followed by observational learning): participants detected a visuomotor sequence by trial and error (TE1), then they observed an actor detecting a sequence different from the one they had previously detected (observational training), and finally they reproduced the observed sequence (OBS2). Condition 2 (observational learning followed by learning by trial and error): participants undertook observational training, then they reproduced the observed sequence (OBS1), and finally they detected by trial and error a different sequence they had not previously observed (TE2). The incorrect positions touched by the actor during the observational training are shown in grey. S, Starting point; F, final point.
Method
Participants
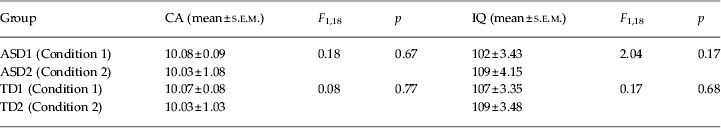
The present study encompassed two experimental conditions: learning by trial and error followed by observational learning (Condition 1) and observational learning followed by learning by trial and error (Condition 2) (Fig. 1). Twenty children with ASD (18 males) with a mean CA of 10 years and 5 months (10.05 s.e.m. ± 0.07 years), and an IQ of 105.85 ± 2.77 and 20 TD children matching the ASD participants for CA (10.05 ± 0.06 years), gender (18 males) and IQ (108 ± 2.36) were examined in the two conditions. No significant differences in CA and IQ (p always > 0.1) among participants performing Conditions 1 and 2 were found (Table 1). Cognitive level was measured by using the Wechsler Intelligence Scale for Children – Third Edition (WISC-III; Wechsler, Reference Wechsler1991). All participants had normal or corrected-to-normal vision and were screened for exclusion criteria (dyslexia, epilepsy, and any other neurological or psychiatric conditions) prior to taking part.
Table 1. Statistical comparisons of chronological age (CA) and IQ between autism spectrum disorder (ASD) groups (ASD1 and ASD2) and typically developing (TD) groups (TD1 and TD2) performing the two experimental conditions
ASD children were diagnosed according to established criteria (DSM-IV; APA, 1994). The diagnosis was made by a licensed clinician not associated with this research. Module 3 of the Autism Diagnostic Observation Schedule (ADOS; Lord et al. Reference Lord, Rutter, Di Lovore and Risi2005) was used to confirm the diagnosis of autistic spectrum disorder (scores 7–10) or autistic disorder (scores >10). The mean ADOS total score was of 13.5 ± 0.85. Based on the ADOS results, 16 children met the criteria for autistic disorder and four for autistic spectrum disorder. (In the current study, all 20 children are described as ASD.)
The study was approved by the local ethics committee and was conducted according to the Declaration of Helsinki. Parents of the participants gave informed written consent. ASD children were tested in a quiet room at Bambino Gesù Children's Hospital and TD children were tested individually in a quiet room at their schools.
Neuropsychological assessment
As difficulties in visuomotor integration, deficits of selective or sustained attention and attentional hyperactivity behaviours may interfere with performance on the learning task, ASD children were evaluated for: visuomotor integration, assessed by the BEERY VMI test (VMI; Beery & Buktenica, Reference Beery and Buktenica2000); visuospatial attention, evaluated by the Bells Test (BELLS, Italian version; Biancardi & Stoppa, Reference Biancardi and Stoppa1997); and attentional hyperactivity behaviors, estimated by Conners’ Parent Rating Scales –Revised: Long Version (CPRS–R:L; Nobile et al. Reference Nobile, Alberti and Zuddas2007).
Experimental procedure
Each participant sat in front of a computer touch screen at a distance of 60 cm. In both Conditions 1 and 2, the experimenter acting as the actor (F.F.) sat near the participant. A 10 × 10 black matrix appeared on the touch screen. The participant was asked to find a hidden sequence of ‘correct’ squares prepared in advance by the experimenters. The sequence was composed of 20 adjacent spatial positions in the matrix, which formed a ‘snake-like’ pattern (Fig. 1). To explain the task to each participant, the experimenter used the same verbal instructions: ‘You have to find a snake formed by twenty squares. When you touch a correct square belonging to snake body it will be turned grey and you will hear a sound; conversely, if you touch a wrong square not belonging to the snake, it will be turned red. In this case, you have to find a new grey square. You have to restart each time you find a new correct square. After finding the whole snake, you have to retouch it three times without making lighted red squares’. The participants started touching a grey square, which was the first element of the sequence representing the snake body and was always lit up. In the search for the second correct square, the participants had to touch one of the four squares bordering the grey square by moving in the matrix vertically or horizontally, but never diagonally. Each touched square (correct or wrong) was lit up for 500 ms and then the light went off again; thus, no trace of the touched sequence remained on the screen.
In learning the sequence by trial and error, the participants tried to find the correct sequence immediately after the verbal instructions. Conversely, in the observational learning task, after the verbal instructions the participants observed the actor while she detected a 20-item sequence by trial and error (observational training). The actor performed the task by always making the same errors in the same positions, so that all participants observed the same pattern of correct and wrong touches. Two minutes after the end of the observational training, the participants were required to reproduce the observed sequence.
A pilot study was conducted to verify that the two sequences arranged to be detected by trial and error (TE) and following observational training (OBS) did not differ as to degree of difficulty. Six TD children (five male) of CA 10.04 ± 0.05 years detected the two different sequences by trial and error; the presentation order was randomized among participants. Errors (wrong touches) made in detecting each sequence were calculated by a one-way ANOVA with repeated measures. The analysis failed to reveal any significant difference between sequences (F 1,5 = 0.094, p = 0.77), confirming that they were of the same difficulty.
Condition 1: learning by trial and error followed by observational learning
Ten (all male) ASD and 10 (all male) TD children (Table 1) first detected a sequence by trial and error (TE1) and, 10 min after the end of the task, they were submitted to the observational training. After 2 min, participants were required to reproduce the observed sequence (OBS2). There was no fixed time limit for executing the task.
Condition 2: learning by observation followed by learning by trial and error
Ten (eight male) ASD and 10 (eight male) TD children (Table 1) first observed the actor detect a sequence (OBS1) and then reproduce it. After 10 min, they had to detect a different sequence by trial and error (TE2). Thus, the difference between the two conditions was that participants reproduced a sequence learned by observation after (Condition 1) or before (Condition 2) the detection of a different sequence by trial and error (Fig. 1).
Mental representative mapping abilities
At the end of the reproduction of each sequence, participants were asked to draw the arrangement of the sequence on a 10 × 10 matrix sketched on a sheet of paper. Thus, each participant drew the arrangement of the two sequences, one learned by observation and the other by trial and error. Mapping abilities were evaluated by tabulating the variable ‘error’ into three categories: ‘no error’, ‘one error’ and ‘more than one error’.
Parameters
Regardless of whether learning took place observationally or by trial and error, the two tasks involved three phases: the detection phase (DP), which ended once the participants found the 20th correct position; the exercise phase (EP), in which they had to repeat the 20-item sequence until their performance was error free; and the automatization phase (AP), which ended when the correct sequence was repeated three consecutive times without errors.
The parameters measured were: DP errors, that is the number of incorrect squares touched in detecting the sequence; EP repetitions, the number of replications needed to achieve an error-free performance; and AP times (in ms), the time spent carrying out the three correct repetitions of the sequence. Considering DP and EP together, we calculated perseverations, consecutive errors touching the same square or a fixed sequence of squares; sequence errors, touching a correct square at the ‘wrong’ moment (i.e. touching E4 before E3; Fig. 1); side-by-side errors, errors in the squares bordering the correct sequence (i.e. D5; Fig. 1); illogical errors, errors in any other square (i.e. C10; Fig. 1); and, exclusively in the observational learning task, imitative errors, errors in the wrong squares unnecessarily touched by the actor during the observational training (i.e. G4; Fig. 1).
Statistical analyses
The data were first tested for normality (using the Shapiro–Wilk test) and homoscedasticity (the Levene test) and then compared by using two-, three- or four-way analyses of variance (ANOVAs) followed by post-hoc multiple comparisons using the Newman–Keuls test. The two-way ANOVAs were performed by applying the mixed model for independent variable (group) and repeated measures (square). Three-way ANOVAs (group × condition × task) were performed on most parameters, and the four-way ANOVA was performed on AP times by applying the mixed model for independent variables (group, condition and task) and repeated measures (times or error). Correlations between data were tested by means of Pearson's r. Error categories of mapping abilities were analysed by χ 2. Statistical analyses were performed by using Statistica 8.0 for Windows and the significance level was set at p < 0.05.
Results
Learning tasks
In comparison with TD children, ASD participants performed a number of DP errors not significantly different after the observational trainings (OBS1–OBS2 tasks) and significantly higher in TE1 (Fig. 2 a), as revealed by post-hoc comparisons on the second-order interaction of the three-way ANOVA (group × condition × task) (F 1,36 = 8.07, p = 0.0073).

Fig 2. Performances of autism spectrum disorder (ASD) and typically developing (TD) children. Data are expressed as mean ± s.e.m. The asterisks indicate the significance level of post-hoc comparisons between groups: *** p < 0.0005. DP, Detection phase; EP, exercise phase; AP, automatization phase. See Fig. 1 for explanation of TE1, TE2, OBS1 and OBS2.
In the EP, ASD participants needed a similar number of repetitions to reach error-free performances in comparison to TD regardless of condition (1 or 2) and task (OBS or TE), as revealed by the lack of a significant group effect of the three-way ANOVA (group × condition × task) (F 1,36 = 1.33, p = 0.25) (Fig. 2 b).
Furthermore, ASD children performed a number of perseverations significantly higher than TD, as revealed by the significant group effect of the three-way ANOVA (group × condition × task) (F 1,36 = 4.53, p = 0.04) (Fig. 2 c). Post-hoc comparisons on the first-order interaction group × task (F 1,36 = 5.83, p = 0.02) revealed that the groups differed significantly only in the TE tasks (p = 0.00036), given the higher number of perseverations displayed by ASD participants.
A four-way ANOVA (group × condition × task × time) on AP times revealed that, although all participants exhibited significantly reduced times as the task continued (time effect: F 2,72 = 9.99, p = 0.00014), ASD children were significantly slower than TD (group effect: F 1,36 = 4.58, p = 0.039), revealing their difficulty in automatizing the sequences (Fig. 2 d).
Analysis of error
A four-way ANOVA (group × condition × task × error) revealed a significant group effect (F 1,36 = 7.43, p = 0.009). Post-hoc comparisons on the significant third-order interaction (F 3,108 = 3.07, p = 0.03) revealed that ASD children differed significantly from TD participants, performing more imitative errors in OBS1 (p = 0.017), along with sequence (p = 0.0011) and side-by-side (p = 0.0001) errors in TE1 (Fig. 3).

Fig 3. Errors of autism spectrum disorder (ASD) and typically developing (TD) children. Data are expressed as mean ± s.e.m. The asterisks indicate the significance level of post-hoc comparisons between groups: * p < 0.05, ** p < 0.005, *** p < 0.0005. See Fig. 1 for explanation of TE1, TE2, OBS1 and OBS2.
As for side-by-side errors, the high number of errors made by ASD children was due to their significantly more frequent wrong touching when a change of direction was required (squares I3, F4, F3, E7) (Fig. 4), as revealed by post-hoc comparisons made on the significant interaction (F 41,738 = 2.39, p < 0.00001) of the two-way ANOVA (group × square).

Fig 4. Incorrect squares touched on the screen by autism spectrum disorder (ASD) and typically developing (TD) children in performing the tasks. On the right, the chromatic scale indicates the sum of incorrectly touched squares (brown and blue denote maximal and minimal values respectively). S, starting point; F, final point. See Fig. 1 for explanation of TE1, TE2, OBS1 and OBS2.
Mapping abilities
No significant difference between groups and among error categories was found in any sequence (always p > 0.3), an index of similar mental representative mapping abilities in both groups.
Neuropsychological findings
The scores of each ASD participant on the VMI, BELLS and CPRS–R:L tasks were transformed into standard scores based on normative data. All ASD children showed scores that fell within a maximum of 1.5 standard deviations below the average (VMI standard scores: 101.9 ± 3.6; BELLS selective attention z score: −0.8 ± 0.3; BELLS sustained attention z score: –1.3 ± 0.4; CPRS–R:L DSM-IV total t score: 60.6 ± 3.1). Moreover, in ASD participants, no significant correlation between learning performances and VMI, BELLS and CPRS–R:L was found (Table 2).
Table 2. Correlations between learning performances (DP, EP and AP parameters) and visuomotor integration (VMI), selective or sustained visuospatial attention (BELLS) and attentional hyperactivity behaviours (CPRS–R:L DSM-IV) in ASD participants
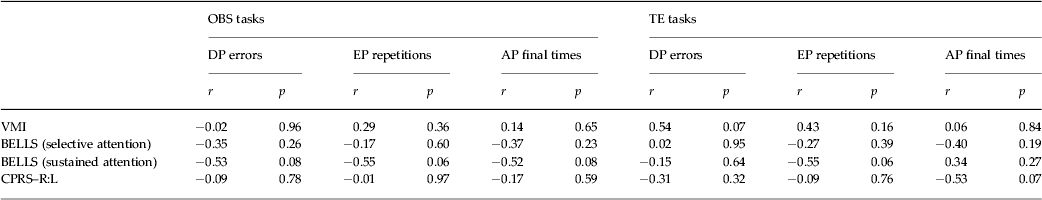
DP, Detection phase, EP, exercise phase; AP, automatization phase; VMI, the BEERY VMI test; BELLS, Italian version of the Bells Test; CPRS–R:L, Conners’ Parent Rating Scales – Revised: Long Version; TE, trial and error; OBS, observational training.
Discussion
ASD participants were severely impaired in detecting a visuomotor sequence by trial and error when the task was first proposed as in TE1, whereas they were as efficient as TD children in reproducing the previously observed sequence as in OBS1. Notably, in the DP the positive effect of observational training was evident not only in reproducing the previously observed sequences as in OBS1 and OBS2 but also in subsequently detecting a sequence by trial and error as in TE2. However, it should be considered that a practice effect, inescapably present in any second task, might have improved performances in both groups (Fig. 2 a). Thus, through the observational training, a kind of visuomotor priming, ASD participants learned to put into action the correct decision-making strategy and the appropriate strategies to discover rules and generate new knowledge to be automated. The high number of errors in the DP in TE1 of ASD participants may reflect a deficit in the executive function. ‘Executive function’ is traditionally used as an umbrella term for functions such as planning, working memory, impulse control, inhibition, set-shifting and monitoring of action (Stuss & Knight, Reference Stuss and Knight2002). Deficits such as planning (Robinson et al. Reference Robinson, Goddard, Dritschel, Wisley and Howlin2009), flexibility (Corbett et al. Reference Corbett, Constantine, Hendren, Rocke and Ozonoff2009) and response inhibition (Agam et al. Reference Agam, Joseph, Barton and Manoach2010), along with initiating, sequencing and monitoring the task (Hill, Reference Hill2004; Robinson et al. Reference Robinson, Goddard, Dritschel, Wisley and Howlin2009), described repeatedly in autism, could influence the capacities of ASD children to perform efficiently in the DP.
In the EP, ASD children were as efficient as TD participants (Fig. 2 b). As the EP mainly requires working memory, memory load to form and maintain the trace of correct sequences, long-term memory and attentional demands to monitor its correct execution, the efficient EP performance of ASD participants could indicate the sparing of these specific abilities. In fact, the ASD high-level ability to arrange simple visual elements (Shah & Frith, Reference Shah and Frith1993; Mottron et al. Reference Mottron, Belleville and Ménard1999; O'Riordan et al. Reference O'Riordan, Plaisted, Driver and Baron-Cohen2001) represents an advantage in performing the EP that involves the encoding and retrieval of visuospatial components. Their efficient EP performance is also in agreement with some studies indicating that ASD children are not impaired in spatial working memory tasks or in search tasks (Klin et al. Reference Klin, Sparrow, de Bildt, Cicchetti, Cohen and Volkmar1999; Ozonoff & Strayer, Reference Ozonoff and Strayer2001). Thus, the autism cognitive style favouring the use of visuospatial coding strategies seems to allow for efficient performance in the EP, which requires mnesic and attentional visuospatial abilities more than problem-solving and planning capacities as in the DP. The efficient EP performance may reflect a way of thinking made up of series of images, instead of words (Grandin, Reference Grandin1995, Reference Grandin2009; Kunda & Goel, Reference Kunda and Goel2011). In addition, the efficient mapping abilities requiring visual imagery found in ASD children in the present research tend in this direction. The relative strength in mental rotation and visual imagery functions described in ASD children supports this proposal (Falter et al. Reference Falter, Plaisted and Davis2008; Soulières et al. Reference Soulières, Zeffiro, Girard and Mottron2011).
In the AP, ASD children displayed longer automatization times than TD participants, although these times diminished progressively as repetitions continued (Fig. 2 d). This suggests a partial deficit in automatization processes linked to the functions of subcortical structures, such as the cerebellum and basal ganglia, and to their bidirectional interconnections with parietal and frontal cortices (Seidler et al. Reference Seidler, Purushotham, Kim, Ugurbil, Willingham and Ashe2005; Menghini et al. Reference Menghini, Hagberg, Caltagirone, Petrosini and Vicari2006; Torriero et al. Reference Torriero, Oliveri, Koch, Lo Gerfo, Salerno, Ferlazzo, Caltagirone and Petrosini2011). Indeed, neuroimaging and autoptic data on ASD individuals have consistently described increased volumes of the caudate nucleus (Sears et al. Reference Sears, Vest, Mohamed, Bailey, Ranson and Piven1999; Luna et al. Reference Luna, Minshew, Garver, Lazar, Thulborn, Eddy and Sweeney2002; Hollander et al. Reference Hollander, Anagnostou, Chaplin, Esposito, Haznedar, Licalzi, Wasserman, Soorya and Buchsbaum2005; Haznedar et al. Reference Haznedar, Buchsbaum, Hazlett, LiCalzi, Cartwright and Hollander2006; Rojas et al. Reference Rojas, Peterson, Winterrowd, Reite, Rogers and Tregellas2006; Langen et al. Reference Langen, Schnack, Nederveen, Bos, Lahuis, de Jonge, van Engeland and Durston2009; Neuhaus et al. Reference Neuhaus, Beauchaine and Bernier2010) in addition to reduced volumes of cerebellar vermis and hemispheres (Bailey et al. Reference Bailey, Luthert, Dean, Harding, Janota, Montgomery, Rutter and Lantos1998; Verhoeven et al. Reference Verhoeven, De Cock, Lagae and Sunaert2010). Moreover, in ASD individuals an altered cerebellar activation has been reported during simple motor tasks (Allen et al. Reference Allen, Muller and Courchesne2004; Martineau et al. Reference Martineau, Andersson, Barthelemy, Cottier and Destrieux2010). Even the types of errors made by ASD children in the present research support the ‘subcortical’ involvement. In both trial-and-error tasks, but not in both observational learning tasks, ASD participants made more perseverative errors than TD children, once more suggesting a deficit in top-down executive control (Fig. 2 c). Perseverations may be symptoms not only of prefrontal dysfunction but also of cerebellar and basal ganglia damage provoking ‘frontal-like’ cognitive deficits (Middleton & Strick, Reference Middleton and Strick2000; Seidler et al. Reference Seidler, Purushotham, Kim, Ugurbil, Willingham and Ashe2005; Clarke et al. Reference Clarke, Robbins and Roberts2008; Ersche et al. Reference Ersche, Roiser, Abbott, Craig, Muller, Suckling, Ooi, Shabbir, Clark, Sahakian, Fineberg, Merlo-Pich, Robbins and Bullmore2011). In effect, the described association of caudate volume with repetitive behaviours emphasizes the striatal role in repetitive behaviours characterizing ASD (Hollander et al. Reference Hollander, Anagnostou, Chaplin, Esposito, Haznedar, Licalzi, Wasserman, Soorya and Buchsbaum2005; Langen et al. Reference Langen, Schnack, Nederveen, Bos, Lahuis, de Jonge, van Engeland and Durston2009). Functional neuroimaging findings in ASD individuals evidenced a disruption in frontostriatal and frontoparietal functional connectivity (Silk et al. Reference Silk, Rinehart, Bradshaw, Tonge, Egan, O'Boyle and Cunnington2006; Just et al. Reference Just, Cherkassky, Keller, Kana and Minshew2007). Moreover, ASD repetitive behaviours correlate with white matter indices in posterior brain pathways, including the cerebellum (Cheung et al. Reference Cheung, Chua, Cheung, Khong, Tai, Wong, Ho and McAlonan2009). Data from mouse models have also indicated that cerebellar pathology may play a causal role in generating repetitive behaviours (Martin et al. Reference Martin, Goldowitz and Mittleman2010; Tsai et al. Reference Tsai, Hull, Chu, Greene-Colozzi, Sadowski, Leech, Steinberg, Crawley, Regehr and Sahin2012).
As for the remaining errors, all participants made an analogously low number of illogical errors, indicating that all participants similarly managed task fundamentals (Figs 3 and 4). Furthermore, ASD children made more sequence errors and side-by-side errors than TD participants in TE1, particularly when a change of direction was required. Errors in stopping the easier ‘keep-straight’ response and performing the more demanding ‘turn-left’ response resulted in suppressing a previously correct but then inappropriate response. Not by change, correctly responding requires processes, such as response inhibition, cognitive flexibility and attentional shifting (Chambers et al. Reference Chambers, Bellgrove, Gould, English, Garavan, McNaught, Kamke and Mattingley2007; Swick et al. Reference Swick, Ashley and Turken2011), already indicated as being impaired in ASD participants (Pellicano, Reference Pellicano2012). Of note, despite the positive effect of the observational training, in OBS1 ASD participants made a high number of imitative errors, indicating their tendency to hyperimitate (Fig. 3). Such an increase in imitation and a reduction in imitation inhibition fit with the ASD symptoms of echolalia and echopraxia (Rutter, Reference Rutter1974; Russell, Reference Russell1997; Spengler et al. Reference Spengler, von Cramon and Brass2010), although a recent report has criticized the notion of an ASD tendency to overimitate (Marsh et al. Reference Marsh, Pearson, Ropar and Hamilton2013).
A tempting interpretation of the present results supports the broken mirror neuron system hypothesis in ASD (Ramachandran & Oberman, Reference Ramachandran and Oberman2006). The mirror system provides the observer with a matching motor representation in one's own motor system (Iacoboni et al. Reference Iacoboni, Woods, Brass, Bekkering, Mazziotta and Rizzolatti1999; Buccino et al. Reference Buccino, Binkofski, Fink, Fadiga, Fogassi, Gallese, Seitz, Zilles, Rizzolatti and Freund2001). Action mirroring is assumed to underlie imitation of observed actions and social understanding (Rizzolatti & Craighero, Reference Rizzolatti and Craighero2004). It has been suggested that, along with emotion sharing and theory of mind, a deficit in perception–action matching is a primary difficulty in ASD (Rogers & Pennington, Reference Rogers and Pennington1991) and that early mirror system dysfunction might lead to a cascade of developmental impairments (Williams et al. Reference Williams, Whiten, Suddendorf and Perrett2001, Reference Williams, Whiten and Singh2004). Furthermore, weaker responses in mirror system regions in ASD individuals during movement observation, execution and imitation have been described (Théoret et al. Reference Théoret, Halligan, Kobayashi, Fregni, Tager-Flusberg and Pascual-Leone2005; Dapretto et al. Reference Dapretto, Davies, Pfeifer, Scott, Sigman, Bookheimer and Iacoboni2006; Bernier et al. Reference Bernier, Dawson, Webb and Murias2007). However, more recent studies do not support the view of a global failure in the mirror system in autism (Leighton et al. Reference Leighton, Bird, Charman and Heyes2008; Southgate & Hamilton, Reference Southgate and Hamilton2008). Thus, rather than the mirror system being ‘broken’, it may be that control over its output or top-down modulation of this system are atypical (Hamilton et al. Reference Hamilton, Brindley and Frith2007; Hamilton, Reference Hamilton2008; et al. Reference Spengler, von Cramon and Brass2010; Kana et al. Reference Kana, Wadsworth and Travers2011; Cook et al. Reference Cook, Barbalat and Blakemore2012, Reference Cook, Swapp, Pan, Bianchi-Berthouze and Blakemore2013). The present results are consistent with this latter hypothesis. Indeed, we evidenced the beneficial effect of the observational training in ASD children so that their ability to reproduce a previously observed visuomotor pattern was almost completely spared. Nevertheless, the large number of imitative errors seems to indicate an impaired function of imitation inhibition, advancing problems in the control of imitative behaviours rather than in imitation per se (Shih et al. Reference Shih, Shen, Ottl, Keehn, Gaffrey and Müller2010).
In conclusion, elucidating the disturbances to the multiple learning and memory systems in autism could have several potential implications for both research and clinical practice. A clear understanding of the learning and memory deficits in ASD could target therapies to remediation of the specific deficits. Understanding the cognitive profile with its relative strengths and weaknesses may ultimately help in developing optimal therapeutic interventions tailored to each individual to facilitate the acquisition of new abilities and competencies. Furthermore, it may allow the best teaching approach along with social integration and development of self-efficacy and self-confidence. Thus, the present results can promote progress in three main areas: early intervention programmes, learning outside of school and formal education. Children are born learning, and how much they learn depends on environmental input. The recognition that the right input at the right time has cascading effects can lead to early interventions in children at risk, as ASD children may be. Indeed, programmes enhancing early social interactions and contingencies can produce significant long-term improvements in academic achievement and social adjustment.
Acknowledgements
We thank the children with ASD and their parents for making this study possible.
Declaration of Interest
None.








