Introduction
Despite available guidelines (Galletly et al., Reference Galletly, Castle, Dark, Humberstone, Jablensky, Killackey and Tran2016; National Institute for Health & Care Excellence, 2014; Schmidt et al., Reference Schmidt, Schultze-Lutter, Schimmelman, Maric, Salokangas, Riecher-Rössler and Ruhrmann2015) discourage the use of antipsychotics (AP) as first-line treatment option for youth who are experiencing clinical high-risk (CHR) for psychosis, baseline exposure to AP in CHR samples is widespread (Raballo & Poletti, Reference Raballo and Poletti2019). For example, the subgroup of studies reporting baseline AP exposure in DSM-5 Attenuated Psychosis Syndrome DSM-5–APS or closely related CHR criteria (Salazar de Pablo, Catalan, & Fusar-Poli, Reference Salazar de Pablo, Catalan and Fusar-Poli2020) indicated a pooled prevalence of about 20% (Raballo, Poletti, & Preti, Reference Raballo, Poletti and Preti2020).
While adolescent and young adult help-seekers attending generalist mental health services might receive AP treatment for a variety of reasons (e.g. age-limited behavioral problems) and prescriptive habits (Olfson, King, & Schoenbaum, Reference Olfson, King and Schoenbaum2015), this should not be the case within specialized CHR programs where AP prescription is allegedly well-pondered and circumscribed. Furthermore, ongoing AP treatment in newly enrolled CHR participants is a non-negligible clinical and prognostic confounder that needs to be adequately acknowledged. Indeed, AP treatment may mitigate the intensity of the clinical presentation and modulate the later outcome trajectory, somehow masking the formal transition to psychosis, which is usually based on the pure psychometric threshold of positive symptoms severity (Raballo & Poletti, Reference Raballo and Poletti2019; Raballo, Poletti, & Carpenter, Reference Raballo, Poletti and Carpenter2019; Raballo et al., Reference Raballo, Poletti and Preti2020; Van Os & Guloksuz, Reference Van Os and Guloksuz2017). Precisely for this reason, in addition to the formal criteria for transition to psychosis (i.e. based on psychometric scores on positive symptoms), the original ultra high-risk model of psychosis explicitly conceptualized a functional equivalent of transition to psychosis, i.e. the threshold at which AP treatment would probably be commenced in common clinical practice (Yung et al., Reference Yung, Phillips, Yuen, Francey, McFarlane, Hallgren and McGorry2003). Along the same conceptual line, the endpoint of the duration of untreated psychosis (aka DUP) allegedly coincides with the instantiation of the first AP treatment (Penttilä, Jääskeläinen, Hirvonen, Isohanni, & Miettunen, Reference Penttilä, Jääskeläinen, Hirvonen, Isohanni and Miettunen2014).
Clearly, distinguishing AP-naïve CHR participants (i.e. truly CHR subjects) from AP-treated CHR (i.e. pharmacologically ‘attenuated first-episode psychosis’: Raballo et al., Reference Raballo, Poletti and Preti2020) is crucial for prognostic stratification. Indeed, the magnitude of such confounder may substantially impact current prognostic estimates and reduce the precision of contemporary prediction models. Therefore, the primary aim of the present study is to quantify through meta-analytical lenses if baseline AP exposure in CHR subjects impacts on transition to psychosis at follow-up. Based on the original ultra high-risk conceptualization (Yung et al., Reference Yung, Phillips, Yuen, Francey, McFarlane, Hallgren and McGorry2003) as well as on previous clinical and conceptual analysis in the field (Raballo & Poletti, Reference Raballo and Poletti2019; Raballo et al., Reference Raballo, Poletti and Preti2020), we expected the CHR subgroup already undergoing AP treatment at baseline to represent a more severe subgroup in terms of risk of transition to psychosis. Indeed, they are a group of individuals with rapidly aggravating mental health states that are severe enough to (a) motivate their referral for CHR evaluation, and (b) require AP prescription even before formal CHR assessment. Compared to AP-naïve CHR, CHR who had already exposed to APs at baseline might include a disproportionate fraction of unrecognized clinical equivalents of ‘first-episode psychosis’. Such subgroup, although psychometrically mitigated at its initial presentation because of the ongoing AP therapy, could harbor a higher risk of imminent transition to full-blown psychosis as compared to simple (i.e. AP-naïve) CHR.
The present meta-analysis focused on the population of CHR individuals, diagnosed according to predefined criteria, with transition to psychosis as outcome and exposure to APs at baseline as the criterion to distinguish between cases (exposed) and comparison (not exposed).
Methods
Study selection
This systematic review and meta-analysis was planned and executed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, Altman, & PRISMA Group, Reference Moher, Liberati, Tetzlaff and Altman2009). We searched PubMed/Medline (https://pubmed.ncbi.nlm.nih.gov/) and the Cochrane library (https://www.cochranelibrary.com/) from inception up to 20 April 2020, by using the following key terms: ‘Ultra high risk’ OR ‘Clinical high risk’ and ‘psychosis’ and ‘transition’ OR ‘conversion’. This search retrieved 1838 articles, of which 98 were systematic review or meta-analysis, in PubMed/Medline, and 196 trials in the Cochrane Central Register of Controlled Trials. Two authors (MP, AP) evaluated the list of extracted articles and decided about inclusion or exclusion according to the following criteria:
-
− written in English;
-
− detailing information about samples with people diagnosed at CHR of psychosis based on a validated diagnostic procedure;
-
− reporting numeric data about the sample and the outcome at a predefined follow-up time; having transition to psychosis as one of the outcomes;
-
− reporting raw data on AP baseline exposure in relation to the transition outcome.
Data extraction
After exclusion of duplicates (including articles repeatedly reporting the results of the same trial or with overlapping samples) and articles that were unrelated to the main topic (i.e. studies on brain imaging or genetic markers), individual studies were included when they matched the inclusion criteria. Discrepancies were resolved consulting a third experienced researcher (AR). The references of the retrieved articles and of the extracted reviews on the topic were scanned to identify potentially missed studies. At the end of this procedure, 14 independent studies were included in the systematic analysis and the subsequent meta-analysis (Fig. 1: PRISMA flow chart).

Fig. 1. PRISMA flowchart of studies reporting conversion to psychosis in CHR help-seeking people according to antipsychotics exposure at baseline (yes or not).
The following variables were extracted from the included studies: authors and year of publication of the study; location of the study; criteria and instrument for diagnosis; criteria for transition to psychosis; sample size at baseline and at follow-up; mean age in the sample; gender ratio in the sample; data on AP exposure (yes/no) on the basis of outcome (transition/no transition); duration of the follow-up; number of cases that transitioned psychosis at the end of follow-up by group. Quality assessment was rated according to the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (nhlbi.nih.gov/health-topics/study-quality-assessment-tools). Discrepancies in extraction of data were solved by discussion within the research team.
Data analysis
All analyses were carried out with the ‘meta’ package (Schwarzer, Carpenter, & Rücker, Reference Schwarzer, Carpenter and Rücker2015) and the ‘metafor’ package (Viechtbauer, Reference Viechtbauer2010) running in R version 3.5.1 (R Core Team, Reference R Core Team2020).
The outcome of the meta-analysis was the proportion of transition to psychosis. All proportions were estimated with the variance-stabilizing Freeman and Tukey (Reference Freeman and Tukey1950) double arcsine transformation, since there is evidence that it outperforms other proposed methods (e.g. logit transformation) of estimating prevalence (Barendregt, Doi, Lee, Norman, & Vos, Reference Barendregt, Doi, Lee, Norman and Vos2013), especially when the proportion of cases is expected to be small. Thereafter, we compared the binary outcome of transition to psychosis by group. Risk ratio (RR) was calculated and the inverse variance method was used for pooling (Fleiss, Reference Fleiss1993). Between-studies variance and variance of the effect size parameters across the population were estimated with the τ 2 statistics using Empirical Bayes estimator (Veroniki et al., Reference Veroniki, Jackson, Viechtbauer, Bender, Bowden, Knapp and Salanti2016); its 95% CI was calculated by using the Q-Profile method (Viechtbauer, Reference Viechtbauer2010) with Knapp and Hartung (Reference Knapp and Hartung2003) correction. Continuity correction of 0.5 was applied in studies with zero cell frequencies.
Both fixed- and random-effects summary estimates were reported, along with a corresponding 95% confidence interval (CI) for each outcome in forest plots. In the interpretation of the results, we gave preference to the fixed-effects model. Our main goal was to make a conditional inference only about the studies included in the meta-analysis (Viechtbauer, Reference Viechtbauer2010), and the estimates that can be drawn from a fixed-effects model provide perfectly valid inferences under heterogeneity when the inference is limited to the investigated studies (Hedges & Vevea, Reference Hedges and Vevea1998). Moreover, the fixed-effects model does not inflate the role of small studies as the random-effects model does (Borenstein, Hedges, Higgins, & Rothstein, Reference Borenstein, Hedges, Higgins and Rothstein2010). Finally, in the attempt to model some (but not all) heterogeneity in the studies, the random-effects model loses power compared to the fixed-effects model (Jackson & Turner, Reference Jackson and Turner2017).
To control the adequacy of the models and outlier detection, the radial plot was considered (Galbraith, Reference Galbraith1994). Studies with estimates that were beyond two standard deviations from the common estimates were assumed to have a poor fit with the model (i.e. potential outlier). When outliers were identified, the model was recalculated without the outliers.
In all analyses, heterogeneity was assessed with Cochran's Q and I 2 statistics (Huedo-Medina, Sánchez-Meca, Marín-Martínez, & Botella, Reference Huedo-Medina, Sánchez-Meca, Marín-Martínez and Botella2006). Cochran's Q test assesses the null hypothesis that the true effect size is the same in all studies (Borenstein, Reference Borenstein2020). A low p value (i.e. p < 0.10) of the Q-statistic indicates that variation in the study-specific effect estimates is due to heterogeneity beyond that depending on sampling error. The I 2 statistic measures the extent to which the variance in observed effects reflects variance in true effects rather than sampling error (Borenstein, Reference Borenstein2020). The higher the I 2, the greater the impact of the variance in true effects. The funnel plot and the Egger's test were used as a proxy index of bias in publication (Egger, Davey Smith, Schneider, & Minder, Reference Egger, Davey Smith, Schneider and Minder1997). We used meta-regression techniques to evaluate the impact of the following clinical variables: gender ratio, mean age of the sample, overall sample size, duration of follow-up, and the quality of the study.
Results
Search results
The literature searching process and study identification are summarized in Fig. 1. Briefly, the initial search identified 1838 records, and study selection procedures yielded 14 articles (Table 1) reporting on meta-analyzable information as regards baseline AP exposure in relation to the binary outcome at follow-up (transition/no transition).
Table 1. Studies included in the meta-analysis and reporting raw baseline data on AP exposure in relation to transition to psychosis

AP, antipsychotic; CAARMS, Comprehensive Assessment of At Risk Mental States; Conv., converters to psychosis at follow-up; CHR, clinical high-risk; Nonconv., non-converters to psychosis at follow-up; PANSS, Positive and Negative Syndrome Scale; SIPS, Structured Interview for Prodromal Syndromes; SD, standard deviation.
Overall, six studies included participants from the USA, five from Asian Countries (two Japan, one China, one South Korea, one Taiwan) and three from Europe (one the Netherlands, one Germany, one Spain). All studies included details about age and gender ratio. Studies do vary hugely as far as sample size and time to follow-up, as well as in terms of age and gender ratio, were concerned.
Mean age in the 14 studies was 20.0 ± 2.5, ranging from 15.3 to 24.9 years old. The proportion of girls was 44% on average, ranging from 24% to 75%. There were one study (7.1%) with a sample including exclusively children or adolescents, one study (7.1%) with only adult participants (aged 18 years old and older) and 12 studies (85.8%) based on mixed samples, with both children/adolescents and adults. Sample size at baseline ranged from 34 to 764, with average sample size = 143. Sample size at follow-up ranged from 31 to 431, being on average = 113. Time to follow-up was up to 12 months in five studies (36%), 13–24 months in five studies (36%), and 25 months or longer in four studies (28%).
As far as the tool for the diagnosis was concerned, there was one study (7.1%) using the Comprehensive Assessment of At Risk Mental States (CAARMS; Yung et al., Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio and Buckby2005); one study (7.1%) using the Positive and Negative Syndrome Scale (PANSS: Kay, Fiszbein, & Opler, Reference Kay, Fiszbein and Opler1987) and 12 studies (85.8%) using the Structured Interview for Prodromal Syndromes (SIPS: McGlashan, Reference McGlashan2001). In 13 studies, the sample was based on help-seeking participants and in one study (Liu et al., Reference Liu, Lai, Liu, Chiu, Hsieh, Hwang and Hwu2011) included a community sample. Quality was good in five studies (Brucato et al., Reference Brucato, Masucci, Arndt, Ben-David, Colibazzi, Corcoran and Girgis2017; Collin et al., Reference Collin, Seidman, Keshavan, Stone, Qi, Zhang and Whitfield-Gabrieli2020; DeVylder et al., Reference DeVylder, Muchomba, Gill, Ben-David, Walder, Malaspina and Corcoran2014; Katsura et al., Reference Katsura, Ohmuro, Obara, Kikuchi, Ito, Miyakoshi and Matsumoto2014; Schultze-Lutter, Klösterkotter, & Ruhrmann, Reference Schultze-Lutter, Klösterkotter and Ruhrmann2014) and fair in the other nine studies.
The proportion of participants with exposure to AP at baseline was substantial, ranging from 5.5% up to 79.6% pending on the study.
Overall, the participants who were already exposed to AP at baseline (from herein upon, ‘cases’) were 370 (range: 7–88; average per sample: 26) (23.3% of the whole sample), while those without exposure to AP at baseline (‘controls’) were 1218 (range: 12–343; average per sample: 87) (76.7% of the whole sample). At the end of the period of observation, i.e. the follow-up as reported in the study, 112 (29%; 95% CI 24–34%; Fig. S1 in Supplementary material) participants developed psychosis among the cases as against 235 among the controls (16%; 14–19%; see Fig. S2 in Supplementary material).
Risk ratio estimates of transition to psychosis by exposure to antipsychotics at baseline
CHR participants who were already under AP treatment at baseline had a higher chance of transition to psychosis than CHR participants who were AP-naïve. The RR was 1.47 (95% CI 1.18–1.83) in the fixed-effects model (z = 3.48; p = 0.0005), and 1.59 (0.86–2.93) in the random-effects model (z = 1.62; p = 0.128) (Fig. 2).
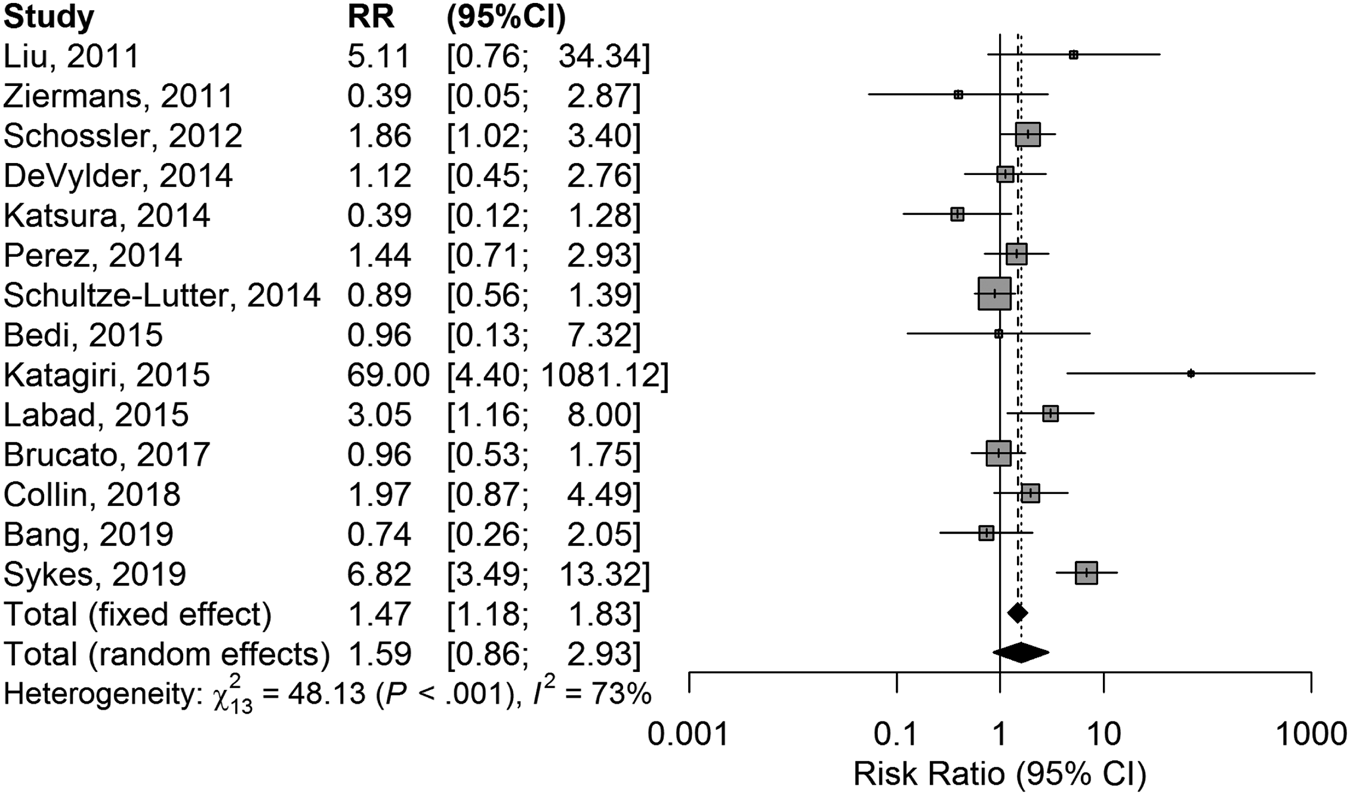
Fig. 2. Forest plot of comparison in risk ratio of conversion to psychosis between CHR who were or were not exposed to antipsychotics at baseline.
There was substantial heterogeneity (Cochran's Q = 48.13; df = 13; p < 0.0001), and a relevant proportion of the variance reflected true variance in the effect across studies than sampling error: I 2 = 73% (95% CI 54–84%). Funnel plot was modestly asymmetric (Fig. S3 in Supplementary material), but without evidence of publication bias at the Egger's test: t = 0.607; df = 12; p = 0.555. There was no impact of age, gender ratio, overall sample size, duration of the follow-up, or quality of the studies on the RR estimates.
At the radial plot, four studies showed estimates that were above (45, 40) or below (30, 33) the predefined threshold of two standard deviations (Fig. 3).

Fig. 3. Galbraith radial plot of comparison in risk ratio of conversion to psychosis between CHR who were or were not exposed to antipsychotics at baseline. Studies beyond (above or below) two standard deviations from the common estimates are outliers.
In the model without the outliers, heterogeneity disappeared (Cochran's Q = 10.74; df = 9; p = 0.293; I 2 = 16%; 0−54%), with still a statistically significant result at the fixed-effects model (RR = 1.42; 1.07–1.87; z = 2.47; p = 0.013) and a near-significant result at the random-effects model (RR = 1.42; 0.98–2.05; z = 2.16; p = 0.059) (Fig. 4).
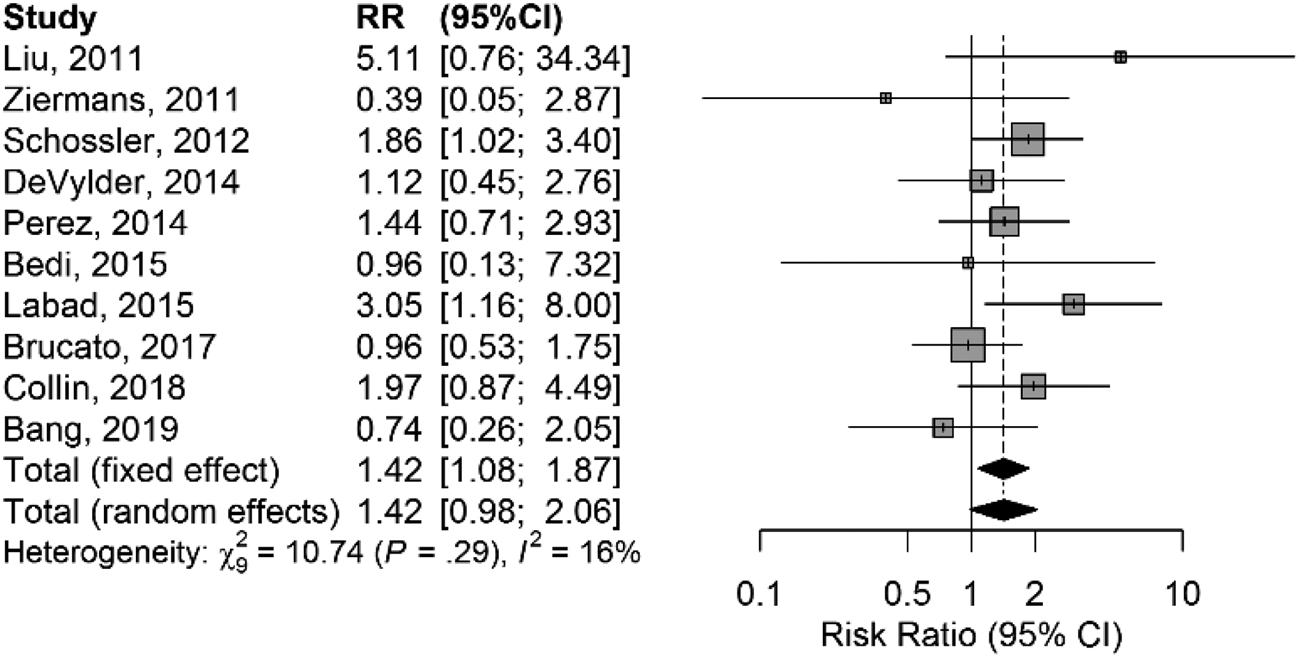
Fig. 4. Forest plot of comparison in risk ratio of conversion to psychosis between CHR who were or were not exposed to antipsychotics at baseline after exclusion of outlier studies.
Funnel plot was symmetric, without evidence of publication bias at the Egger's test (Fig. S4 in Supplementary material).
Again, there was no impact of age, gender, duration of follow-up, or quality of the studies on the estimates.
Discussion
There is a substantial portion of CHR help-seeking people who are already under AP therapy at the referral for CHR evaluation. In the meta-analyzed studies, one out of 4–5 enrolled CHR individuals has already an ongoing exposure to AP at baseline. This has an impact on the risk of transition to psychosis over time. Indeed, CHR people who have been exposed to AP at baseline had a greater risk of transition to psychosis than those who were not. The difference is clear-cut at the fixed-effects model. Crucially, fixed-effects models produce perfectly valid conditional inference on the studies included in the meta-analysis (Viechtbauer & Cheung, Reference Viechtbauer and Cheung2010). Indeed, the fixed-effects model provides a more direct answer when someone wants to know what is the evidence about an outcome in the collected studies. The random-effects model attempts to correct for some (not all) heterogeneity that is present in the distribution of the effects and aims to provide an inference about the average effect in the population considered. This model, by its nature, has less power to calculate the estimates, which is probably one reason for the not statistically significant results for the analysis based on them in this study.
When tested with meta-regression, sample composition by age and gender, the duration of follow-up, or the quality of the studies did not affect the estimates. Four studies were identified as especially influential in the estimates, and when they were phased out from the calculation, the heterogeneity was greatly reduced. Overall, the heterogeneity in the distribution of effects is likely to depend on variables that are not measured in the studies, and one potential variable might be whether or not the AP were discontinued during the course of the follow-up, or whether those CHR who were not exposed to AP at baseline were later prescribed AP during the study.
Ultimately, the hypothesis that exposure to AP at baseline may affect the probability of transition to psychosis is confirmed in the analyzed studies, but it cannot be generalized to future studies on the basis of the results of the random-effects model.
Implications for the clinics
Given the established pharmacological actions of AP drugs and their major prescriptive indications, the higher risk of transitions to psychosis in baseline AP-exposed v. AP-naïve CHR is apparently counter-intuitive. However, in light of the high prevalence of AP exposure in newly identified CHR individuals, such prognostic difference needs to be acknowledged and ongoing AP prescription at baseline should be regarded as an indicator of a higher level of imminent psychopathological risk. This might be due to two different and not mutually exclusive mechanisms.
First, clinically, in line with the original ultra high-risk conceptualization (Yung et al., Reference Yung, Phillips, Yuen, Francey, McFarlane, Hallgren and McGorry2003), AP prescription (usually a collegial and consensual decision of the treating staff especially) is indicative of an emerging, severe mental state that should be regarded as a functional equivalent of transition to psychosis (even when positive symptoms remain below the psychometric severity threshold); therefore, AP-exposed CHR would be akin to undetected or masked ‘first-episode psychosis’ states thereby intrinsically endowed with a higher predisposition to incur in severity fluctuations.
Alternatively, such increased risk of transition might be due to the possible discontinuation of AP treatment during the follow-up. Indeed, according to major guidelines, the ‘primary aim [of low-dose second-generation AP in adult CHR patients] is to achieve a degree of symptomatic stabilization’ and ‘any long-term antipsychotic treatment with a primarily preventive purpose is not recommended’ (Galletly et al., Reference Galletly, Castle, Dark, Humberstone, Jablensky, Killackey and Tran2016; National Institute for Health and Care Excellence, 2014; Schmidt et al., Reference Schmidt, Schultze-Lutter, Schimmelman, Maric, Salokangas, Riecher-Rössler and Ruhrmann2015). This AP suspension at follow-up is somehow encouraged once a form of symptomatic remission/stabilization is achieved. In this case, however, initially AP-exposed CHR participants, once the AP is suspended, could be at a higher risk due to the loss of the protective anti-D2 action or by a putative AP-induced dopamine supersensitivity (Chouinard et al., Reference Chouinard, Samaha, Chouinard, Peretti, Kanahara, Takase and Iyo2017). Another contributing factor could be the perceived harm due to potential stigma, which has been recently emphasized among the social stressors predicting transition to psychosis (Rüsch et al., Reference Rüsch, Heekeren, Theodoridou, Müller, Corrigan, Mayer and Rössler2015) and which could be amplified by the prescription of AP.
In sum, ongoing AP exposure at inception in CHR help-seekers should be a motivated cautionary criterion for the ascription of an outright CHR state or, at least, a red flag warning for imminent risk monitoring.
Implications for research
Although both formally satisfying CHR criteria at inception, AP-naïve CHR and AP-exposed CHR constitute two prognostically different subgroups that should not be conflated together. While this conflation is widespread in the literature and it is probably an unintended consequence of the neglect of the original notion of functional equivalent of transition to psychosis (Yung et al., Reference Yung, Phillips, Yuen, Francey, McFarlane, Hallgren and McGorry2003), it certainly has important consequences that should be duly noted and amended. First, current prediction models and prognostic algorithms based on CHR populations (Sanfelici, Dwyer, Antonucci, & Koutsouleris, Reference Sanfelici, Dwyer, Antonucci and Koutsouleris2020), if combining baseline AP-exposed and AP-naïve CHR, are likely to be substantially flawed (e.g. Ciarleglio et al., Reference Ciarleglio, Brucato, Masucci, Altschuler, Colibazzi, Corcoran and Girgis2019; Zhang et al., Reference Zhang, Li, Tang, Niznikiewic, Shenton, Keshavan and Wang2018). Second, a re-analysis of AP exposure in available CHR datasets is imperative to correct such non-trivial bias. Third, a new wave of CHR studies with transparent and systematic reporting of AP exposure at baseline and follow-up is urgent.
Strengths and limitations
State-of-the-art methods were used to investigate the topic. However, several limitations have to be taken into account: we only had access to summary statistics rather than individual data. While there is robust evidence that the results of aggregate data meta-analysis agree with the results of individual patient data meta-analysis, the latter allows more subgroup analyses and of interaction terms (Huang et al., Reference Huang, Tang, Tam, Mao, Yuan, Di and Yang2016).
Moreover, more fine-grained information on clinical severity, CHR subgroups with potentially different need of care (e.g. APS, BLIPS or GRFD), and AP dosage were not available in the source literature thereby preventing the meta-regression of these variables.
Finally, we had no information on whether or not the prescribed APs at baseline were maintained up to the follow-up or whether some of the CHR APs-naïve participants had received a prescription of APs during the study. Nonetheless, this first meta-analysis examines the impact of ongoing AP treatment at baseline on the risk of transition to psychosis in CHR cohorts.
Conclusion
Disentangling the complex architecture of psychosis risk requires a constant scrutiny of potential blind spots and overlooked confounders. While the CHR construct, its relative cultural dominance in the early detection field, and its operative implementations have been widely debated (Ajnakina, David, & Murray, Reference Ajnakina, David and Murray2018; Moritz, Gawęda, Heinz, & Gallinat, Reference Moritz, Gawęda, Heinz and Gallinat2019), this the first meta-analytic evidence of the impact of a widespread, non-negligible confounder in the field. Indeed, the current study confirms that a substantial proportion of CHR individuals were already exposed to AP at baseline (i.e. they were ascribed to a CHR state while under ongoing AP treatment) and that such subgroup had greater incidence rates of transition to psychosis. Therefore, conflating at baseline AP-naïve and AP-exposed CHR participants surreptitiously increases the heterogeneity within the CHR population, distorts current prediction models, and ultimately hinders more reliable and reproducible translational implementations of precision psychiatry in the field of early detection (Raballo & Poletti, Reference Raballo and Poletti2019; Raballo et al., Reference Raballo, Poletti and Preti2020).
A comprehensive revision of the conceptual and reporting habits in the field is deeply needed. First, the field requires a more transparent reporting of AP exposure in CHR cohorts; second, in line with the original ultra high-risk concept (Yung et al., Reference Yung, Phillips, Yuen, Francey, McFarlane, Hallgren and McGorry2003), such exposure to AP should be regarded as a functional equivalent of transition to psychosis (even when positive symptoms remain below the psychometric severity threshold); third, ongoing AP exposure at inception should be a motivated cautionary criterion for the ascription of a CHR state.
Future studies are warranted to investigate whether the greater incidence rates of transition to psychosis in those CHR who were on ongoing AP treatment at baseline are an epiphenomenon of different factors. For example, global pretest risk enrichment (Fusar-Poli et al., Reference Fusar-Poli, Rutigliano, Stahl, Schmidt, Ramella-Cravaro, Hitesh and McGuire2016) (i.e. AP-exposed CHR cohorts present overall more severe cases as compared to fully AP-naïve cohorts), clinical fluctuations toward escalating severity in those who were already experiencing a progressive transition to psychosis (yet delayed by AP prescription at baseline), or rebound effects due to the possible discontinuation of AP treatment during the follow-up (i.e. loss of the protective anti-D2 action or, on the contrary, a mild variant of AP-induced dopamine supersensitivity: Chouinard et al., Reference Chouinard, Samaha, Chouinard, Peretti, Kanahara, Takase and Iyo2017).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720004237
Funding
This study received no source of external funding.
Conflict of interest
None.








