Introduction
Auditory neuropathy spectrum disorder reflects hearing conditions characterised by altered function of the auditory nerve in the presence of normally functioning outer hair cells. This is evidenced by intact cochlear microphonic potentials and/or otoacoustic emissions (OAEs) with absent or severely desynchronised auditory brainstem responses (ABRs).Reference Starr, Picton, Sininger, Hood and Berlin1 Various factors – genetic, immunological, infectious, toxic and inflammatory conditions – have been shown to be causative in auditory neuropathy spectrum disorder.Reference Starr, Sininger, Winter, Derebery, Oba and Michalewski2 Clinically, auditory neuropathy spectrum disorder is characterised by normal examination findings on otoscopy, and mild-to-severe sensorineural hearing loss with impaired speech discrimination, which is out of proportion to the hearing loss and pure tone thresholds. The clinical syndrome of auditory neuropathy varies in several measures, such as age of onset, cause, presence of peripheral neuropathy and physiological measures of auditory function.Reference Berlin, Hood, Morlet, Wilensky, Li and Mattingly3 The site of abnormality could be anywhere ranging from the inner hair cells to the auditory nerve synapse, dendrites, axons or the myelin sheath of the auditory nerve, as reflected by the pathophysiological processes occurring at these sites.Reference Berlin, Hood, Morlet, Wilensky, Li and Mattingly3–Reference McMahon, Patuzzi, Gibson and Sanli7
Treatment of auditory neuropathy spectrum disorder aims to restore the processing of auditory information either through conventional amplification and/or alternative forms of communication, or by cochlear implantation.Reference Starr, Rance, Aminoff, Boller and Swaab8 Success with hearing aids has been reported previously.Reference Rance and Barker9,Reference Roush, Frymark, Venediktov and Wang10 Some researchers have also explained the transient nature of the pathology and the possibility of spontaneous recovery.Reference Psarommatis, Riga, Douros, Koltsidopoulos, Douniadakis and Kapetanakis11,Reference Madden, Hilbert, Rutter, Greinwald and Choo12 Cochlear implantation is reserved for patients who demonstrate poor progress in speech understanding and auditory language development, despite being fitted with appropriate acoustic amplification. Varied results have been reported with cochlear implantation. Most studies show significant improvement in sound detection, speech perception and communication skills, but some have reported otherwise.Reference Shallop, Facer and Peterson13,Reference Breneman, Gifford and DeJong14 The rationale behind cochlear implantation is the possibility of direct electrical stimulation of the neural elements. However, whether the cochlear neural elements are electrically excitable or not is a point of concern.
Pre-operative transtympanic electrically evoked ABR testing is a proven electrophysiological diagnostic assessment; it is used to determine whether the cochlear nerve is electrically excitable or not and, if it is, establishes the most appropriate ear for implantation.Reference Gibson, Sanli, Kim, Chang and Lim15 According to Kim et al.Reference Kim, Kileny, Arts, El-Kashlan, Telian and Zwolan16 and Kileny and Zwolan,Reference Kileny and Zwolan17 this testing can also decrease the likelihood of placing a cochlear implant in a non-excitable ear. Transtympanic electrically evoked ABR has been used as part of a pre-operative test battery to identify the site of pathology in auditory neuropathy. It is also used to provide clinicians with information about the prognosis of cochlear implantation, based on whether a clear waveform of electrically evoked ABRs is present or not.Reference Gardner-Berry, Gibson and Sanli18 However, no previous attempts have been made to correlate the results of pre-operative electrically evoked ABR testing with the post-operative results in auditory neuropathy spectrum disorder.
The current paper describes the role of transtympanic electrically evoked ABRs in predicting the outcomes of cochlear implantation in auditory neuropathy spectrum disorder by correlating the findings with post-operative results. The paper discusses the serial neural response telemetry measurements and post-operative cochlear implant electrically evoked ABRs following cochlear implantation in auditory neuropathy spectrum disorder patients.
Materials and methods
The study included three children and one adult. All patients had undergone medical, audiological and radiological investigations prior to the study. Standard audiological assessments, including OAE, ABR, behavioural audiometry, and speech reception and recognition measures, were carried out. All the patients were diagnosed as having auditory neuropathy spectrum disorder. Radiological evaluation showed normal cochlear nerves bilaterally. The patient data, hearing loss type, Categories of Auditory Performance scoresReference Archbold, Lutman and Marshall19 and Speech Intelligibility RatingsReference Allen, Nikolopoulos and O'Donoghue20 are shown in Table 1. All the patients received low or insufficient benefit from hearing aids and hence were considered for cochlear implantation.
Table 1. Hearing loss type and pre-operative CAP score and SIR

CAP = Categories of Auditory Performance; SIR = Speech Intelligibility Rating; pt no. = patient number; y = years; pre-op = pre-operative; F = female; SNHL = sensorineural hearing loss; M = male
The patients underwent pre-operative bilateral transtympanic electrically evoked ABR testing and measurement of neural response telemetry intra-operatively, and at 3, 6 and 12 months after switch-on. Out-patient cochlear implant electrically evoked ABRs were measured at one year. Categories of Auditory Performance and Speech Intelligibility Ratings scores were measured at one year after switch-on.
Pre-operative transtympanic responses
A technique of recording transtympanic electrically evoked ABRs previously described by Pau et al. was used.Reference Pau, Gibson and Sanli21 Under general anaesthesia, a posterior segment myringotomy was performed to visualise the round window niche. A subdermal needle electrode placed in the ipsilateral temporal region acted as a ground electrode. A ‘golf club’ electrode was introduced through the myringotomy and placed at the round window niche. Cochlear Custom Sound™ electrophysiology software in electrically evoked ABR mode was used through the ‘implant in a box’ interfaced via a sound processor. Biphasic current pulses were delivered with stimuli presented at rate of 11 per second, with a pulse width of 100–150 μs, with basic, alternating and reverse polarity. A GSI Audera™ electrophysiology machine with surface electrodes was used for recording the waveforms. Wave V morphology, latency and repeatability were recorded. A classification proposed by Dutt et al. (the Kumar–Dutt classification) (Table 2) was used to classify the wave V responses obtained.Reference Dutt and Kumar22
Table 2. Classification of transtympanic electrically evoked ABRs*

* Kumar–Dutt classification. ABR = auditory brainstem response
The ear for implantation was decided based on the type of transtympanic electrically evoked ABR waveform. Specifically, the ear with a better waveform and type of transtympanic electrically evoked ABRs according to the Kumar–Dutt classification was chosen. Informed consent was obtained, and the prognosis was explained to the patient or their parents.
Cochlear implantation surgery was performed by a routine posterior tympanotomy technique around one to two months after transtympanic electrically evoked ABR testing, ensuring that the tympanic membrane defect had healed. All patients were implanted with the Cochlear Nucleus® system. Complete electrode insertion was achieved in all cases.
Neural response telemetry
Neural response telemetry was measured using the automatic neural response telemetry mode available in Cochlear Custom Sound™ electrophysiology software. Changes in parameters and advanced neural response telemetry mode were not used in these patients.
Cochlear implant responses
Cochlear Custom Sound electrophysiology software with electrically evoked ABR mode was used to stimulate electrode 1. The stimulation rate was 35 Hz and the pulse width was 25 μs, using basic, alternating and reverse polarity. A GSI Audera machine with surface electrodes was used to record the waveforms. Wave V morphology, latency and repeatability were recorded.
Results
Patient one
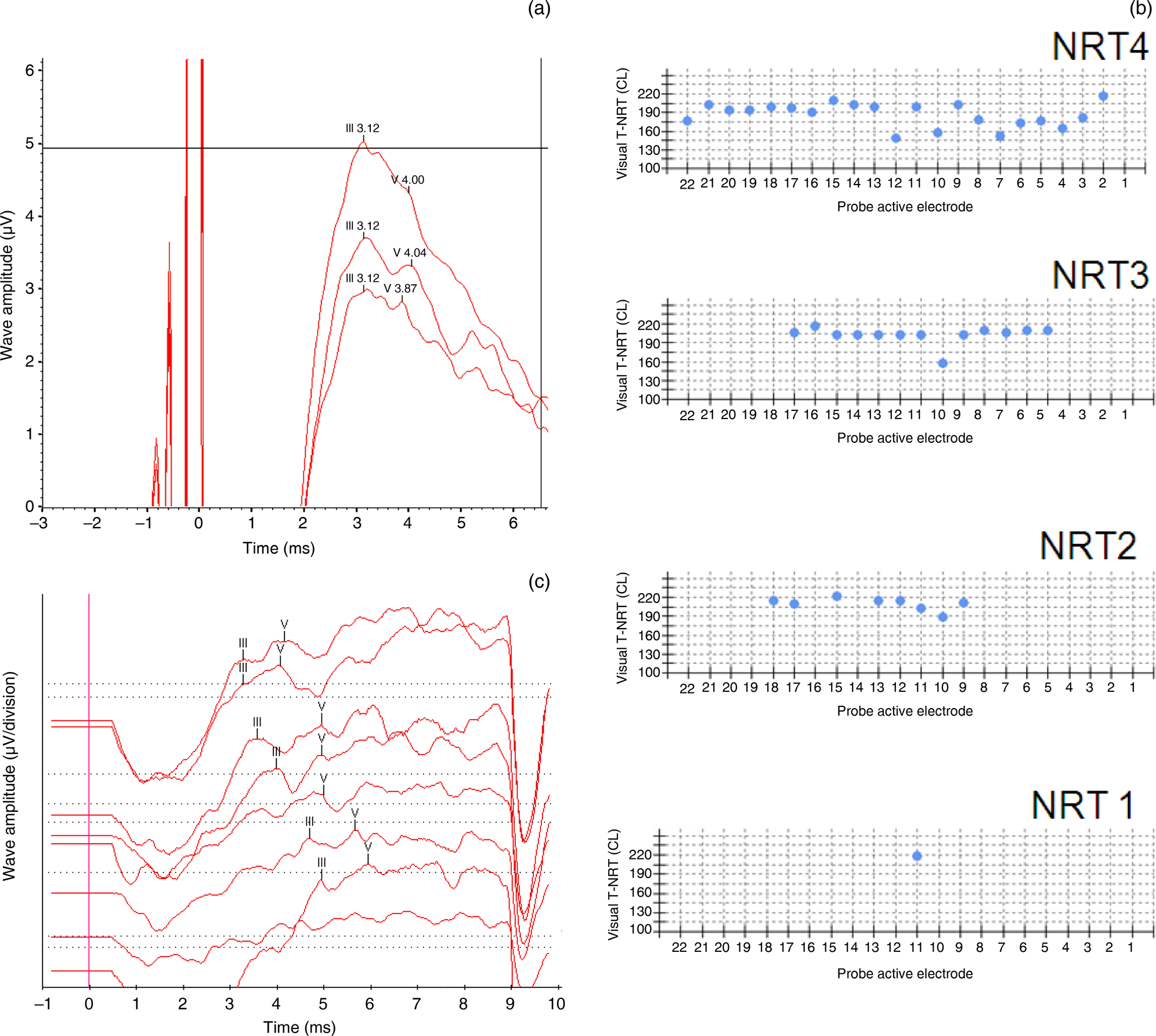
In patient one, type B transtympanic electrically evoked ABRs were obtained in the right ear. No response was obtained in the left ear. Hence, the patient was implanted in the right ear. Intra-operatively, responses were obtained only in electrode 11. Subsequent neural response telemetry measurements performed at 3 months, 6 months and 12 months post switch-on showed progressive improvement in the responses. Cochlear implant electrically evoked ABRs at one year showed a good wave V morphology (Tables 3 and 4, and Figure 1a–c). At the end of one year, a Categories of Auditory Performance score of 6 and a Speech Intelligibility Rating of 5 were achieved.

Fig. 1. Patient one. (a) Pre-operative transtympanic electrically evoked auditory brainstem responses (ABRs) in the right ear – type B response. (b) Neural response telemetry measures intra-operatively (‘NRT1’), at 3 months (‘NRT2’), at 6 months (‘NRT3’) and at 12 months (‘NRT4’) post switch-on. (c) Cochlear implant electrically evoked ABRs at 12 months post switch-on. T-NRT = threshold – neural response telemetry; CL = current level
Table 3. Transtympanic and cochlear implant electrically evoked ABRs, CAP score, and SIR

ABR = auditory brainstem response; CAP = Categories of Auditory Performance; SIR = Speech Intelligibility Rating; pt no. = patient number; pre-op = pre-operative; post-op = post-operative; CL = current level; pw = pulse width
Table 4. Serial neural response telemetry measurements

Pt no. = patient number; intra-op = intra-operatively; NRT = neural response telemetry
Patient two
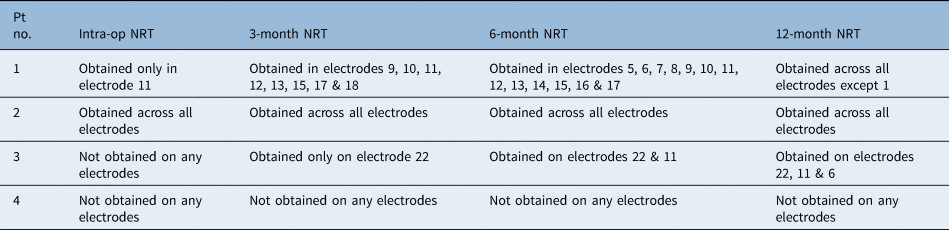
Patient two had robust type A pre-operative transtympanic electrically evoked ABRs (as per the Kumar–Dutt classification) in both ears. The right ear was implanted. Intra-operatively, neural response telemetry measures were obtained in all electrodes. Subsequently, robust neural response telemetry findings were obtained in each electrode in all post-operative mappings. One year after switch-on, cochlear implant electrically evoked ABRs also showed robust wave V morphology (Tables 3 and 4, and Figure 2a–c); moreover, the Categories of Auditory Performance score had improved to 7 and the Speech Intelligibility Rating had improved to 5.
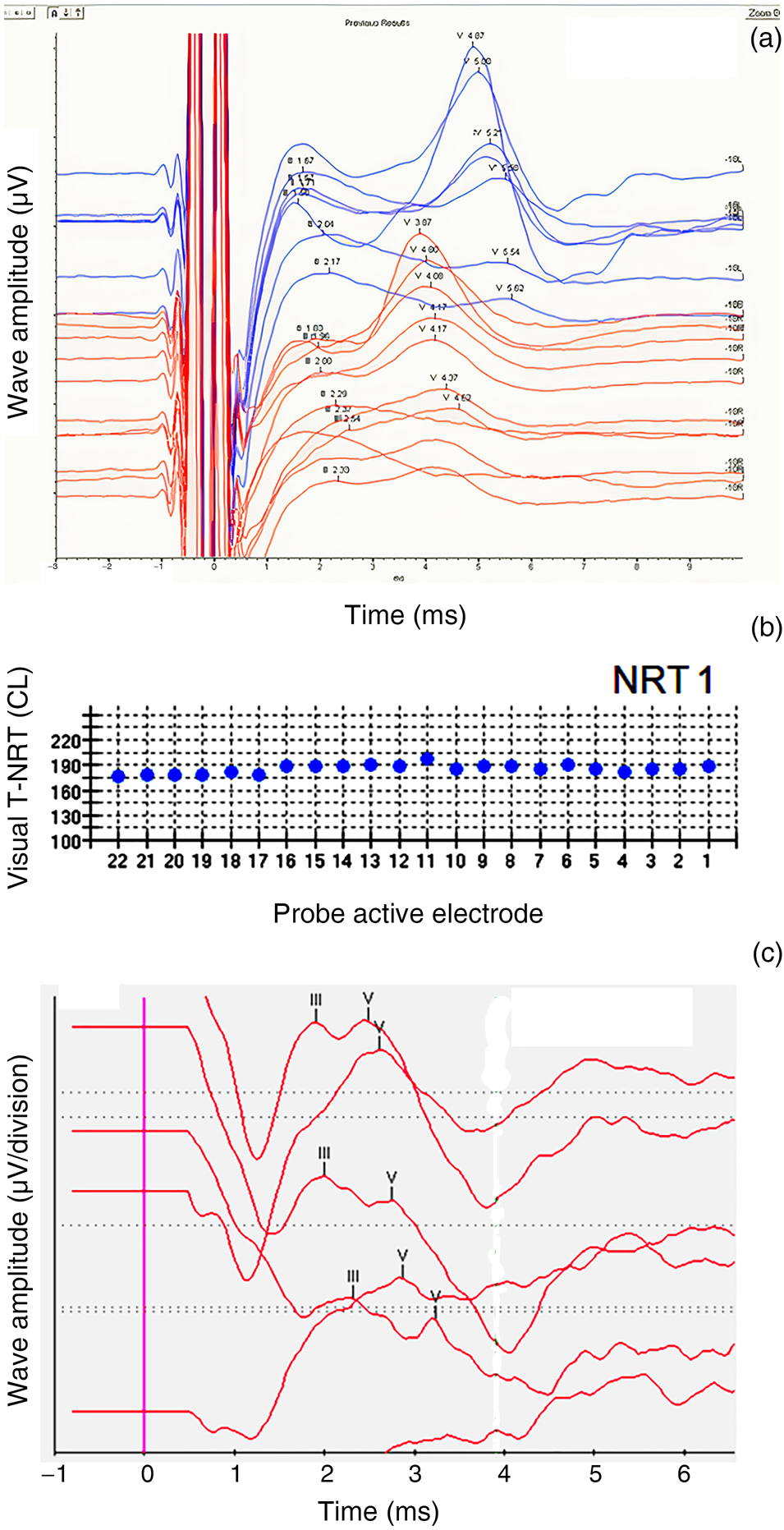
Fig. 2. Patient two. (a) Pre-operative transtympanic electrically evoked auditory brainstem responses (ABRs) in right and left ears – type A response. (b) Neural response telemetry measures intra-operatively (‘NRT1’). (c) Cochlear implant electrically evoked ABRs at 12 months post switch-on. T-NRT = threshold – neural response telemetry; CL = current level; L = left; R = right
Patient three
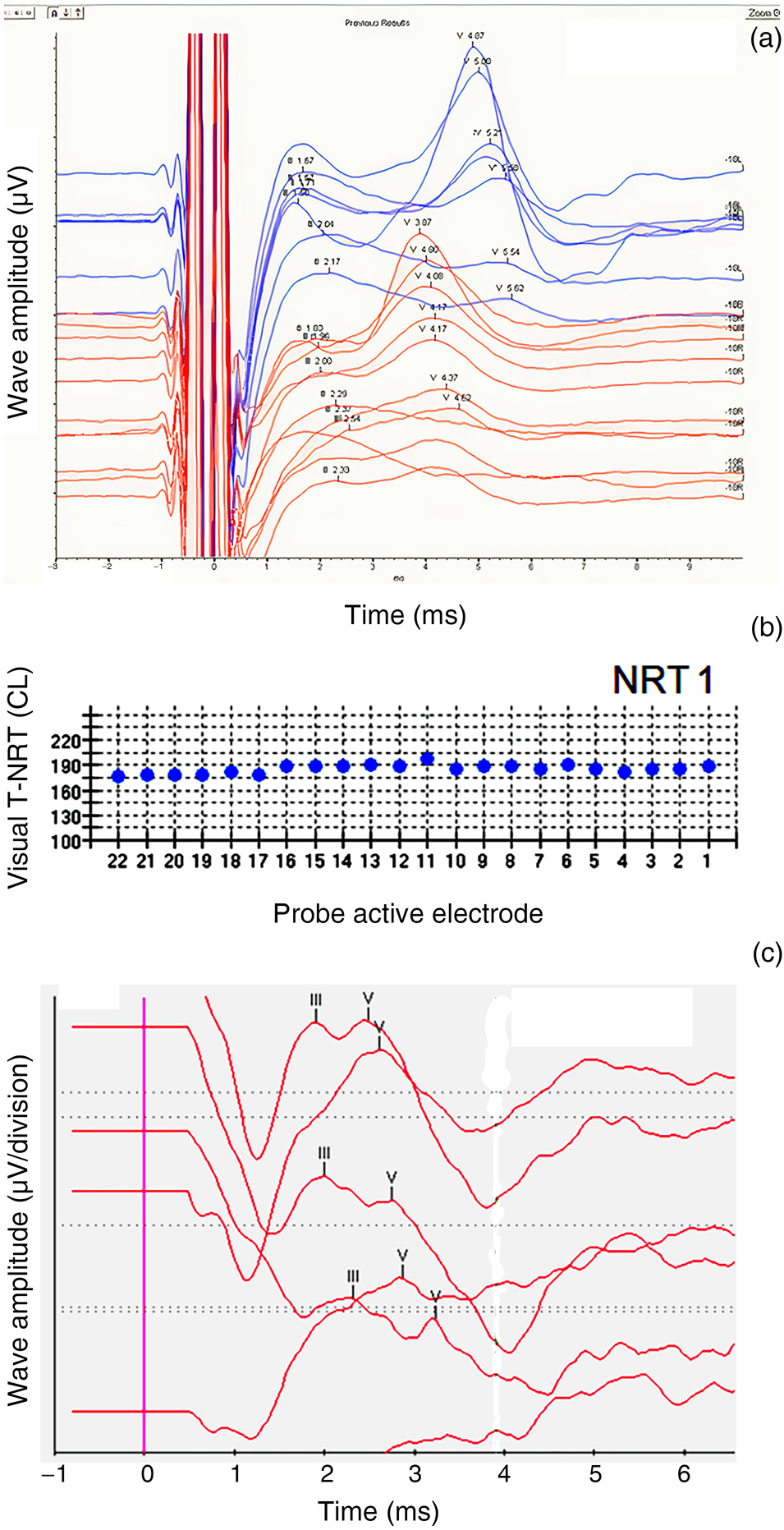
This patient did not have a wave V on pre-operative transtympanic electrically evoked ABRs (Kumar–Dutt type D) in either ear (Figure 3a). With limited expectations and guarded prognosis, cochlear implantation was performed in the right ear. Intra-operatively, neural response telemetry measures were not obtained in any electrode (Table 4). However, at 3 months, 6 months and 12 months, neural responses were obtained in one, two and three electrodes, respectively (Figure 3b). Cochlear implant electrically evoked ABRs measured at one year showed a repeatable wave V (Figure 3c). The patient's Categories of Auditory Performance score improved to 5 and her Speech Intelligibility Rating improved to 4 after one year of implant use.

Fig. 3. Patient three. (a) Pre-operative transtympanic electrically evoked auditory brainstem responses (ABRs) in the right ear – type D response. (b) Neural response telemetry measures intra-operatively (‘NRT1’), at 3 months (‘NRT2’), at 6 months (‘NRT3’) and at 12 months (‘NRT4’) post switch-on. (c) Cochlear implant electrically evoked ABRs at 12 months post switch-on. T-NRT = threshold – neural response telemetry; CL = current level; L = left
Patient four
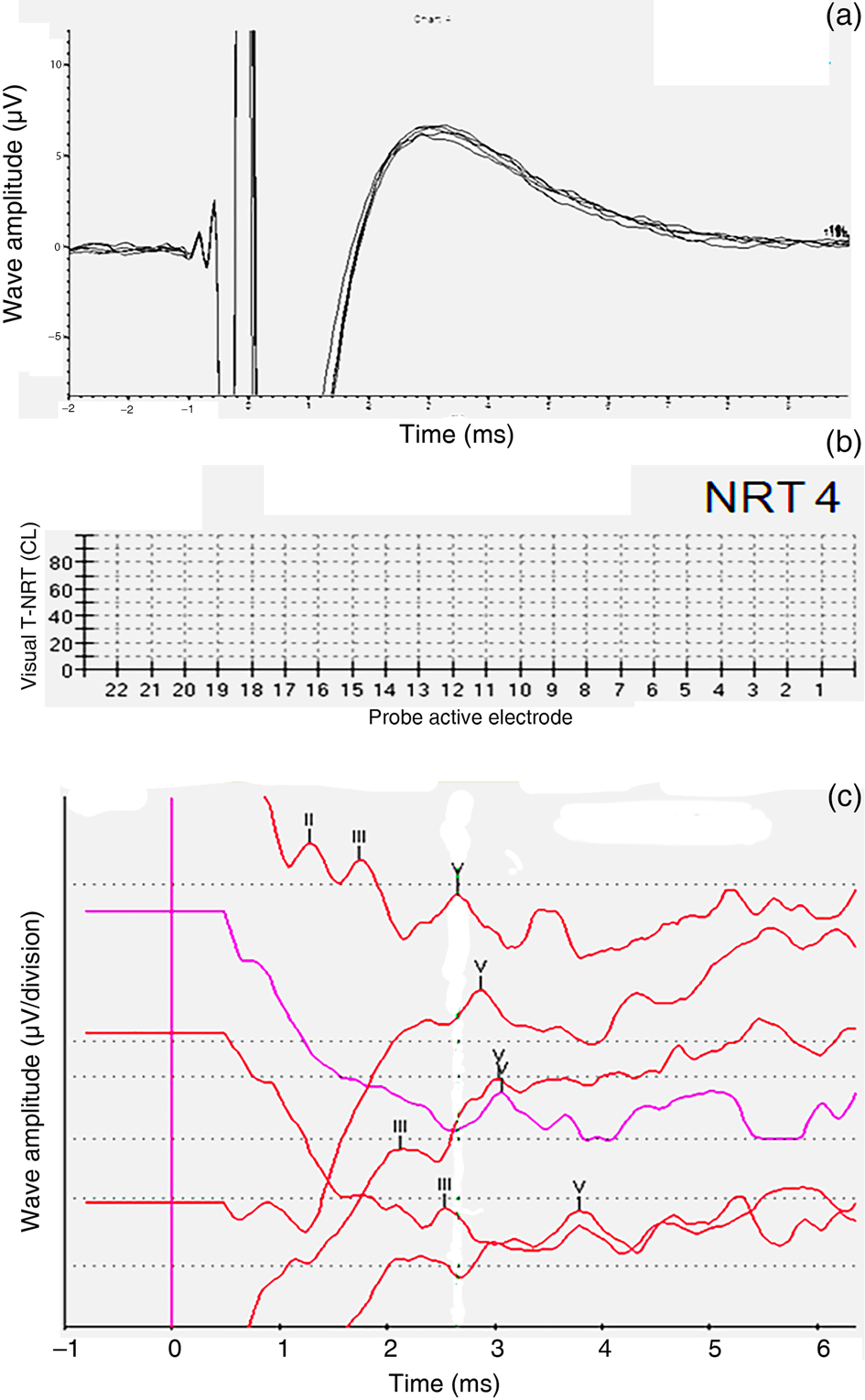
Patient four was a case of post-lingual progressive hearing loss. Transtympanic electrically evoked ABRs showed no identifiable wave V (Kumar–Dutt type D) (Figure 4a). With informed consent concerning limited expectations and poor prognosis, a decision was taken to perform cochlear implantation in the right ear. No neural response telemetry measures were obtained intra-operatively or even at 12 months post switch-on (Figure 4b and Table 4). However, cochlear implant electrically evoked ABRs were obtained at 12 months post switch-on (Figure 4c), and subsequently a Categories of Auditory Performance score of 4 and a Speech Intelligibility Rating of 4 were attained.

Fig. 4. Patient four. (a) Pre-operative transtympanic electrically evoked auditory brainstem responses (ABRs) in the right ear – type D response. (b) Neural response telemetry measures at 12 months post switch-on (‘NRT4’). (c) Cochlear implant electrically evoked ABRs at 12 months post switch-on. T-NRT = threshold – neural response telemetry; CL = current level; L = left
Discussion
Audiological management and speech and language intervention for patients with auditory neuropathy spectrum disorder are challenging. As the developmental consequences of auditory neuropathy spectrum disorder cannot be predicted and the disorder is unique in every patient, a special rehabilitative, patient-oriented approach is necessary. Management aims to restore the ‘synchrony’ of the action potentials in the auditory pathway.
When compared with acoustic stimulation, most patients produce normal ABRs following electrical stimulation with cochlear implantation, suggesting that a larger number of neural elements contribute to the electrical excitability. This could be the result of bypassing the peripheral abnormality and of direct stimulation of the spiral ganglion and/or first node of Ranvier by the cochlear implant, or an improvement in the synchrony of neural firing. Hence, cochlear implants offer a viable means of improving functional hearing and are currently the most promising and established form of intervention for auditory neuropathy spectrum disorder.Reference Starr, Rance, Aminoff, Boller and Swaab8 Previous studies have demonstrated improved audiological and speech perception performance with cochlear implantation.Reference Mason, De Michele, Stevens, Ruth and Hashisaki23–Reference Peterson, Shallop, Driscoll, Breneman, Babb and Stoeckel25 However, the behavioural outcome of these patients following cochlear implantation is often unpredictable because of the varied nature of the disease in different individuals.Reference Harrison, Gordon, Papsin, Negandhi and James26
Different pre-operative and intra-operative tests have been proposed to assess the functionality of the cochlear nerve, such as round window stimulation and transtympanic electrically evoked ABRs.Reference Pau, Gibson and Sanli21 Pre-operative transtympanic electrically evoked ABRs have been used as part of the pre-operative test battery to identify the location of the abnormality in auditory neuropathy spectrum disorder. However, there is a paucity of literature demonstrating the use of transtympanic electrically evoked ABRs in predicting the effects of cochlear implantation on speech and language development. Furthermore, very few attempts have been made to objectively demonstrate the response to cochlear implantation in auditory neuropathy spectrum disorder.
Kileny and Zwolan studied 59 patients aged 10–60 months, who had received cochlear implantation (for bilateral profound sensorineural hearing loss), using peri-operative transtympanic electrically evoked ABRs.Reference Kileny and Zwolan17 They concluded that the procedure aids selection of the ear for implantation and helps to avoid placement of the implant in a non-excitable ear by providing quantitative information regarding residual auditory function.
Gardner-Belly et al. described a pre-operative test battery consisting of electrocochleography, and acoustic ABR and electrical ABR testing in 246 paediatric patients.Reference Gardner-Berry, Gibson and Sanli18 Their classification of the disorders into sensory hearing loss (primary cochlear pathology), auditory dys-synchrony (abnormal or absent acoustic ABR with normal electrical ABR), auditory neuropathy (absent acoustic ABR and delayed wave V on electrical ABR) and brain stem auditory neuropathy (both acoustic and electrical ABR absent) is based on the results of the pre-operative tests. They concluded that patients with sensory hearing loss and auditory dys-synchrony have better outcomes with cochlear implantation than patients with auditory neuropathy and brain stem auditory neuropathy.Reference Gardner-Berry, Gibson and Sanli18 However, electrical ABR testing had been used as part of the pre-operative test battery, and its direct correlation with the post-operative results was not discussed.
Gibson and Sanli considered the utility of round window electrocochleography and electrically evoked ABRs in predicting the outcome following cochlear implant surgery in ears affected by auditory neuropathy.Reference Gibson and Sanli27 They concluded that in auditory neuropathy spectrum disorder, the presence of normal electrically evoked ABRs may indicate a significantly better outcome after cochlear implant surgery than for ears with abnormal electrically evoked ABRs.
Al Shaikh et al. reported significant improvements in the auditory skills and language development of patients with auditory neuropathy spectrum disorder following intervention by cochlear implantation.Reference Al Shaikh, Eldin and Abusetta28 The parameters assessed were the Auditory Skills Checklist and the Arabic language test (receptive, expressive and total language quotients). They compared these findings with those of patients with sensorineural hearing loss who had also undergone cochlear implantation and found that the results were comparable in the two groups. However, no objective evidence of improvement was demonstrated in terms of neural response telemetry measures and electrically evoked ABR wave V latency.Reference Al Shaikh, Eldin and Abusetta28
A study was carried out in the Sydney Cochlear Implant Centre to evaluate the longitudinal outcomes of early implantation in auditory neuropathy spectrum disorder children with thin or hypoplastic cochlear nerves.Reference Chisholm, Gibbons, Psarros, Bate and Gardner-Berry29 The study reported the speech perception and speech and language outcomes of five children who received cochlear implants. Two implantees had no evidence of auditory brainstem activity with the cochlear implant, two had activity only in a few electrodes, and only one had clear electrically evoked ABRs with the cochlear implant. Four children achieved auditory integration within the first year of cochlear implant use, whilst one required several years after cochlear implantation to achieve integration. Integration gradually improved over the years in all five patients.Reference Chisholm, Gibbons, Psarros, Bate and Gardner-Berry29
Jeon et al. retrospectively analysed 11 auditory neuropathy spectrum disorder patients who underwent cochlear implantation and compared them with 9 control subjects with sensorineural hearing loss who did not have neural pathology.Reference Jeon, Bae, Song, Noh, Choi and Choi30 The electrically evoked ABR thresholds of the auditory neuropathy spectrum disorder patients were almost within the values of the disease controls. However, the wave V latency displayed variable lengths and the amplitude showed a wider distribution compared with the values of the disease controls. The data suggested that all auditory neuropathy spectrum disorder patients require cochlear implantation and that electrically evoked ABR results can help establish realistic expectations about future performance. Even if electrical stimulation fails to generate a sufficiently synchronised signal for eliciting electrically evoked ABRs, cochlear implantation provides at least partial, measurable auditory benefit in auditory neuropathy spectrum disorder.Reference Jeon, Bae, Song, Noh, Choi and Choi30
Although previous studies have shown demonstrable speech and language benefits from cochlear implantation in auditory neuropathy spectrum disorder patients, pre-operative prediction of cochlear implantation outcome has not been discussed. Pre-operative transtympanic electrically evoked ABR has never been directly used to correlate with the post-operative results.
In our study, the first patient showed type B transtympanic electrically evoked ABR. Even though the post-operative neural response telemetry measures were obtained in very few electrodes initially, the number of electrodes becoming stimulated increased at subsequent evaluations. Cochlear implant electrically evoked ABRs measured one year after implantation showed a robust wave V, indicating an extremely favourable response. The second patient had a type A transtympanic electrically evoked ABR, and robust neural response telemetry responses were obtained across all electrodes. The post-operative cochlear implant electrically evoked ABRs also showed a robust repeatable wave V, with latencies between 2.5 and 3.2 ms. Subjectively, this child showed marked development in speech and language, and is currently multilingual.
In the third patient, transtympanic electrically evoked ABRs showed no wave V and the intra-operative neural response telemetry showed responses only in one electrode. With continuous stimulation and therapy, the number of electrodes showing responses gradually increased, and the cochlear implant electrically evoked ABRs measured at one year after switch-on showed a fair wave V morphology. Our findings were consistent with the findings of Chisholm et al., who also found improvement in responses with continuous stimulation.Reference Chisholm, Gibbons, Psarros, Bate and Gardner-Berry29
The fourth patient, who had post-lingual progressive hearing loss, had a very guarded prognosis. The absence of transtympanic electrically evoked ABRs and neural response telemetry were also suggestive of this. Despite poor objective evidence of improvement following implantation, the patient had considerable subjective benefit. These findings were consistent with the study by Cinar et al., which found some discrepancies between the electrophysiology results and actual hearing ability.Reference Cinar, Yarali, Atay, Bajin, Sennaroglu and Sennaroglu31 A large proportion of neurons are required to be activated simultaneously for electrically evoked ABRs, whereas for actual hearing not as much simultaneous activation may be needed. This may be the reason why the patient showed no objective evidence of improvement but demonstrated a fairly good subjective response.Reference Cinar, Yarali, Atay, Bajin, Sennaroglu and Sennaroglu31
• Treatment of auditory neuropathy spectrum disorders is challenging
• Cochlear implantation is an established treatment for auditory neuropathy spectrum disorders
• Transtympanic electrically evoked auditory brainstem response testing is a diagnostic measure used to determine excitability of cochlear neural elements
• Such pre-operative responses aid inferences regarding cochlear implantation outcomes in auditory neuropathy spectrum disorder patients
• Patients with better waveforms of said responses have better speech perception and audiological performance after cochlear implantation
• Serial neural response telemetry measurements help monitor the progress of auditory pathways after implantation in auditory neuropathy spectrum disorder patients
The two patients with better types of transtympanic electrically evoked ABRs (types A and B) scored better on Categories of Auditory Performance and Speech Intelligibility Ratings. The patient with a robust wave V (type A transtympanic electrically evoked ABRs) can use the telephone with a familiar talker (Categories of Auditory Performance score of 7) and has speech that is intelligible to all listeners (Speech Intelligibility Rating of 5). On the other hand, two patients without a measurable wave V (type D transtympanic electrically evoked ABRs) and neural response telemetry measures scored relatively lower on Categories of Auditory Performance and Speech Intelligibility Rating. These results suggest that electrical stimulation with cochlear implantation may facilitate the delivery of some sound information to the auditory cortex and that transtympanic electrically evoked ABRs help the clinician to establish some expectations about future performance.
Conclusion
Measuring neural response telemetry and electrically evoked ABRs at different intervals can provide information on the changes or improvements in the auditory pathway. In three out of four patients with auditory neuropathy spectrum disorder, the neural response telemetry measurements were obtained on three or more electrodes within the first year of implant use. Two patients showed changes starting from ‘no neural response telemetry’ to ‘presence of neural response telemetry’ to ‘obtaining neural response telemetry on more electrodes’. In one patient, the neural response telemetry measurements were obtained across all electrodes from intra-operative testing; however, in another patient, no neural response telemetry measures were obtained on any electrode, even after one year of implant use.
Two patients had no peak V on transtympanic electrically evoked ABR measurements; however, a repeatable peak V was obtained in out-patient cochlear implant electrically evoked ABR testing one year after implantation. The other two patients, who had better waveforms on transtympanic electrically evoked ABR testing, showed improvements in the latency of responses after implantation over the one-year time period. Regardless, we make no direct comparisons between the transtympanic electrically evoked ABRs and cochlear implant electrically evoked ABRs because of the variable parameters and obvious differences in electrode position (extracochlear vs intracochlear). A practical response classification of transtympanic electrically evoked ABRs also helps to predict excitability and hence prognosis of a cochlear implantation.
Although there are varied results with cochlear implantation, improvements in electrically evoked ABRs and neural response telemetry over time with cochlear implant use indicate that a patient with auditory neuropathy spectrum disorder can show significant benefits with electrical stimulation in terms of audiological and speech perception performance. This also provides an objective way to monitor changes and progress in the auditory pathway after cochlear implantation.
Pre-operative transtympanic electrically evoked ABRs are one of the prognostic indicators of cochlear implantation outcome in auditory neuropathy spectrum disorder patients. These responses guide the clinician to give appropriate counselling regarding prognosis post implantation. It can be assumed, based on our results, that patients with type A transtympanic electrically evoked ABRs show better post-operative improvements in auditory performance, electrical excitability and speech perception scores than patients with other types. However, adequate patient numbers would be useful for performing statistical correlations and analysis of the role of transtympanic electrically evoked ABRs and their relation with post-operative outcomes.
Acknowledgements
We acknowledge Cochlear Corporation's support with the ‘implant in a box’. We also acknowledge Professor Halit Sanli from Sydney for supplying us with the ‘golf club’ electrodes.
Competing interests
None declared