INTRODUCTION
Thalassinideans are burrowing benthic decapods, with more than 95% of species inhabiting shallow waters (0 to 200 m) in marine and estuarine environments (Dworschak, Reference Dworschak2005). These organisms exert considerable influence over the structure of benthic communities via their ability to bioturbate the sediments (Kinoshita et al., Reference Kinoshita, Nakayama and Furota2003), with effects on the infauna and seagrasses in coastal environments (Berkenbusch et al., Reference Berkenbusch, Rowden and Myers2007) and some species are considered pests in oyster aquaculture, reducing the stability of the bottom substrate where oysters are raised (Dumbauld et al., Reference Dumbauld, Booth, Cheney, Suhrbier and Beltran2006). Moreover, species of commensal invertebrates are often associated with their burrows, such as shrimp from the family Alpheidae (Anker et al., Reference Anker, Jeng and Chan2001) and larval phoronid primarily (Santagata, Reference Santagata2004).
The infraorder Thalassinidea has been split in two infraorders, Axiidea de Saint Laurent, Reference Saint Laurent1979, that includes Lepidophthalmus siriboia Felder and Rodrigues, Reference Felder and Rodrigues1993, and Gebiidea de Saint Laurent, Reference Saint Laurent1979, that includes Upogebia vasquezi Ngoc-Ho, Reference Ngoc-Ho1989, with a total of 615 thalassinidean species already described (De Grave et al., Reference De Grave, Pentcheff, Ahyong, Chan, Crandall, Dworschak, Felder, Feldmann, Fransen, Goulding, Lemaitre, Low, Martin, Ng, Schweitzer, Tan, Tshudy and Wetzer2009), distributed along a latitudinal gradient, less diverse at high latitudes and more frequent at low latitudes. Thirty-six per cent of the species are concentrated in the western Indo-Pacific Ocean and 22% are found in the south-eastern Atlantic (Dworschak, Reference Dworschak2005), and 43 thalassinidean species are reported for the coast of Brazil (Melo, Reference Melo1999; Nucci & Melo, Reference Nucci and Melo2001).
Many species have been exploited for use as live bait in artisanal and recreational fishing in diverse locations (Pezzuto, Reference Pezzuto1998; Hodgson et al., Reference Hodgson, Allanson and Cretchley2000; Souza & Borzone, Reference Souza and Borzone2003; Contessa & Bird, Reference Contessa and Bird2004; Botter-Carvalho et al., Reference Botter-Carvalho, Santos and Carvalho2007), which could lead to the overexploitation of some groups. Moreover, the disturbance created by both the bait-pumping and associated trampling of adjacent mudflat harvesting shrimp, may alter the habitat and influence resident communities (Contessa & Bird, Reference Contessa and Bird2004).
Despite their ecological importance, little is known about the biology of many thalassinidean species (Candisani et al., Reference Candisani, Sumida and Pires-Vanin2001), primarily because of their cryptic lifestyle and difficulties in capturing specimens (Rodrigues, Reference Rodrigues1976; Coelho et al., Reference Coelho, Cooper and Rodrigues2000). In relation to larval development of thalassinidean species, according to Pohle et al. (Reference Pohle, Santana, Jansen and Greenlaw2011) larval information is available for about one-eighth of the species and one-quarter of known genera. Therefore, studies on the population and reproductive biology of these species, such as those previously carried out by Rodrigues (Reference Rodrigues1976), Dworschak (Reference Dworschak1988), Tamaki & Ingole (Reference Tamaki and Ingole1993), Dumbauld et al. (Reference Dumbauld, Armstrong and Feldman1996), Nates & Felder (Reference Nates and Felder1999), Berkenbusch & Rowden (Reference Berkenbusch and Rowden2000), Tamaki & Miyabe (Reference Tamaki and Miyabe2000), Candisani et al. (Reference Candisani, Sumida and Pires-Vanin2001), Kinoshita et al. (Reference Kinoshita, Nakayama and Furota2003), Botter-Carvalho et al. (Reference Botter-Carvalho, Santos and Carvalho2007) and Rotherham & West (Reference Rotherham and West2009) are important to understand the lifecycle of thalassinideans and the management of this group.
Lepidophthalmus siriboia (Callianassidae) and Upogebia vasquezi (Upogebiidae) are found on the north-eastern coast of the State of Pará (northern Brazil) in the present study. Descriptions of the larval morphology of L. siriboia are available (Abrunhosa et al., Reference Abrunhosa, Pires, Lima and Coelho-Filho2005), but there are no ecological studies on the larval and adult forms of these species in the equatorial region. The aim of the present study was to determine the reproductive period of thalassinidean species in the Marapanim River estuary in the State of Pará (northern Brazil), using data on the density of larval and adult forms.
MATERIALS AND METHODS
Collection of juvenile and adult thalassinideans
Adult shrimp sampling was carried out monthly from August 2006 to July 2007, perpendicular to the shoreline together with sediment from bare sand and between boulders from the intertidal region. Four sites were established (two on each bank of the Marapanim River, east and west) in two areas comprising the upper and lower portion of the mesolittoral region. In each site, there were three replicates of 0.5 m2 quadrat placed randomly, totalling 16 monthly samples (4 sites (A1 and A2 on western bank; B1 and B2 on eastern bank) × 2 microhabitats (bare sand and between boulders) × 2 areas (upper and lower portion of the mesolittoral region) × 12 months), totalling 192 samples, with three subsamples each (Figure 1).

Fig. 1. Geographical location of study area with indication of sampling sites of adult thalassinideans in the Marapanim River estuary (Pará, northern Brazil), August 2006 to July 2007; A1 and A2, western bank; B1 and B2, eastern bank; detail in upper left corner: schematic of sampling location in intertidal regions.
Subsamples were demarcated with a polyvinyl chloride (PVC) quadrat (0.5 m in length by 0.5 m in width). Burrowing shrimp were captured by digging in each quadrat, with a PVC suction tube (‘yabby pump’), with 0.9 m in length and 0.05 m in diameter. The five first centimetres of the substrate were collected following the removal of rock fragments. Samples were sieved over a 3 mm mesh and washed in running water from the estuary for the separation of the organisms and retained shrimp were stored in labelled recipients, which were initially kept in ice and subsequently fixed in 70% alcohol until analysis.
Aliquots of water were removed from the burrows with a syringe (3 ml), for the determination of salinity using an optical refractometer. Data on total and mean monthly precipitation were obtained from the Brazilian National Water Agency (Agência Nacional de Águas, 2007). All material was identified at the species level using identification keys from studies carried out by Melo (Reference Melo1999) and Rodrigues & Pezzuto (Reference Rodrigues, Pezzuto, Buckup and Bond-Buckup1999) at the Laboratory of Fish Biology and Management of Aquatic Resources of the Universidade Federal do Pará (Brazil).
The total length (TL, from the tip of the rostrum to the posterior margin of the telson) measure was taken using a digital caliper (nearest 0.01 mm) and the density was expressed as number of individuals/1002. The Kruskal–Wallis test was used to compare mean values of the abiotic factors (temperature, salinity and pH) between months. Spearman's correlation coefficient was used to determine associations between these factors and density of thalassinidean species.
Collection of thalassinidean larvae
Zooplankton samples were collected from six sites distributed in two areas (A and B) in shallow waters (bank of the main channel) of the estuary (A1: 0°38′12″S and 47°38′74″W; A2: 0°40′35″S and 47°38′31″W; A3: 0°42′38″S and 47°41′23″W; B1: 0°36′14″S and 47°35′15″W; B2: 0°40′35″S and 47°36′29″W; B3: 0°43′43″S and 47°39′35″W).
Area A corresponded to the western bank of the Marapanim River, on which the city of Marapanim and the fishing communities of Araticum, Aracumirim and Alegria are located. Area B corresponded to the eastern bank of the river, which has virtually no urban communities. Sites A1, A2 and A3 were aligned with the position of Sites B1, B2 and B3 on the opposite margin of the estuary, except when sand banks or rocks impeded the exact alignment of sites. The establishment of these sites was based on the gradient of salinity in the estuary, considering three zones: Zone I (A1 + B1), nearest to the open sea; Zone II (A2 + B2), intermediate; and Zone III (A3 + B3), innermost portion of the estuary with lowest salinity. Three distinct climatic periods were considered: dry season (August to December), transition periods (January, June and July) and rainy season (February to May).
Twelve monthly field expeditions were carried out between August 2006 and July 2007, encompassing the characteristics of the dry season, transition periods and rainy season. Zooplankton sampling was performed during the daytime outgoing tide, totalling 72 samples (6 sites × 12 months), with two replicates per site.
Thalassinidean larvae were collected in horizontal neuston hauls. Sampling was carried out for three minutes at a velocity of approximately one to 1.5 knots using conical–cylindrical plankton net with a mesh size of 200 µm. A previously calibrated Hydrobios flow meter was coupled to the mouth of the net to calculate the volume of water filtered during the sampling. Samples were fixed in 4% buffered formaldehyde. The following abiotic factors were also determined during the sampling using a multi-parameter YSI analyser: water temperature (°C), hydrogen potential (pH) and salinity.
In the laboratory, zooplankton samples were divided into smaller aliquots with a Folsom Plankton Splitter. A volume of 250 ml was defined for sorting and identification of thalassinidean larvae. The larvae were analysed using a Zeiss optical stereomicroscope and Leica optical microscope with a micrometric grid. Organisms were identified to the lowest possible taxa, based on descriptions contained in previous studies (Sandifer, Reference Sandifer1973; Ngoc-Ho, Reference Ngoc-Ho1981; Nates et al., Reference Nates, Felder and Lemaitre1997; Strasser & Felder, Reference Strasser and Felder1999; Santos & González-Gordillo, Reference Santos and González-Gordillo2004; Abrunhosa et al., Reference Abrunhosa, Pires, Lima and Coelho-Filho2005). The megalops and juveniles of L. siriboia collected in the zooplankton samples were identified according to Abrunhosa et al. (Reference Abrunhosa, Pires, Lima and Coelho-Filho2005).
RESULTS
Temperature was higher between August and December, reaching its lowest value in February (27.5°C) and rising once again from March to July. The pH ranged from 7.5 to 9.0, with the highest values recorded from January to March. The salinity of the water and burrows was higher in drier months (August to December), with an intermediate value in January and lower values in more rainy months (February to July) (Figure 2). Although the mean temperature throughout the study was similar, the variation in this factor was significant between climatic periods: dry season—August to December, transition periods—January, June and July, and rainy season—February to May (H = 46.5; P < 0.0001). Salinity and pH also varied significantly (H = 117.5; P = 0.03 and H = 7.1; P < 0.0001, respectively).

Fig. 2. Abiotic factors recorded monthly during collection of thalassinidean larvae and adults in the Marapanim River estuary (Pará, Brazil) from August 2006 to July 2007; (A) temperature; (B) pH; (C) salinity—recorded concomitantly to collection of larvae; (D) salinity recorded in burrows of adult thalassinideans. KW-H, Kruskal–Wallis test values plus P values. Different letters symbolize significant differences with α = 0.05.
Larvae of Callichirus major (Say, Reference Say1818), Lepidophthalmus siriboia and Upogebia vasquezi were collected, whereas only adult specimens of the latter two species were collected. Table 1 displays the number of individuals and density of each taxon identified according to stage of development. Upogebia vasquezi was the most abundant species in the meroplankton, accounting for 92% of the total, followed by L. siriboia (5%) and C. major (3%). The first zoea stage was the most frequent in the samples of the three thalassinidean species. Upogebia vasquezi was also the dominant species among the adults, accounting for 82% of the samples, whereas L. siriboia accounted for the remaining 18%. The density of thalassinidean larvae was not significantly correlated to abiotic factors (temperature, salinity and pH). The density of adult U. vasquezi was negatively correlated with the salinity recorded in the burrows (Table 2). The mean density of L. siriboia adults was highest in June, whereas mean density of the larval form was highest in February (Figure 3). Upogebia vasquezi adults were abundant from January to April, with the greatest abundance of larvae in December (Figure 3).

Fig. 3. Mean density ± standard error of Lepidophthalmus siriboia and Upogebia vasquezi in larval and adult forms in the Marapanim River estuary (Pará, Brazil) from August 2006 to July 2007.
Table 1. Number of individuals and total density of thalassinidean species collected from the Marapanim River estuary (Pará, Brazil) according to stage of development; N, number of individuals; D, total density expressed in number of larvae/100 m3 for larval stages and number of ind./100 m2 for adults (relative density).

Table 2. Spearman's correlation coefficient (R) for larval density (number of larvae/100 m3) and adult density (number of ind./100 m2) of thalassinidean species in relation to abiotic factors (temperature, pH, salinity and salinity in burrows); *significant results (P<0.05).

The smallest L. siriboia adult measured was a female with 14.31 mm TL and the largest was an ovigerous female with 55.01 mm TL. The smallest U. vasquezi adult measured was a male with 10.96 mm TL and the largest was an ovigerous female with 61.50 mm TL (Figure 4). Lepidophthalmus siriboia megalops and juveniles were collected on zooplankton samples and were not measured. The unidentified sex individuals are probably still juveniles, but they were grouped in ‘others’ (Figure 5).
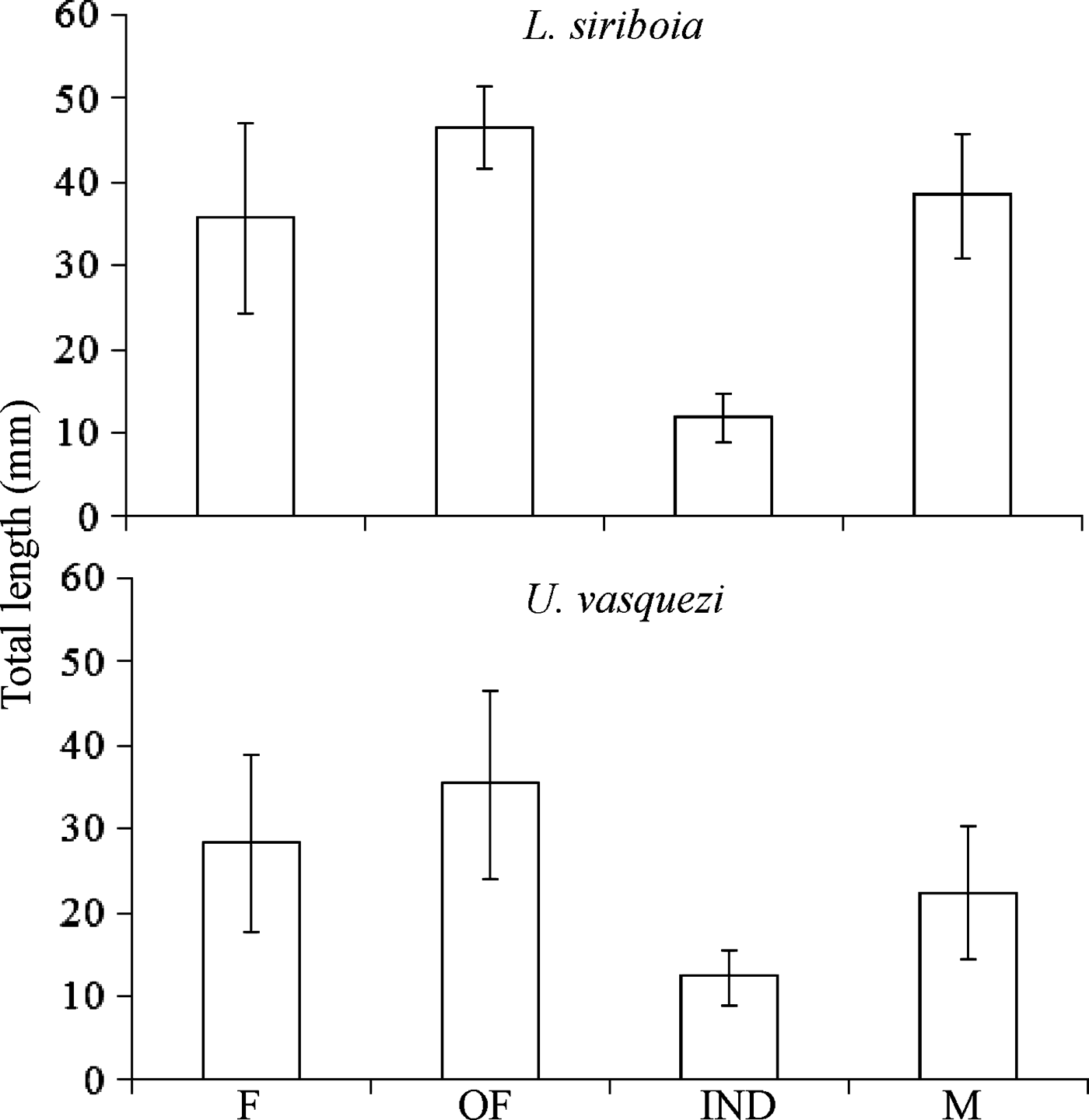
Fig. 4. Mean of total length for females (F), ovigerous females (OF), indeterminate sex (IND) and males (M) of Lepidophthalmus siriboia and Upogebia vasquezi. Vertical bars, standard deviation.

Fig. 5. Relationship between total larval density (number of larvae/100 m3) and frequency (%) of adults collected per month in the Marapanim River estuary (Pará, Brazil) with representation of ovigerous females, non-ovigerous females and others (category includes males and juveniles); (A) Lepidophthalmus siriboia; (B) Upogebia vasquezi.
Peak density of L. siriboia and U. vasquezi larvae occurred in months in which there were ovigerous females or in consecutive months following the presence of ovigerous females in the estuary. Ovigerous L. siriboia females were collected in the months of September, November, February, April, May, June and July, with the greatest frequency registered in June. In the months following those in which ovigerous females were collected (August, October, December and July), there were small peaks in larval density, with the greatest frequency of L. siriboia larvae recorded in February (Figure 5A). Ovigerous U. vasquezi females were collected from December to July, with the exception of April. Larval peaks for this species were recorded in December, January, February and July, coinciding with the months of occurrence of ovigerous females. Small peaks in larval density were also recorded in August, October and November, but no ovigerous females were collected in these months (Figure 5B). Most of the larvae present in zooplankton samples were Stage 1 zoeae and peaks in their abundance coincided with the presence of ovigerous females (February for L. siriboia; January, February and July for U. vasquezi; Figure 6).

Fig. 6. Monthly density (number of larvae/100 m3) of different larval stages of Lepidophthalmus siriboia and Upogebia vasquezi collected in the Marapanim River estuary (Pará, Brazil).
DISCUSSION
Physical factors and adults
A number of authors report the influence of physical factors, such as temperature, over patterns of growth, distribution and abundance among thalassinidean species (Thessalou-Legaki, Reference Thessalou-Legaki1990; Pezzuto, Reference Pezzuto1998; Botter-Carvalho et al., Reference Botter-Carvalho, Santos and Carvalho2007; Rotherham & West, Reference Rotherham and West2009). Other authors also report temperature to be a determinant of the reproductive period among thalassinidean species (Wooldridge & Loubser, Reference Wooldridge and Loubser1996; Botter-Carvalho et al., Reference Botter-Carvalho, Santos and Carvalho2007). Moreover, in the case of Callianassa filholi Milne-Edwards, Reference Milne-Edwards1878, the availability of food sources has been reported to be a determinant of the reproductive period (Berkenbusch & Rowden, Reference Berkenbusch and Rowden2000). In the Marapanim River estuary, temperature exerted no significant influence over the density of L. siriboia and U. vasquezi adults and was not a determinant of reproductive activity for these species.
Physical factors and ovigery
Patterns in the frequency of ovigerous females and abundance of larvae and adults in this study demonstrate that Lepidophthalmus siriboia and Upogebia vasquezi reproduce throughout the year in the estuary of the Marapanim River (Pará, Brazil). The peak of greatest reproductive intensity was in June for L. siriboia, whereas peaks of greater reproductive intensity occurred in January, June and July for U. vasquezi. Rodrigues (Reference Rodrigues1976) collected ovigerous females of Callichirus major Say, Reference Say1818 at Santos Bay, Brazil, during March, June, July, November and December, suggesting reproduction all over the year. Candisani et al. (Reference Candisani, Sumida and Pires-Vanin2001) found berried females of Upogebia noronhensis Fausto-Filho, Reference Fausto-Filho1969 throughout the year, suggesting reproduction is continuous at Ubatuba, south-eastern Brazil.
Physical factors and larvae
In coastal and estuarine environments, decapod larvae are subjected to temporal and spatial variability in salinity, undergoing osmotic stress, which can reduce growth and survival rates (Anger, Reference Anger2001; Torres et al., Reference Torres, Giménez and Anger2002). Since that larval stages of crustaceans species in estuaries, undergo development under this range of environmental conditions, Paula et al. (Reference Paula, Mendes, Paci, McLaughlin, Gherardi and Emmerson2001) studied the temperature and salinity effects on the larval development of Upogebia africana Ortmann, Reference Ortmann and Semon1894, in relation to survival and duration of larval stages. Also Newman et al. (Reference Newman, Papadopoulos, Vorsatz and Wooldridge2006) investigated the influence of temperature on the larval development of two Upogebia species. In the Marapanim River estuary larval density of U. vasquezi was significantly greater in the dry season and transition periods, in which the salinity of the water was greater (mean: 28.5 ± 4.3 in dry season and 18.2 ± 3.3 in transition periods) in comparison to the rainy season (mean: 13.5 ± 3.7), therefore low salinity is not favourable to the development of the larvae of this species. Most of laboratory rearing of Upogebia species larvae occurs in salinity range of 30–35 (Konishi, Reference Konishi1989; Siddiqui & Tirmizi, Reference Siddiqui and Tirmizi1995; Shy & Chan, Reference Shy and Chan1996; Melo & Brossi-Garcia, Reference Melo and Brossi-Garcia2000). However, the significant negative correlation between the density of U. vasquezi adults and salinity in the burrows (R = –0.7; P < 0.05) is an indication that the adults may be more euryhaline than larvae.
Although there were no significant differences in the density of L. siriboia larvae and adults between months and there were also no significant correlation to abiotic factors, the larvae were caught at salinities ranging from 20 to 32, with the occurrence of only three larvae at a salinity of 8. The larval development under laboratory conditions of both L. siriboia (Abrunhosa et al., Reference Abrunhosa, Pires, Lima and Coelho-Filho2005) and U. vasquezi (Oliveira et al., unpublished data) occurs at higher degrees of salinity, as for other thalassinidean species, such as Callianassa tyrrhena (Petagna, Reference Petagna1792), which reaches its maximal development in the laboratory at a salinity of 29 or higher (Thessalou-Legaki, Reference Thessalou-Legaki1990). Moreover, the spawning of callianassid species is also favoured at salinities greater than 20 (Botter-Carvalho et al., Reference Botter-Carvalho, Santos and Carvalho2007).
Decapod larvae are generally present in zooplankton communities of coastal waters throughout the year and their presence is commonly associated with the reproductive period and spawning of adults (Fehlauer & Freire, Reference Fehlauer and Freire2002). In tropical estuaries, the spawning of these crustaceans can occur throughout the year, contrasting with the pattern found in temperate estuaries (Dittel & Epifanio, Reference Dittel and Epifanio1990), in which the majority of decapods release their larvae in a particular period of the year, when environmental conditions are favourable (Gonçalves et al., Reference Gonçalves, Ribeiro and Soares2003). For example, Paula (Reference Paula1987) encountered two peaks of decapod larval abundance at São Torpes bay, south-western Portugal. Moreover, specific taxa may exhibit distinct seasonality.
Species that reproduce in an estuarine environment generally have a complex lifecycle that involves mechanisms of larval retention and/or exportation, depending on the natural habitat of the species in the adult phase (Wooldridge & Loubser, Reference Wooldridge and Loubser1996). In the present study, U. vasquezi and L. siriboia larvae were found at higher salinities, which may indicate that these larvae develop in the open sea and return to the estuary in the final stages of development for settlement. This larval exportation mechanism is very important to the reproductive success for U. vasquezi, as the adults occur in greater abundance at lower salinities, which are incompatible with the larval development of the species. This reproductive strategy has also been reported for U. africana, which reaches its reproductive peak in summer, exporting the hatched eggs to the open sea, with the re-invasion of the post-larvae in the estuarine environment (Wooldridge & Loubser, Reference Wooldridge and Loubser1996). The absence of larval stages Zoea II and Zoea III for L. siriboia in plankton during the present study also supports the hypothesis that these larvae are not retained in the Marapanim River estuary like Petrolisthes armatus (unpublished data), suggesting different mechanisms of larval transportation among sympatric decapods.
In the Marapanim River estuary, C. major larvae were also caught, especially under conditions of high salinity (20 to 33), with only one larva caught at a salinity of 18. Unlike L. siriboia and U. vasquezi, which occurred in both larval and adult forms, no C. major adults were caught. The relatively long duration of the plankton stage of the genus Callichirus contributes to the greater dispersion of larvae. Adult populations are distributed throughout the intertidal open coastline, especially in locations made up mostly of silica sand (Rodrigues & Shimizu, Reference Rodrigues, Shimizu, Absalão and Esteves1997). It is possible that this preference for a silica substrate/sediment on the part of species of Callichirus is not compatible with sedimentological characteristics of the Marapanim River estuary. This would explain the absence of adults in the A1, A2, B1 and B2 samples, as muddy sediments predominate at these sites. Granulometric analysis of the sediment in which the thalassinidean species were collected in the Marapanim River estuary are being carried out in order to elucidate the type of sediment preferred by the species in the region.
There is little knowledge on the dynamics of thalassinidean larvae in the natural environment at the different latitudes at which the species are found, especially in tropical regions, where the greatest number of species from this group occur. Thus, the data presented here make a fundamental contribution to the knowledge on the life history of these species.
ACKNOWLEDGEMENTS
The authors are grateful to the colleagues who helped in the field collections, to Richard Boike for the translation into English and to anonymous referees for their suggestions and contributions toward improving this paper. All experiments conducted in this study complied with current applicable state and federal laws of Brazil (Proc. DIFAP/IBAMA No. 02018.008516/2005-51; number 94-2005). This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) through research project CT-AMAZÔNIA (J.M.M., grant number 553106/2005-8) and through a postgraduate fellowship (D.B.O., grant number 132847/2008-6) and by the Pró-Reitoria de Pesquisa/Universidade Federal do Pará (PROPESP/UFPA) and Fundação de Amparo e Desenvolvimento da Pesquisa (FADESP). The authors thank a referee for critical review of the manuscript and constructive comments.










