Introduction
Coronavirus disease 2019 (COVID-19) was first identified in December 2019, in Wuhan city, China. It likely occurred via zoonotic transmission in a seafood market trading live animals. 1 Following a rigorous investigation, a new viral strain belonging to the family Coronaviridae family was identified as the causative agent. 1 Soon after COVID-19 was pronounced as Public Health Emergency of International Concern (PHEIC) on January 30, 2020, after it spread to 18 countries. 1 To date, 214 countries and territories worldwide have reported over 24 million confirmed cases and 820,000 deaths. While intermediate hosts for SARS-CoV were believed to be Asian palm civets and dromedary camels for MERS-CoV, respectively, pangolins are suspected to be likely intermediate hosts for SARS-CoV-2 but the evidence is not sufficient to prove the link. Reference Guan, Zheng and He2–Reference Lam, Shum and Zhu4
Considering the urgency to identify the host–pathogen mechanisms for COVID-19, we offer important insights into the host immune pathogenesis. Specifically, we focused on the dysfunctional immune response and explore the clinical manifestations to be considered as concerted vaccine efforts are being made.
Relevance of developmental origins of health and disease (DOHAD)
The first 1000 days of life including gestation are critical periods for human development. Infants in their first 2 years of life are vulnerable since they undergo rapid growth and development. While the neurological and linear growth development is compromised chronically following nutritional deficiency, environmental factors contributing to compromised immunity cannot be ignored. Reference Martorell5 Rapid development during the first 2 years of life, if dysregulated, can manifest later in adulthood. Age-dependent immunity has been observed due to the lack of adaptive immunity and differentiated innate immunity in children. Reference Maggini, Pierre and Calder6 The phenomenon is not pathological as environmental priming of the immunological system may be attainable after 2 years of age. Immunological responses are the combination of gene–environment interactions. The developmental origins of the health and disease (DOHaD) hypothesis have gained prominence since the last few decades that correlates adult disease to childhood, or in utero exposures. Reference MacGillivray and Kollmann7 The immunological responses are the prototype example of the DOHaD concept due to the complex gene–environmental foreplay, especially through epigenetic modifications. The differential environmental exposures owing to socio-geographic contributors result in epigenetic reprogramming and lead to variable trained innate immunity. Cell ontogeny in the context of immunology epigenetics may contribute to the global variation of COVID-19 mortality and outcomes. Reference MacGillivray and Kollmann7 As global efforts are being made to optimize vaccines, it is fundamental to carry out regional assessments of population-based immunological responses.
Methodology
This study reviewed databases including MEDLINE, Scopus, CINAHL Plus, and WHO Global Database. No restrictions were applied on the data search and the search encompassed articles from inception until September 1, 2020. We used the following search terms with Boolean operators including “immunology”, “age”, “innate”, “adaptive”, “vaccine”, and “COVID-19”. We did not exclude any type of study and the reference list of included articles was also searched (umbrella review). Journals including NEJM, the Lancet, JAMA, and the BMJ were manually searched for relevant articles. Two investigators (AS, ZS) searched for data from the included studies with a third investigator (NTI) solving any disagreements for the narrative review.
Structure, genome, and viral life cycle
The coronavirus consists of a positive-sense, single-stranded RNA (+ssRNA) molecule. The size of the coronavirus genome ranges between 26,000 and 32,000 bases and it is among the largest known RNA viruses. Reference Gorbalenya, Enjuanes, Ziebuhr and Snijder8 The genome size of SARS-CoV-2 varies from 28.9 kb to 29.9 kb and incorporates up to 9–14 open reading frames (ORFs). Reference Gorbalenya, Enjuanes, Ziebuhr and Snijder8,Reference Khailany, Safdar and Ozaslan9 The gene segments translate ORF1a and ORF1b, constituting two-thirds of the genome length and is present at the 5′end, to produce two overlapping polyproteins, pp1a and pp1ab. Reference Masters, Kuo, Ye, Hurst, Koetzner and Hsue10 Four major structural proteins and different accessory proteins encoded by other ORFs are located at the 3′ end. Structural proteins contribute to the viral assembly and host tropism including spike (S) protein (recognizes host cell receptors), envelope (E) protein (assembles and releases virion), membrane (M) glycoprotein (shapes the virion), and nucleocapsid (N) protein packs the RNA genome. Reference Siu, Teoh and Lo11 The life cycle of SARS-CoV-2 is summarized in Fig. 1.
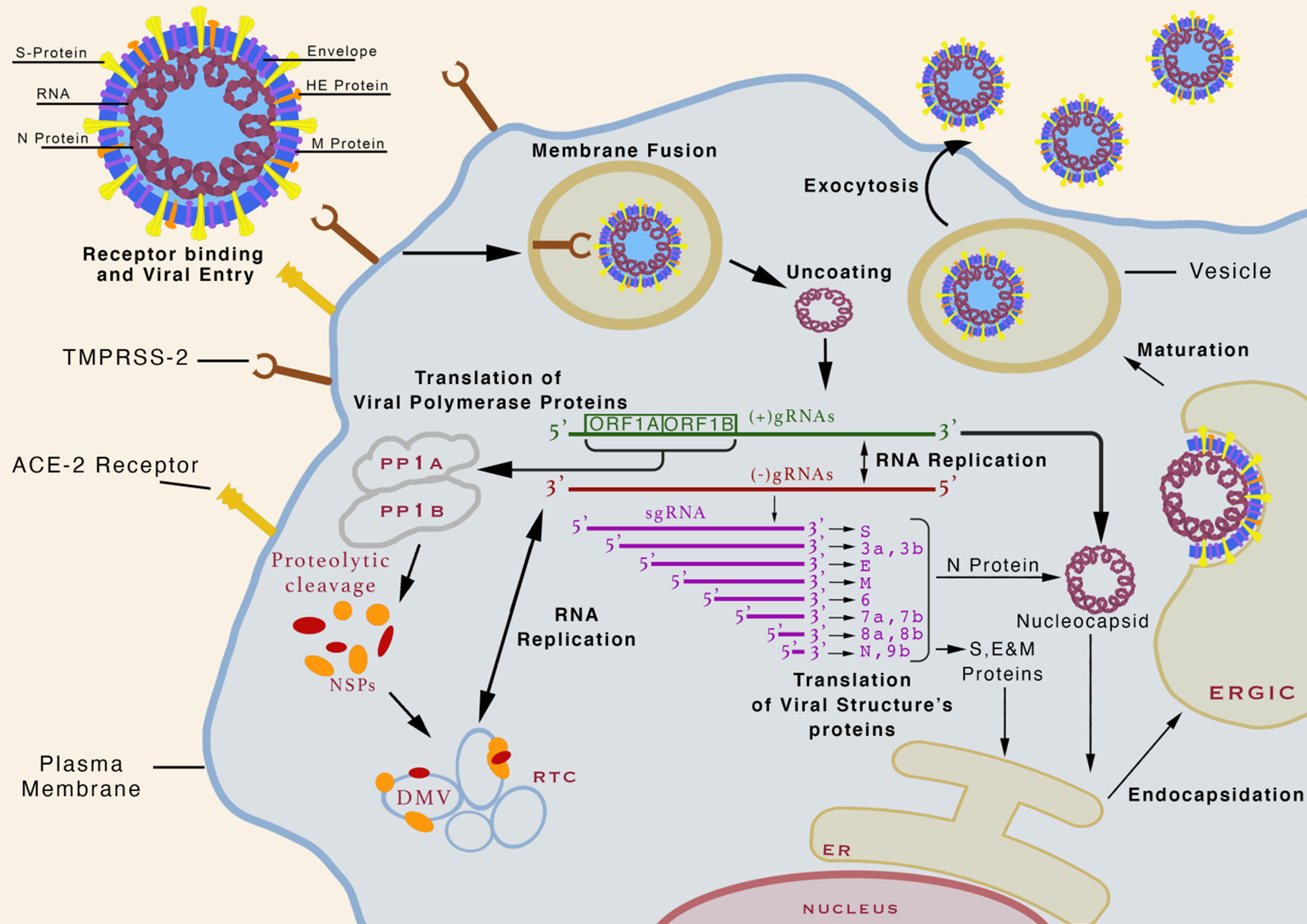
Fig. 1. The life cycle of SARS-CoV-2. The virion binds to its receptor angiotensin-converting enzyme 2 (ACE2) on the cell surface through its spike protein (S) with the help of either cellular transmembrane serine protease 2 (TMPRSS2) or cathepsin L as mediator. Receptor-bound virion particles enter into the cell through endocytosis and ultimately viral envelop fuses with endocytic vesicle membrane releasing viral nucleocapsid inside the cytoplasm. The helical viral nucleocapsid is uncoated and the viral genomic ORFs 1a and 1b encoding the viral replicase complex translates into two polyproteins, pp1a and pp1ab. Proteolysis of pp1a and pp1ab leads to 16 non-structural proteins (NSPs). The NSPs contribute to the formation of a replication–transcription complex (RTC) in double-membrane vesicles (DMV). RTC in DMV mediates positive-sense, negative-sense, subgenomic, and genomic RNA synthesis to promote replication and cellular evasion. The viral genome encodes structural proteins S, E, and M, and other accessory proteins translated by ribosomes attached to the endoplasmic reticulum. Structural protein N is assembled with newly synthesized viral genomic RNA in small vesicles and fuses with virion precursor and is transported to the endoplasmic-reticulum–Golgi intermediate compartment (ERGIC). Following virion encapsidation, budding and maturation occur after which the newly assembled virions are exocytosed to out of host cell. Adapted from. Reference Jiang, Hillyer and Du55
Age-dependent immunological responses
The immune system is derived from the hematopoietic stem cells (HSCs) that differentiate into the innate and adaptive immune systems. Reference Montecino-Rodriguez, Berent-Maoz and Dorshkind12 The immune system undergoes maturation throughout the life due to different environmental stimuli. The innate immune system, formed by the neutrophils, macrophages, and dendritic cells, are the gatekeepers of immunological responses. The adaptive immune system, constituted by the T cells and B cells, is also markedly impaired in the first 2 years of life. Reference Maggini, Pierre and Calder6
Innate immunity in SARS-CoV-2
In adults, the entry of SARS-CoV-2 initiates a local innate immune activation through pattern recognition receptors (PRRs) that identify pathogen-associated molecular patterns (PAMPs) of unique viral components. Reference Akira, Uematsu and Takeuchi13 The subsequent immune response recruits monocytes and macrophages at the site of infection. Reference Guo, Cao and Hong14 The innate immune response dictates the activation of an adaptive immune response. The release of cytokines from immune cells eventually leads to cytokine storm syndrome (CSS), which has been correlated with severe clinical manifestations of COVID-19. The innate immune responses are derived by neutrophils, monocytes, dendritic cells, macrophages, and NK cells as part of the first line of defense. Reference Hato and Dagher15 These cells carry specialized receptors known as PRRs which recognize a specific pattern of protein, carbohydrate moiety present on pathogens known as PAMPs. Reference Akira, Uematsu and Takeuchi13 The identification of PAMPs by PRRs promotes a cascade of events including transcription of pro-inflammatory cytokine genes regulated by the NF-kB pathway. Reference Akira, Uematsu and Takeuchi13 The activation of macrophages releases cytokines that either interfere with viral replication or recruit more macrophages and immune cells at the site of activation. Reference Duque and Descoteaux16 Host innate immune signaling is mainly initiated by toll-like receptors (TLRs), RIG-I-like receptors (RLRs), and NOD-like receptors (NLRs) but other PRRs and free-molecule receptors also have minor contributions. Reference Akira, Uematsu and Takeuchi13 Collectively, intracellular PRRs to PAMPs dictate the immune response of the host to successfully abrogate infections of SARS-CoV-2 and prevent severe disease manifestations. Reference Akira, Uematsu and Takeuchi13
A milder disease has been observed in children and it has been associated with age-dependent immune responses. In neonates and particularly exaggerated preterm infants, neutrophils have reduced efficacy due to poor responses to inflammation, reduced adhesion to endothelial cells, and impaired chemotaxis. Reference Simon, Hollander and McMichael17 Newborn infants also have reduced TLR4 expressions resulting in diminished cytokine responses. Reference Förster-Waldl, Sadeghi and Tamandl18 There is also a lower concentration of dendritic cells of myeloid origin. Consequently, neonates have a higher risk of contracting viral infections due to reduced priming of CD8+ T cells and shift from Th1 to Th17 responses. Reference De Kleer, Willems, Lambrecht and Goriely19 NK cells are critical in limiting viral replication and are regulated by human leukocyte antigen (HLA) genes which are limited in childhood. Reference Ivarsson, Loh and Marquardt20 Overall, the innate immune system in infants is controlled by the alternative and lectin-binding activation pathways.
Adaptive immunity in SARS-CoV-2
The nature of host adaptive immune responses may depend on timing, composition, and magnitude. Three distinct adaptive immune responses have been recognized in SARS-CoV-2 infection in adults. Reference Mathew, Giles and Baxter21 The first immunotype had robust CD4+-specific T cell response with modest activation of CD8+ terminally differentiated effector memory T cells re-expressing CD45RA+ (TEMRA), highly activated or exhausted CD8+ T cells, T-bet+ plasmablasts (PB), and paucity of circulating T follicular helper cells (cTfh). The second immunotype had mainly T-betbright effector-like CD8+ T cell responses along with proliferating Ki67+ PB, memory B cells, and less robust CD4+ T cell activation. The third immunotype had minimal to no immune responses suggesting a failure of immune activation. The first immunotype suggests an association between CD4+ T cell activation with increased severity as observed in COVID-19 patients with organ failure and acute kidney injury. The second immunotype containing CD8+ T cell responses were observed among patients with preexisting immunosuppressive states. The third immunotype did not correlate with disease severity or specific symptoms. Strong PB responses observed in SARS-CoV-2 did not correlate with cTfh activation and suggest T cell-independent B cell responses. Reference Mathew, Giles and Baxter21 SARS-CoV-2-specific antibodies against COVID-19 patients admitted to the ICU were observed in the same cohort suggesting the role of antigen-specific PB responses. Reference Mathew, Giles and Baxter21
In pediatric populations, plausible adaptive immune responses include an antibody or T cell recognition of self-antigens suggesting autoantibodies or viral mimicry of the host. Reference Jiang, Tang and Levin22 Consequently, immune complex deposition across multiple organs results in the activation of inflammatory immune responses. Following inflammatory pathways, viral superantigen sequences further aggravate immune responses in pediatric populations. Underlying genetic association with inositol 1,4,5-triphosphate 3-kinase C (ITPKC) gene, which maintains T cell activity, may amplify COVID-19 severity. Reference Onouchi, Gunji and Burns23
T cell immunity
There is a limited understanding of the T cell immunity in SARS-CoV-2. In adults, combined Th1/Th2 responses are observed with Th1- (IL-1β, IL-2, IFNγ, IP-10, MCP1, TNF) and Th2-mediated (IL-6, IL-10) pro-inflammatory chemokines and cytokines responses in severe COVID-19 patients. Reference Huang, Wang and Li24,Reference Zhang, Zhou and Qiu25 Prior studies have reported the predominance of Th1-mediated CD4+ responses in COVID-19 patients. However, an increase in Th2-specific responses combined with an increase in G-CSF, MIP-1-α provides a mixed picture of Th1/Th2 polarization. A large number of CCR6 + Th17 cells were observed in COVID-19 patients with severe disease, supporting the relevance of Th17 cells in CSS. Reference Xu, Shi and Wang26 It is pertinent to quantify CD4+ and CD8+ T cells specific to the virus. A lack of protective immunity may be due to an insufficient magnitude and timing of CD4+ T cell response leading to sub-optimal priming of CD8+ T cells and neutralizing antibody responses. Reference Crotty27 The combined evidence of clinical lymphopenia, the aberrant response of CD4+ T cells, reduced functional diversity of CD4+ T cells, and subsequent exhaustion of CD8+ T cells in critically ill patients indicate the relevance of local T cell immunity in SARS-CoV-2 infections. Reference Huang, Wang and Li24,Reference Zhang, Zhou and Qiu25 Despite evidence of protective T cell immunity, previous vaccine formulations for SARS-CoV identified TH2-mediated eosinophil infiltration causing immunopathology. Reference Bolles, Deming and Long28
While developmental changes in immunity are age-dependent, young infants have less prominent adaptive immunity. Maternal antibodies in neonates may not act against SARS-CoV-2. Prominent Th1-type responses observed in adults may not be activated in children. With minimal lymphocytopenia in children, less aggressive adaptive immune responses are expected. Neonatal peripheral Foxp3+ CD25+ regulatory T cells (Treg) are strongly self-tolerant and tilt toward Th2 immunity enhanced by epigenetic and dendritic cell activity. γδ T cell receptor (TCR)-positive and innate-like αβ TCR-positive T cells are also present in newborns and infants that promote the release of IFN, mucosal-associated invariant T (MAIT) cells and CXCL8. Overall, the immune responses during the first 2 years of life are characteristic of tolerogenic reactivity with reduced ability to respond to foreign antigens and alloantigens.
B cell immunity
The kinetics of antibody responses to SARS-CoV-2 is understood by the increase in virus-specific IgG and IgM antibody titers within 3 weeks of infection. Reference Long, Liu and Deng29 IgM antibodies are being evaluated for their potential as recent markers of infection. Reference Guo, Ren and Yang30 The IgM antibody titers are detected about 5 days after SARS-CoV-2 infections and may be useful for the confirmation of COVID-19 patients with negative RT-PCR results. Reference Guo, Ren and Yang30 The detection of IgG antibodies against SARS-CoV-2 is associated with viral neutralization and becomes detectable after 8–14 days of infection. Reference Zhao, Yuan and Wang31 The IgG antibodies are associated with protective immunity and are thought to last for about 2–3 months. Reference Long, Tang and Shi32 However, not all IgG antibodies to SARS-CoV-2 are found to be neutralizing. Those found to be most effective for virus neutralization are spike proteins. Reference Wu, Wang and Liu33 Hence, determining total IgG to SARS-CoV-2 may not be reflective of the “protective” status of the host. Similarly, immune-compromised individuals may never seroconvert and others may take longer. Reference Long, Deng and Chen34
Two different types of B cells are recognized in humans, with B1 cells comprising 40% of the total B cells in neonates and infants. B cells in children aged 2 months or lower have decreased somatic hypermutation compared to adults. Additionally, B cells respond to co-receptors of T cells of which neonates have lower expressions. With minimal long-term support of plasmablast survival, IgG antibodies are not effective at maintaining long-term survival even after vaccinations.
In theory, individuals with seroconversion are at lower risk for reinfection. Consequently, the importance of serological testing lies in diagnosing suspected cases and quantifying the temporal duration of protective immunity after infection. The current understanding of the kinetics of antibody responses to SARS-CoV-2 is limited and the varied serological responses in symptomatic versus asymptomatic individuals are required to determine the temporality of antibody-mediated immunity. Developing strong neutralizing antibody responses toward SARS-CoV-2 is an important goal of vaccine development. Reference Burton and Walker35 Identifying seroconversion is relevant among individuals with asymptomatic infections and negative RT-PCR results.
It is particularly important to evaluate potentially protective IgG antibody responses to SARS-CoV-2 in the context of convalescent plasma, which has been authorized for emergency use in COVID-19. SARS-CoV-2-specific IgG antibodies to spike protein in a high dose may be used for treating patients with COVID-19. Studies conducted on the safety of convalescent plasma have shown a severe adverse reaction of <1%. Reference Joyner, Wright and Fairweather36 The efficacy of convalescent plasma studies has shown to be variable possibly, due to the level of pre-screening and selection of the sera used for treating patients. Further, the timing of plasma collection from donors is key for the favorable outcome of treatment. The highest peak of IgG is about 21 days after the initial infection. Reference Long, Liu and Deng29 While immunity is thought to wane 2–3 months after infection, some B memory cells could likely be present afterward. In this context, sero-surveillance studies are important to determine if the population in question has acquired herd immunity or there is any herd immunity at all exists for COVID-19. It is currently unknown what proportion of individuals needs to be protective to impart herd immunity. The antibody response is also important for screening individuals for vaccine trials, return to work, and finish lockdown in certain sensitive areas but the sensitivity and specificity of the test should be reliable to make these decisions. Despite the good specificity of the test, false-positive rates do exist (2.5%–3.4%) for autoantibodies and previous antibodies against the influenza vaccine. Reference Theel, Slev, Wheeler, Couturier, Wong and Kadkhoda37 IgG levels in asymptomatic individuals are lower compared to the acute phase of symptomatic individuals. Asymptomatic individuals also have a rapid decline in IgG responses in the convalescent phase of infection compared to symptomatic individuals. Reference Long, Tang and Shi32
Inflammatory pathogenesis in adult populations
As part of its natural replicative cycle, SARS-CoV-2 is a cytopathic virus that induces the death of cells and tissues. Reference Park, Kwon and Choi38 Epithelial and endothelial cells undergo apoptosis and vascular leakage triggers the rise of cytokines and chemokines to induce an inflammatory state. Reference Yang39 Importantly, pyroptosis is an inflammatory programmed cell death of infected cells via activation of NLP3 inflammasome and release of interleukin-18 (IL-18) and interleukin-1 beta (IL-1β) for active recruitment of immune cells at the site. Reference Yang39 In some people, a dysfunctional immune response is followed by massive immune cell proliferation and overproduction of cytokines resulting in cytokine storm syndrome. Reference Tay, Poh, Rénia, MacAry and Ng40 The induction of an aberrant inflammatory response is associated with acute respiratory distress syndrome (ARDS) and multi-organ involvement. Reference Zhang, Zhou and Qiu25 The clinical manifestations of severe COVID-19 are associated with the release of proinflammatory cytokines known as cytokine storm syndrome. The induction of an aberrant inflammatory response is associated with ARDS and multi-organ involvement. Reference Zhang, Zhou and Qiu25
Serum ferritin, D-dimer, c-reactive protein (CRP), erythrocyte segmentation rate (ESR), procalcitonin, lactate dehydrogenase (LDH), interleukin-2 receptor (IL-2), interleukin-6 (IL-6), and interleukin-10 (IL-10) were found to be elevated and platelet levels were reduced in non-survivors in a cohort of COVID-19 cases in Wuhan, China suggesting the role of hyper-inflammation. Reference Huang, Wang and Li24,Reference Chen, Wu and Chen41–Reference Du, Liang and Yang44 Elevated granulocyte-colony stimulating factor (G-CSF levels) was observed and were notably higher among patients requiring ICU admission. Reference Huang, Wang and Li24 Neutrophilia was reported in COVID-19 non-survivors and may be associated with cytokine storm syndrome induced by viral evasion. Reference Wang, Hu and Hu42 Higher mortality rates may be due to SARS-CoV-2-induced hyper-inflammation observed in a cytokine storm. Using immune-inflammatory laboratory trends such as increased ferritin, D-dimer, IL-6, and CRP may help identify patients who require immunosuppressive treatments such as steroids and selective cytokine blockade along with supportive treatment may reduce mortality rates. The severity of the disease is observed to increase with age and comorbidities such as obesity, cardiovascular disease, and pulmonary disease increase the mortality rates in COVID-19. Reference Pirofski and Casadevall45 The mechanisms of dysfunctional immune pathogenesis are described in Fig. 2(a–f).

Fig. 2. Pathological immune response in SARS-CoV-2. (a) SARS-CoV-2 enters the lungs due to a high affinity to angiotensin-converting enzyme-2 (ACE-2). (b) Pyroptosis and cytopathic processes along with pattern recognition receptors (PRRs) that identify pathogen-associated molecular patterns (PAMPs) of viral RNA invoke the release of pro-inflammatory cytokines including IL-2, IL-6, IL-7, IL-10, granulocyte colony-stimulating factor (GCSF), interferon-gamma (IFN-γ), interferon gamma-induced protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1 alpha (MIP-1-α), and tumor necrosis factor-α (TNF-α). (c) As opposed to healthy immune responses whereby host cells recruit innate and adaptive immune responses to suppress infection, pathological immune responses promote a pro-inflammatory loop leading to cytokine storm syndrome (CSS). Monocytes and macrophages are recruited possibly due to direct infection by virus leading to the recruitment of helper and suppressor T cells. T cells, monocytes, and macrophages further attract pro-inflammatory cytokines and cytokines thereby establishing a pathogenic pro-inflammatory loop. (d) Cytokine storm causes a systemic pro-inflammatory state across the host system. (e) Lung architecture is compromised leading to pulmonary edema and microvascular leakage observed in severe acute respiratory syndrome (SARS). Antibody-dependent enhancement (ADE), molecular mimicry, and epitope spreading contribute to the severity of pathological immune responses. (f) Damage to the lung architecture and pro-inflammatory state leads to multi-organ damage. Adapted from. Reference Tay, Poh, Rénia, MacAry and Ng40
Inflammatory pathogenesis in pediatric populations
Most COVID-19 cases are known to be mild or asymptomatic in children. However, a small number develop a multi-system inflammatory syndrome in children (MIS-C) that present with elevated markers of inflammation. The clinical features are similar to those of Kawaski disease and toxic shock syndrome with features unique to COVID-19. Reference Whittaker, Bamford and Kenny46 MIS-C was not reported in the early phases of the pandemic with pediatrics populations having already developed antibodies suggesting the role of adaptive immunological responses. Preliminary case definitions include fever for at least 1 day with elevated inflammatory markers, involving two or more organs, requiring hospitalization, no other plausible diagnosis and diagnosed by RT-PCR, serology, antigen test, or exposure history in the last 4 weeks before the onset of symptoms for SARS-CoV-2. 47 Antibody-dependent enhancement against spike proteins are suspected to cause the underlying inflammatory pathology in SARS-CoV-2. The distribution of MIS-C follows a u-shaped curve with severe symptoms manifesting commonly in children younger than 1 year of age. Reference Götzinger, Santiago-García and Noguera-Julián48 Associations with underlying genetics and ethnicities (e.g. Black) have been reported. Reference Jiang, Tang and Levin22
Gaps in literature
There is a need to advance research in addressing health inequity as certain groups are more vulnerable. Reference Xafis, Schaefer, Labude, Zhu and Hsu49 Certain subpopulations have had a disproportionately higher rate of incidence and mortality of COVID-19 infection. Reference Xafis, Schaefer, Labude, Zhu and Hsu49 The DOHaD concept identifies a causal association between epigenetic changes and disease susceptibility later in life with the disease risk having also been observed to transmit across generations. Reference Heindel and Vandenberg50 Less than 1% of the clinical trials have been conducted in low-income countries. It may be possible that the vaccine efficacy is compromised when being administered to these populations due to their varied underlying response to COVID-19 vaccinations. There is renewed urgency to identify the underlying immunological mechanisms across various subpopulations at a global level, including low- and middle-income countries, as the vaccines are being rolled out.
Implications for vaccine
During the initial phase of the COVID-19 pandemic, neutralizing antibodies after infection were closely monitored with reports suggesting gradual waning until 3 months after SARS-CoV-2 infection. Reference Seow, Graham and Merrick51 T cells responses were also shown to be important as part of protective immunity among patients with COVID-19 infection. Reference Le Bert, Tan and Kunasegaran52 T cell-mediated immune responses may provide sustained immunity even after B cell-mediated immune responses wane. As the world is racing to develop various vaccine strategies and platforms, a new pandemic vaccine development paradigm has been set up that has reduced the development timeframe from 10–15 years to 1–2 years. However, there is a lack of clarity as to how to categorize successful vaccine efficacy testing. Reference Jeyanathan, Afkhami, Smaill, Miller, Lichty and Xing53 With the accelerated development process, there is overlap in preclinical, clinical, and manufacturing of the vaccines. Vaccine candidates have been hailed as safe and effective, thereby resulting in global deployment. As of March 17, 2020, 7 vaccines are available for public use in over 128 countries. The Center for Disease Control and Prevention (CDC) approves and recommends three vaccines including Pfizer-BioNTech, Moderna, and Johnson & Johnson. 54 All the approved vaccines are administered via intramuscular injection of the upper arm. Pfizer-BioNtech and Moderna both require two doses 3 weeks and 1 month apart, respectively; Johnson & Johnson only requires one dose. To achieve herd immunity, equitable and robust global access is essential. The ongoing vaccination of high-risk populations is an evolving process such that there may be waning immunity. Given the challenges in the implementation of vaccination programs, it is pertinent that different vaccination platforms and strategies be employed.
Conclusion
As the world is developing COVID-19 vaccination strategies, an effective vaccination against COVID-19 may be able to mount innate and adaptive immune responses. The DOHaD hypothesis identifies immunological responses as a prototype to explain the socio-geographic differences in severity of SARS-CoV-2 infection. As global efforts are being made to manufacture and administer vaccines, assessments of population-based immunological responses are fundamental. While the longevity of the vaccine-induced protective immunity remains uncertain, it is necessary to continue administering vaccinations to high-risk populations till further robust testing is carried out.
Acknowledgments
No acknowledgments.
Financial support
No funding was received for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
Azza Sarfraz: Conceptualization, data curation, writing – original draft preparation and editing. Saman Hasan Siddiqui: Conceptualization, data curation. Junaid Iqbal: writing – original draft preparation. Syed Asad Ali: Conceptualization, supervision. Zahra Hasan: writing – reviewing and editing. Zouina Sarfraz: writing – original draft preparation. Najeeha Talat Iqbal: Conceptualization, data curation, writing – original draft preparation and reviewing.





