INTRODUCTION
In decapod crustaceans, investigation of the functional morphology of the foregut and mouthparts of larvae and post-larvae has demonstrated an intimate relationship between feeding behaviour and the morphological characteristics of the digestive system (Factor, Reference Factor, Felgenhauer, Watling and Thistle1989; Nishida et al., Reference Nishida, Quigley, Booth, Nemoto, Kittaka, Biology and May1990; Minagawa & Takashima, Reference Minagawa and Takashima1994; Abrunhosa, Reference Abrunhosa1997; Abrunhosa & Kittaka, Reference Abrunhosa and Kittaka1997a; Abrunhosa & Melo, Reference Abrunhosa and Melo2002, Reference Abrunhosa and Melo2008; Abrunhosa et al., Reference Abrunhosa, Melo and Abrunhosa2003; Queiroz et al., Reference Queiroz, Abrunhosa and Maciel2011). Analyses of the mouthparts and digestive system during larval development have demonstrated an abrupt morphological transformation of feeding to a non-feeding stage.
An absence of feeding behaviour has been observed in the larvae of lobsters of the genera Jasus Parker, 1883 and Palinurus Weber, 1795 during the metamorphosis of the puerulus (Kittaka, Reference Kittaka1988; Kittaka & Ikegami, Reference Kittaka and Ikegami1988; Kittaka et al., Reference Kittaka, Ono and Booth1997). The evidence for this was based on the morphological alterations of the digestive system, such as a reduction in the number of setae on the mouthparts, atrophied mandibles, and underdeveloped foregut (Nishida et al., Reference Nishida, Quigley, Booth, Nemoto, Kittaka, Biology and May1990; Mikami & Takashima, Reference Mikami and Takashima1993). A similar situation was observed during the megalopal stage in Paralithodes Brandt, 1846 (Abrunhosa & Kittaka, Reference Abrunhosa and Kittaka1997a) and in the zoeae of Lepidophthalmus siriboia Felder & Rodrigues, Reference Felder and Rodrigues1993 (Abrunhosa et al., Reference Abrunhosa, Melo, Lima and Abrunhosa2006).
The comparative morphology of the feeding appendages and foregut during different stages is expected to provide important information for understanding the appropriate nutrients for each larval stage or the feasibility of an initial no-feeding period in the case of a non-feeding larval stage (Abrunhosa, Reference Abrunhosa1997; Abrunhosa & Kittaka, Reference Abrunhosa and Kittaka1997a; Rocha et al., Reference Rocha, de Souza, Maciel, Maciel and Abrunhosa2016). Unnecessary addition of feed to rearing tanks may even reduce productivity, given that the introduction of live food, such as Artemia nauplii, may result in a deterioration in water quality, which may eventually affect the wellbeing of the larvae (Abrunhosa & Kittaka, Reference Abrunhosa and Kittaka1997b). Therefore, such information may contribute to the identification of the feeds and captive conditions that may allow an increase in the survival and growth rates of freshwater prawn cultures.
Three palaemonid species with a good potential for shrimp farming are found in Brazil – Macrobrachium acanthurus (Wiegmann, 1836), M. amazonicum (Heller, 1862) and M. carcinus (Wiegmann, 1836) (Valenti, Reference Valenti1993). However, the only species that has been cultured on a commercial scale is the exotic M. rosenbergii (De Man, 1879) (Marques & Moraes-Riodades, Reference Marques and Moraes-Riodades2012). In this species, the functional morphology of the foregut indicates facultative lecithotrophy from the zoea I stage onwards, with exogenous nutrients only being required in stage III (Abrunhosa & Melo, Reference Abrunhosa and Melo2002). In the larvae of M. amazonicum (Heller, 1862), on the other hand, important changes in feeding behaviour are observed during the developmental process, with no feeding being observed during the first stage (Araujo & Valenti, Reference Araujo and Valenti2007), in which the morphology of the foregut is consistent with obligate lecithotrophy (Queiroz et al., Reference Queiroz, Abrunhosa and Maciel2011).
Studies of the functionality of the digestive system of freshwater prawns have found considerable variation in the duration of the ontogenetic development of the larva, which may be prolonged, abbreviated or partially abbreviated (Magalhães & Walker, Reference Magalhães and Walker1988). Up to the present time, however, this process has been investigated in detail in only two long-cycle palaemonid species of the Macrobrachium Bate, 1868 – M. rosenbergii and M. amazonicum (Abrunhosa & Melo, Reference Abrunhosa and Melo2002; Queiroz et al., Reference Queiroz, Abrunhosa and Maciel2011) and only one with abbreviated development, M. jelskii (Rocha et al., Reference Rocha, de Souza, Maciel, Maciel and Abrunhosa2016). The initial larval stages of the M. jelskii (stages I and II) and first stage of M. amazonicum have undeveloped external mouthparts, a lack of setae, and structureless foregut, all of which indicate the lack of functionality for feeding (Queiroz et al., Reference Queiroz, Abrunhosa and Maciel2011). This variation may have a direct effect on the feeding mode in these species, providing important insights into the onset of feeding behaviour in the cultured larvae.
Ontogenetic changes related to feeding behaviour have yet to be investigated in M. acanthurus, however. Given this, the present study investigated the morphological changes in the mouthparts and foregut of the larvae and first juvenile associated with the functionality of these organs in this species, which are compared with the data available for other species.
MATERIALS AND METHODS
Ovigerous female M. acanthurus were captured using baited shrimp traps, known locally as matapis, in the Taíci tidal creek in the estuary of the Caeté River in north-eastern Pará, Brazil (00°58′216″S 46°44′370″W). In the laboratory, the specimens were disinfected in a solution of formaldehyde (25 mg l−1) and transferred to hatching boxes (15 l) with constant aeration, water with salinity of 5 ppt, mean pH of 7.8 ± 0.2 and temperature of 29 ± 0.1°C.
Once hatched, the larvae were transferred to 50 l raising tanks, coupled to biological filters, where they were raised in a dynamic closed system, with constant aeration temperature of 29 ± 0.1°C, pH of 8.0 and salinity of 16 ppt. During rearing, the larvae were fed with recently hatched Artemia nauplii at an initial density of 4 nauplii ml−1.
The larval stages were identified according to Choudhury (Reference Choudhury1970) and de Quadros et al. (Reference de Quadros, Maciel, Bastos and Sampaio2004). When at the first (zoea I), second (zoea II) and last (zoea X) larval stages, and the first juvenile, 30 individuals were selected for dissection and analysis for the production of drawings of the mouthparts and foreguts.
The specimens were fixed in a 10% solution of formaldehyde during 24 h, and then immersed in a 5% aqueous solution of potassium hydroxide (KOH), and heated in a stove at 80°C for ~30 min, in the case of the ZI and ZII, or 60 min for the ZX and first juvenile. The specimens were then washed in distilled water, immersed in a solution (1:1) of 70% ethanol and glycerine, and then stained with a solution of methylene blue.
Dissection was conducted under an optical microscope (Leica), using fine needles. The foreguts and mouthparts were observed and described using the terminology adopted by Abrunhosa & Kittaka (Reference Abrunhosa and Kittaka1997a) and Abrunhosa et al. (Reference Abrunhosa, Melo and Abrunhosa2003). Setal armature of appendages was described from proximal to distal segments and in order of endopod to exopod.
RESULTS
Morphology of the mouthparts
The mouthparts of larval stages I, II and X, and the first juvenile are listed here in a standard sequence beginning with the third maxillipeds, followed by the anterior appendages. This description follows the sequence in which food is processed by the animal, with the most important feeding structures being described in detail for each stage.
Zoea I
Mandible (Figure 2I) – Rudimentary. Incisive process with a short tooth; presence of 1 spine-like tooth between the incisive and molar processes; molar process rounded and lacking protuberances.
Maxillule (Figure 2E) – Unsegmented endopod with 2 short setae on the apical portion; Basal endite with 1 spine and 3 small simple terminal setae; coxal endite with 4 simple terminal setae.
Maxilla (Figure 2A) – Scaphognathite with 4–5 plumose setae; bilobed endopod, distal lobe with 1 plumose seta, proximal lobe with 2 simple setae; basal endite with distal and proximal lobes incompletely fused, each lobe with 2 simple setae; coxal endite with distal and proximal lobes fused with 4 simple setae.
First maxilliped (Figure 1I) – Smooth coxa and base; short endopod with 3 + 1 setae; long exopod with 4 distal natatory setae.
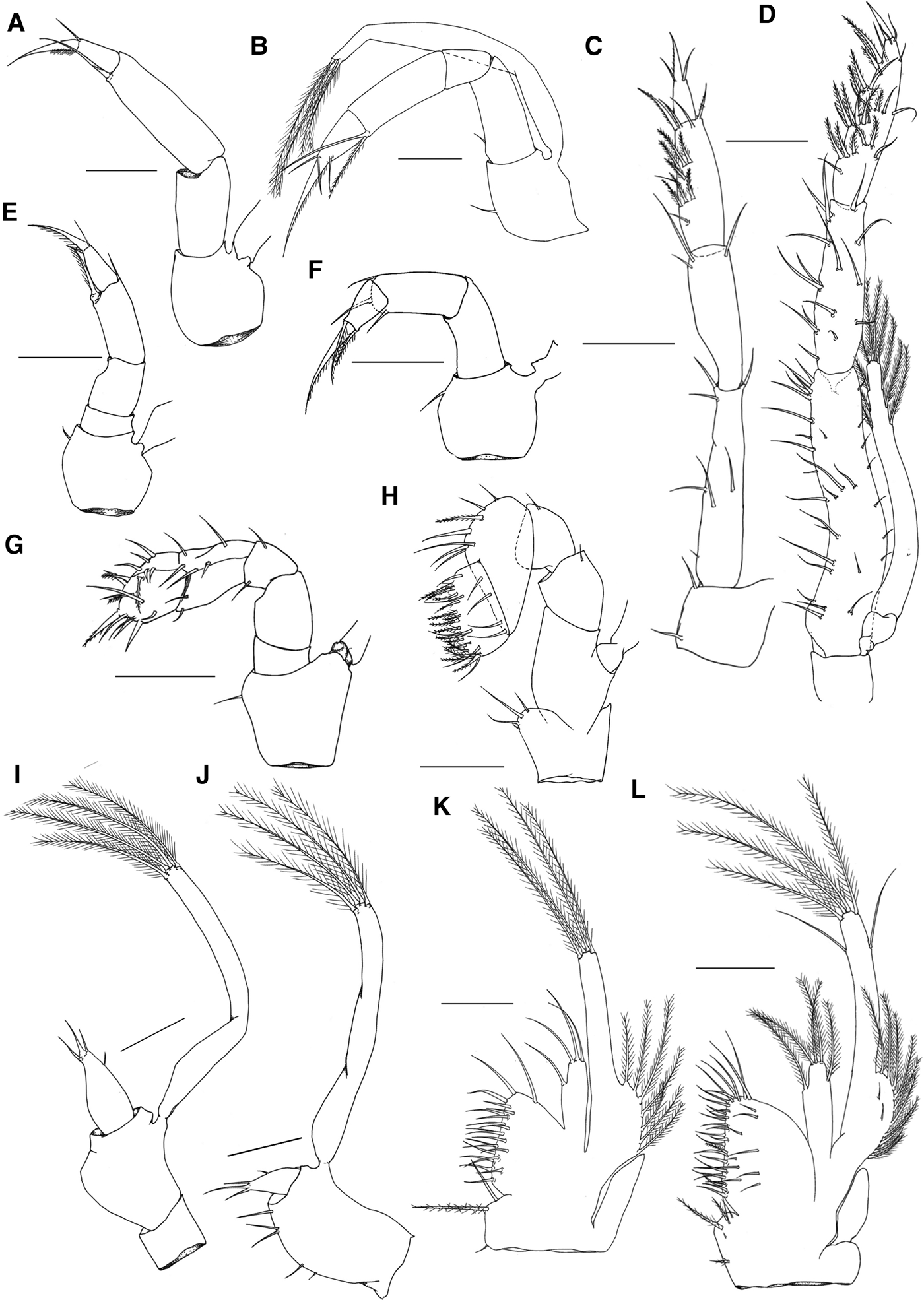
Fig. 1. Mouthparts of M. acanthurus. (A–D) Third maxillipeds: (A) zoea I; (B) zoea II; (C) zoea X; (D) post-larva. (E–H) Second maxillipeds: (E) zoea I; (F) zoea II; (G) zoea X, (H) post-larva. (I–L) First maxillipeds: (I) zoea I; (J) zoea II; (K) zoea X; (L) post-larva. Scale bar: I = 0.05 mm; A, B, E, F, J = 0.075 mm; C, G, H, K, L = 0.15 mm; D = 0.2 mm.
Second maxilliped (Figure 1E) – Base with 1 small simple seta; endopod 4-segmented with 0,0,2,3 setae; long exopod with 4 distal natatory and 2 subdistal small simple setae.
Third maxilliped (Figure 1A) – Base lacking setae; endopod 3-segmented with 0,1,2 setae; long exopod with 4 distal natatory and 2 subdistal small simple setae.
Zoea II
Mandible (Figure 2J) – Incisive process with 3 acute teeth; 2 smooth teeth between the processes; molar process with 3 denticles.

Fig. 2. Mouthparts of M. acanthurus. (A–D) Maxillae: (A) zoea I; (B) zoea II; (C) zoea X; (D) post-larva. (E–H) Maxillules: (E) zoea I, (F) zoea II; (G) zoea X; (H) post-larva. (I–L) Mandibles: (I) zoea I; (J) zoea II; (K) zoea X; (L) post-larva. Scale bar: E, F, I = 0.025 mm; B, J = 0.033 mm; A = 0.05 mm; G, L = 0.075 mm; H, K = 0.1 mm; C, D = 0.15 mm.
Maxillule (Figure 2F) – Unsegmented endopod with 2 short apical setae; basal endite with 4 + 3 simple terminal setae; coxal endite with 4 simple terminal setae.
Maxilla (Figure 2B) – Scaphognathite with 4–7 plumose setae; bilobed endopod with distal lobe presenting 1 plumose seta, proximal lobe with 1 plumose seta and 1 simple seta; basal endite with distal and proximal lobes incompletely fused, proximal lobe with 1 plumose and 2 simple setae on the distal portion, distal lobe with 1 plumose seta and 1 simple seta on the proximal portion; coxal endite with 1 plumose and 1 simple setae on the distal portion and 2 simple proximal setae.
First maxilliped (Figure 1J) – Base with 5 simple setae; endopod and exopod unchanged.
Second maxilliped (Figure 1F) – Base with 1 small simple inner seta; endopod 3–4 segmented, smooth 1st and 2nd segment (sometimes fused), 3rd and 4th segments with 3,3 setae, respectively.
Third maxilliped (Figure 1B) – Base with a simple seta; endopod 4-segmented, with 1,0,2,4 setae; long exopod with 4 distal natatory setae.
Zoea X
Mandible (Figure 2K) – Incisive process with 3 robust teeth; 8 elongate and smooth teeth between the processes; molar process with 2 small protuberances, each one with 5 or 4 denticles at the tip.
Maxillule (Figure 2G) – Unsegmented endopod with a small subterminal protuberance bearing 1 simple seta; basal endite with 10 terminal simple setae and 3 placed laterally; coxal endite with 5 simple and 1 plumose setae at the terminal portion.
Maxilla (Figure 2C) – Scaphognathite well-developed with 35–39 plumose setae; bilobed endopod with 0 + 2 long simple setae; basal endite distinctively bilobed, each lobe with 5–6 simple setae; coxal endite with 4 simple setae.
First maxilliped (Figure 1K) – Smooth epipod; coxa with 3 setae (2 simple and 1 pappose); base with 20–21 simple setae; endopod with 3 + 1 simple setae; exopodal lobe well-developed with 7–8 plumose setae, long exopod with 4 natatory setae.
Second maxilliped (Figure 1G) – Base with a small simple seta on the inner margin; endopod 5-segmented with 0,0,2,7,17 setae; long exopod with 6 natatory setae.
Third maxilliped (Figure 1C) – Base with 2 simple setae; endopod 4-segmented with 7,3,13,3 setae; long exopod with 8 natatory setae.
First juvenile
Mandible (Figure 2L) – Molar and incisive processes Y-shaped; incisive process with 2 robust teeth, one of which is bifurcate; molar process sub-square shaped with 2 robust teeth at the end and surrounded by numerous denticles.
Maxillule (Figure 2H) – Bilobed endopod with 1 + 0 simple seta; basal endite with 9–11 simple setae on the terminal portion, 2–3 simple and 1 pappose setae placed laterally; coxal endite with 5–7 simple terminal setae and 4 lateral setae.
Maxilla (Figure 2D) – Scaphognathite with 42–48 plumose setae; bilobed endopod with 0 + 2 simple setae; Basal endite bilobed, distal lobe with 6–8 simple setae and proximal lobe with 5–7 simple setae; coxal endite with 3 simple setae.
First maxilliped (Figure 1L) – Smooth epipod; coxa with 1–3 simple and 3 pappose setae; base with 35 simple and 1 plumodenticulate setae; endopod with 5 long plumose setae; exopodal lobe with 10 plumose and 2 simple setae on the surface; long exopod with 6 distal natatory setae and 2 simple subdistal setae.
Second maxilliped (Figure 1H) – Coxa with 4 simple setae; base lacking setae; curved endopod, 4-segmented with the 2 distal segments larger than previous with 1,1,7,23–26 setae; long exopod with 6 natatory and 2 simple setae.
Third maxilliped (Figure 1D) – Smooth base; endopod 4-segmented with 30,15,27,3 setae; long exopod with 10 natatory setae.
Morphology of the foregut
Zoea I
Rudimentary, clearly non-functional, lacking setae, hard structures (ossicles or teeth) and filter-press. Cardiac and pyloric chambers not clearly distinguished (Figure 3A).
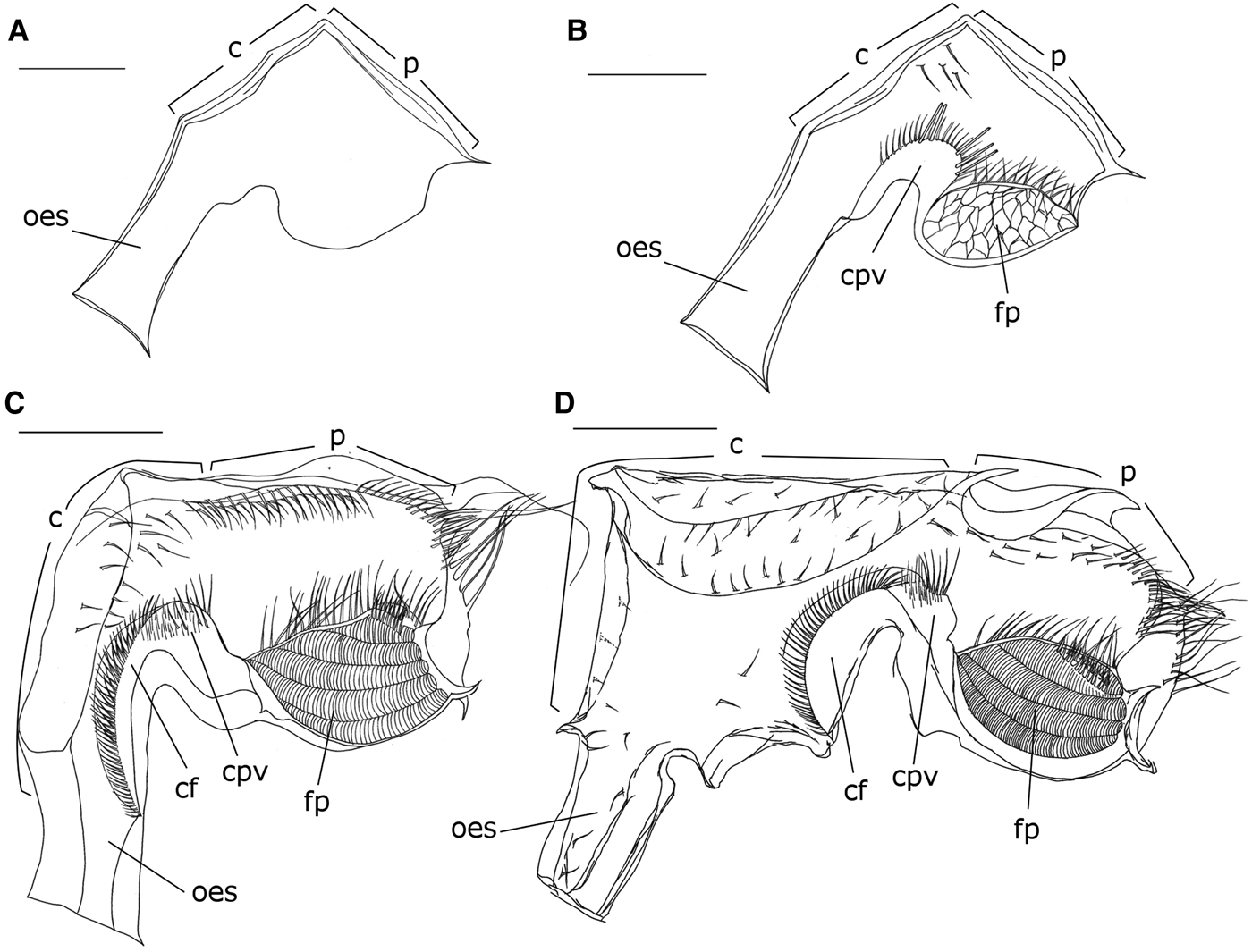
Fig. 3. Foregut of the zoea I (A), zoea II (B), zoea IX (C), and post-larva (D) of M. acanthurus in lateral view, right side. (c) Cardiac chamber, (cf) cardiac floor, (cpv) cardiopyloric valve, (fp) filter-press, (oes) oesophagus, (p) pyloric chamber. Scale bars: A, B = 0.05 mm; C = 0.1 mm; D = 0.2 mm.
Zoea II
Foregut apparently functional with numerous setae. Cardiopyloric valve and filter-press well-developed (Figure 3B).
Cardiac chamber – Narrow, similar in size to the pyloric chamber; fine and short setae distributed on the roof; cardiopyloric valve rounded with fine and robust setae on the anterior and posterior portions.
Pyloric chamber – Similar in length and width to the cardiac chamber; lacking setae; filter-press beehive-shaped filling entire anterior portion of the chamber; row of numerous elongate setae comprising the interampullary crista.
Zoea X
Foregut more developed and complex than the previous stages, with an increased number of setae (Figure 3C).
Cardiac chamber – Narrow, smaller than the pyloric chamber; numerous setae of medium length arranged sparsely along the wall; cardiopyloric valve rounded and more robust than previous stages, presence of numerous fine, short and medium-sized setae on the cardiac surface.
Pyloric chamber – larger and longer than the cardiac chamber; numerous setae arranged in a row along the roof; filter-press larger and specialized, with an increase in the number of setae on the interampullary crista.
First juvenile
Foregut more robust, with an increase in the number of setae and the presence of an oesophageal valve (Figure 3D).
Cardiac chamber – oesophageal valve well-developed; cardiac chamber enlarged, about twice the size of the pyloric chamber, numerous short setae scattered on the cardiac wall and laterally; cardiopyloric valve robust with numerous fine and medium setae arranged in rows.
Pyloric chamber – Shorter than the cardiac chamber, with short setae scattered over the roof; filter-press showing rounded shape, structurally similar to the previous stage.
DISCUSSION
The structure and setation of the various mouthparts play an important role in the detection, capture and mastication of the prey being guided into the foregut for further processing. That morphological alterations in the mouthparts and foregut during larval development may influence changes in larval diet has been demonstrated in several crustacean larvae (Factor, Reference Factor1982; Wolfe & Felgenhauer, Reference Wolfe and Felgenhauer1991; Lavalli & Factor, Reference Lavalli, Factor and Factor1995; Abrunhosa & Kittaka, Reference Abrunhosa and Kittaka1997a, Reference Abrunhosa and Kittakab; Abrunhosa & Melo, Reference Abrunhosa and Melo2008; Castejon et al., Reference Castejon, Ribes, Durfort, Rotlant and Guerao2015a, Reference Castejon, Rotlant, Ribes, Dufort and Gueraob). These changes in feeding habit are likely to be reflected morphologically in the mouthpart structures and foregut of the larvae.
The findings of the present study indicate the occurrence of profound alterations in the structure of the mouthparts and the foregut during the larval development of M. acanthurus. These morphological transformations can be perceived most clearly when comparing the zoeal stages (I and II) and the final stage with the first juvenile. These transformations are closely related to the changes in function and feeding capacity.
Zoea I
In the zoea I stage, the mouthparts are characterized by rudimentary structures and a reduced number of setae. The mandibles, maxillule and maxilla, as well as the maxillipeds, present simplified structures, which indicate extremely limited functioning. These traits appear to indicate a lack of feeding behaviour, given that the structure and the arrangement of the setae on the mouthparts play an important role in the detection, capture and mastication of prey items before they are relayed to the foregut for processing (Queiroz et al., Reference Queiroz, Abrunhosa and Maciel2011).
This conclusion is further reinforced by the analysis of the foregut of the zoea I larvae, which is rudimentary and non-functional, with no differentiation of cardiac and pyloric chambers, lacking setae, and no pyloric filter. These characteristics reflect the lack of any capability for the processing of foodstuffs during this stage, which is consistent with obligate lecithotrophic behaviour. Lecithotrophic behaviour has been observed in the larval stages of a large number of crustaceans, and is considered to be an adaptation to the lack of feeding resources in the environment in which the larvae develop. However, the larval development of many freshwater prawns occurs in brackish water, where the production of plankton tends to be relatively high. In this case, the larvae hatch in freshwater habitats and are transported by river currents to estuarine or marine waters, and during this process, their nutritional requirements are satisfied by their endogenous reserves (Anger, Reference Anger2001).
Evidence of non-feeding behaviour in other decapods such as puerulus of spiny lobster has been reported by the observation of the morphological aspects of the mouthparts (Nishida et al., Reference Nishida, Quigley, Booth, Nemoto, Kittaka, Biology and May1990; Wolfe & Felgenhauer, Reference Wolfe and Felgenhauer1991; Lemmens & Knott, Reference Lemmens and Knott1994) and megalopae of king crab species (Abrunhosa & Kittaka, Reference Abrunhosa and Kittaka1997a, Reference Abrunhosa and Kittakab). Obligate lecithotrophy has also been reported for zoea I stages, which is reflected in the rudimentary structure of the foregut, and has been observed in a number of other crustacean species, in which it may occur during a specific stage or throughout the larval development process. Abrunhosa et al. (Reference Abrunhosa, Melo, Lima and Abrunhosa2006) observed a lack of digestive structures in the foregut of the larvae of the ghost shrimp, Lepidophthalmus siriboia, throughout their development, indicating complete lecithotrophy. However, in brachyurans with abbreviated larval development but the larvae of which are not lecithotrophic (Majoidea), the first zoea has a well-developed pyloric filter. This situation is rather different to other brachyuran species with extended larval development, where their first zoea lacks the pyloric filter or it is undeveloped (Guerao et al., Reference Guerao, Simeó, Anger, Urzúa and Rotllant2012; Castejon et al., Reference Castejon, Ribes, Durfort, Rotlant and Guerao2015a, Reference Castejon, Rotlant, Ribes, Dufort and Gueraob).
In the freshwater palaemonid prawn M. amazonicum, in contrast, Queiroz et al. (Reference Queiroz, Abrunhosa and Maciel2011) recorded partial lecithotrophy, based on the structure of the mouthparts and foregut of the zoea I. This condition was considered to be obligate lecithotrophy due to the highly simplified mandible and the poorly developed setae on the other mouthparts, involved in the detection and capture of prey. The foregut of this species was also rudimentary and lacked setae or a filter-press. Lovett & Felder (Reference Lovett and Felder1989) observed the lack of a lumen in the foregut of the nauplii of Penaeus setiferus, which is consistent with a lack of functionality of the organ in this stage.
However, the occurrence of lecithotrophy in some stages of the larval development of a given species does not mean that the same process occurs in all the species of the same family or genus. In the freshwater prawn M. rosenbergii, for example, Abrunhosa & Melo (Reference Abrunhosa and Melo2002) observed that the larvae have functional foreguts throughout their development, with no lecithotrophic stage whatsoever.
The identification of non-functional morphology in the digestive system of a given stage or stages is essential for the adequate management of the feeding regime in farming systems, given that provisioning during the lecithotrophic stages may have a negative effect on water quality and the welfare of the larvae (Abrunhosa & Kittaka, Reference Abrunhosa and Kittaka1997b).
Zoea II and X
Like the mouthparts, the structure of the foregut of the M. acanthurus larvae undergoes abrupt alteration, shifting from a rudimentary, non-functional organ in the zoea I stage to the appearance of a well-developed pyloric filter and numerous setae. There is an increase in the number of setae on the structures that make up the mouthparts, which become more complex in comparison with the previous stage. Queiroz et al. (Reference Queiroz, Abrunhosa and Maciel2011) recorded a similar pattern in the larvae (zoea I and II) of M. amazonicum, in particular in the structure of the foregut.
As in M. amazonicum, the zoea II stage of M. acanthurus presents a clear differentiation of cardiac and pyloric chambers, more robust cardiopyloric valves with innumerable setae, and the appearance of a filter-press. There are some morphological differences between the two species, however, in the structure of the filter-press. In M. acanthurus, this structure is beehive-shaped, similar to those found in the brachyurans Sesarma curacaoense (Melo et al., Reference Melo, Abrunhosa and Sampaio2006) and S. rectum (Abrunhosa & Melo, Reference Abrunhosa and Melo2008). This is not the typical form of the filter-press in the larvae of M. amazonicum or most other palaemonids, anomurans and brachyurans (e.g. Abrunhosa & Kittaka, Reference Abrunhosa and Kittaka1997a; Abrunhosa & Melo, Reference Abrunhosa and Melo2002; Melo et al., Reference Melo, Abrunhosa and Sampaio2006; Abrunhosa & Melo, Reference Abrunhosa and Melo2008), which may indicate that it is not fully functional, a question that requires further investigation.
The modifications observed in the mouthparts and foregut of the zoea II stage of M. acanthurus indicate that these larvae are able to feed on phytoplankton and soft food items, such as rotifers and Artemia nauplii, as reported in the zoea II of M. amazonicum (Queiroz et al., Reference Queiroz, Abrunhosa and Maciel2011) and S. curacaoense (Melo et al., Reference Melo, Abrunhosa and Sampaio2006). On reaching zoea X, the last larval stage in M. acanthurus, no marked alterations were observed in the digestive system. An increase in the number of setae was observed in the mouthparts, however, as well as the foregut, which becomes more complex, has a cardiopyloric valve with innumerable setae and a specialized and clearly functional pyloric filter, but no ossicles or lateral teeth.
The absence of rigid structures in the foregut has been recorded in palaemonids such as M. rosenbergii (Abrunhosa & Melo, Reference Abrunhosa and Melo2002) and M. amazonicum (Queiroz et al., Reference Queiroz, Abrunhosa and Maciel2011). These structures enable the foregut to mix fine organic particles with digestive enzymes (Factor, Reference Factor, Felgenhauer, Watling and Thistle1989; Nishida et al., Reference Nishida, Takahashi and Kittaka1995) and, together with the relative complexity of the foregut observed in this last stage, indicate that the zoea X larvae of M. acanthurus are able to exploit a diet with richer nutrients, and thus complete their moult from zoea to the first juvenile stage.
Considering the transitional or metamorphic stage (e.g. puerulus, megalopa) as the final larval stage, some evidence of a lack of feeding behaviour related to the non-functionality of the digestive system has been found in other decapods. Mandibles of reduced size with a small number of setae and a poorly developed foregut have been recorded in the pueruli of Jasus edwardsii (Nishida et al., Reference Nishida, Quigley, Booth, Nemoto, Kittaka, Biology and May1990), Panulirus argus (Wolfe & Felgenhauer, Reference Wolfe and Felgenhauer1991), P. cygnus (Lemmens & Knott, Reference Lemmens and Knott1994) and the glaucothoe of Paralithodes camtschaticus, P. brevipes and P. platypus (Abrunhosa & Kittaka, Reference Abrunhosa and Kittaka1997a). This intermediate or metamorphic stage does not occur in the palaemonids, however, and there is no evidence of the atrophy of the mouthparts and foregut in the stage prior to the post-larva.
First juvenile
The first juvenile has benthonic habits, and thus has more well-developed mouthparts, which reflect the demands of this novel lifestyle. Morphological alterations mark the transition from a planktonic lifestyle and feeding behaviour to a benthonic mode of life, characterized by robust mandibles and strong teeth that permit the slicing and tearing of foods, maxillipeds with larger endopods, increased setae, and a reduction in the size of the exopods. These findings are consistent with previous studies, which indicated that the exopods of the maxillipeds have a natatory function, while the endopods are able to capture food items. In this case, the reduction in the exopods may be related to their redundancy in the benthonic behaviour pattern of the prawns following metamorphosis in case of brachyuran and anomuran species (Felgenhauer, Reference Felgenhauer and Harrison1992; Bauer, Reference Bauer2004; Abrunhosa & Kittaka, Reference Abrunhosa and Kittaka1997a, Reference Abrunhosa and Kittakab) and for anamorphic species of Caridea (Queiroz et al., Reference Queiroz, Abrunhosa and Maciel2011).
The adult species of Palaemonidae have been reported lacking gastric mills, the structures like dorsal and lateral teeth are absent (Patwardan, Reference Patwardan1935; Lilliana & Patriella, Reference Lilliana and Patriella2006; Carvalho, et al., Reference Carvalho, Collins and Bonis2011; Lima et al., Reference Lima, Garcia and Tavares2016) including for M. acanthurus (Felgenhauer & Abele, Reference Felgenhauer, Abele, Felgenhauer, Watling and Thistle1989). The foregut of the first juvenile also lack ossicles and teeth, and thus appears to maintain its mixing function, although it is considerably more complex in the zoea X stage, with the presence of well-developed oesophageal and cardio-pyloric valves, and an enlarged pyloric filter, with a specialized inter-ampullary crista. These structures permit the ingestion of a greater diversity of foods found in the substrate, and the development follows the pattern observed in the foreguts of the first juveniles of M. rosenbergii and M. amazonicum (Abrunhosa & Melo, Reference Abrunhosa and Melo2002; Queiroz et al., Reference Queiroz, Abrunhosa and Maciel2011).
Overall, then, the evidence indicates that the M. acanthurus larvae have obligate lecithotrophic behaviour in the zoea I stage, with an unstructured and clearly non-functional foregut, followed by marked morphological alterations in the zoea II, which apparently leave the system functional until the juvenile stage, when more complex structural alterations occur in the transition to the first juvenile, which acquire benthonic habits, which permit the capture and ingestion of a diversity of food items found in the substrate. However, a more detailed investigation of the functionality of the digestive system of other palaemonid species, during both the larval development and the adult phase, will be necessary for a better understanding of the feeding behaviour of these crustaceans.
FINANCIAL SUPPORT
This study is part of the doctoral dissertation of the first author (CPR), which was financed by the Federal University of Pará (UFPA) and the Brazilian Higher Education Training Program (CAPES). Repapaq (by FAPESPA) provided financial resources.





