Background
Postpartum depression (PPD) is a subtype of major depressive disorder (MDD) that affects approximately 500 000 women annually in the USA (Flynn et al. Reference Flynn, Davis, Marcus, Cunningham and Blow2004; Marmorstein et al. Reference Marmorstein, Malone and Iacono2004; Wisner et al. Reference Wisner, Moses-Kolko and Sit2010; Hamilton et al. Reference Hamilton, Martin, Osterman, Curtin and Matthews2015). PPD is a common complication of the perinatal period and one the greatest causes of maternal mortality and morbidity (Gaynes et al. Reference Gaynes, Gavin, Meltzer-Brody, Lohr, Swinson, Gartlehner, Brody and Miller2005; Gavin et al. Reference Gavin, Gaynes, Lohr, Meltzer-Brody, Gartlehner and Swinson2005). Moreover, PPD has been associated with increased risk for infanticide (Lindahl et al. Reference Lindahl, Pearson and Colpe2005), poorer maternal–infant attachment, and impaired parenting behaviors (Flynn et al. Reference Flynn, Davis, Marcus, Cunningham and Blow2004; Britton, Reference Britton2007; Stein et al. Reference Stein, Pearson, Goodman, Rapa, Rahman, Mccallum, Howard and Pariante2014; Junge et al. Reference Junge, Garthus-Niegel, Slinning, Polte, Simonsen and Eberhard-Gran2017).
The major known risk factor for PPD is a past history of MDD, including PPD or MDD outside of the perinatal period (O'Hara & Swain, Reference O'Hara and Swain1996; Gaynes et al. Reference Gaynes, Gavin, Meltzer-Brody, Lohr, Swinson, Gartlehner, Brody and Miller2005; Howard et al. Reference Howard, Molyneaux, Dennis, Rochat, Stein and Milgrom2014; Suri et al. Reference Suri, Stowe, Cohen, Newport, Burt, Aquino-Elias, Knight, Mintz and Altshuler2017). Perinatal anxiety, parity, marital conflict, perceived lack of partner support, stressful or adverse life events, unplanned pregnancy, and adverse pregnancy/birth outcomes have also been reported as risk factors (Heron et al. Reference Heron, O'CONNOR, Evans, Golding, Glover and Team2004; O'Hara & McCabe, Reference O'Hara and Mccabe2013; Norhayati et al. Reference Norhayati, Hazlina, Asrenee and Emilin2015). In addition, there are complex biological changes that occur during the perinatal period that may contribute to vulnerability. These include significant fluctuations in estrogen and progesterone during the transition from pregnancy to the postpartum period. Estrogen and progesterone steadily increase to their highest physiological levels during pregnancy and drop precipitously with parturition. However, the levels of estrogen and progesterone do not predict the occurrence of PPD (Okun et al. Reference Okun, Luther, Prather, Perel, Wisniewski and Wisner2011; Studd, Reference Studd2011). Similarly, no consistent abnormality has been observed in PPD in levels of other hormones and biological markers including brain-derived neurotrophic factor (BDNF) (Christian et al. Reference Christian, Mitchell, Gillespie and Palettas2016; Gao et al. Reference Gao, Wang, Yao, Cai and Cheng2016), oxytocin (Stuebe et al. Reference Stuebe, Grewen and Meltzer-Brody2013; Cox et al. Reference Cox, Stuebe, Pearson, Grewen, Rubinow and Meltzer-Brody2015), and allopregnanolone (Romeo et al. Reference Romeo, Strohle, Spalletta, Di Michele, Hermann, Holsboer, Pasini and Rupprecht1998; Uzunova et al. Reference Uzunova, Sheline, Davis, Rasmusson, Uzunov, Costa and Guidotti1998; Strohle et al. Reference Strohle, Romeo, Hermann, Pasini, Spalletta, Di Michele, Holsboer and Rupprecht1999; Strous et al. Reference Strous, Maayan and Weizman2006).
The role that race/ethnicity plays as an independent risk factor for PPD is largely unknown and has been inadequately studied (Liu & Tronick, Reference Liu and Tronick2014; Di Florio et al. Reference Di Florio, Putnam, Altemus, Apter, Bergink, Bilszta, Brock, Buist, Deligiannidis, Devouche, Epperson, Guille, Kim, Lichtenstein, Magnusson, Martinez, Munk-Olsen, Newport, Payne, Penninx, O'HARA, Robertson-Blackmore, Roza, Sharkey, Stuart, Tiemeier, Viktorin, Schmidt, Sullivan, Stowe, Wisner, Jones, Rubinow and Meltzer-Brody2017). While the prevalence of PPD is approximately 10–15% of women in the general population (largely based on studies in women of European ancestry) (Gavin et al. Reference Gavin, Gaynes, Lohr, Meltzer-Brody, Gartlehner and Swinson2005), the rate of PPD in Black women in the USA has been estimated to be up to twofold greater (Yonkers et al. Reference Yonkers, Ramin, Rush, Navarrete, Carmody, March, Heartwell and Leveno2001; Liu & Tronick, Reference Liu and Tronick2014). For Latinas living in the USA, the prevalence is 30–43% for new mothers, around three times higher than that of the general US population (Zayas et al. Reference Zayas, Jankowski and Mckee2003; Kuo et al. Reference Kuo, Wilson, Holman, Fuentes-Afflick, O'Sullivan and Minkoff2004; Lucero et al. Reference Lucero, Beckstrand, Callister and Sanchez Birkhead2012). Among studies examining the role of race/ethnicity with PPD manifestation, there are conflicting conclusions (Liu & Tronick, Reference Liu and Tronick2013; Liu & Tronick, Reference Liu and Tronick2014; Di Florio et al. Reference Di Florio, Putnam, Altemus, Apter, Bergink, Bilszta, Brock, Buist, Deligiannidis, Devouche, Epperson, Guille, Kim, Lichtenstein, Magnusson, Martinez, Munk-Olsen, Newport, Payne, Penninx, O'HARA, Robertson-Blackmore, Roza, Sharkey, Stuart, Tiemeier, Viktorin, Schmidt, Sullivan, Stowe, Wisner, Jones, Rubinow and Meltzer-Brody2017). This may be due to using self-reported race, which refers to a person's physical characteristics, and ethnicity, which refers to belonging to cultural, linguistic, or societal groups (Fujimura & Rajagopalan, Reference Fujimura and Rajagopalan2011; Mersha & Abebe, Reference Mersha and Abebe2015). Further, individuals identified with a given ethnicity (i.e. Hispanic) may have divergent racial backgrounds. Thus, the evaluation of race and ethnicity using self-report might be more influenced by societal or environmental factors rather than biological or genetic factors. In contrast, estimations of genetic ancestry group individuals on the basis of shared genetic variation, allowing an examination of how underlying genetic structure contributes to health disparities that may otherwise be attributed to race/ethnicity. The use of genetic ancestry is a more accurate indicator of the unique genetic composition of an individual, as it takes into account the complex and heterogeneous nature of an individual's genome. It is important to note that genetic ancestry estimation is not the same as genome-wide association studies (Cross-Disorder Group of the Psychiatric Genomics Consortium et al. Reference Lee, Ripke, Neale, Faraone, Purcell, Perlis, Mowry, Thapar, Goddard, Witte, Absher, Agartz, Akil, Amin, Andreassen, Anjorin, Anney, Anttila, Arking, Asherson, Azevedo, Backlund, Badner, Bailey, Banaschewski, Barchas, Barnes, Barrett, Bass, Battaglia, Bauer, Bayes, Bellivier, Bergen, Berrettini, Betancur, Bettecken, Biederman, Binder, Black, Blackwood, Bloss, Boehnke, Boomsma, Breen, Breuer, Bruggeman, Cormican, Buccola, Buitelaar, Bunney, Buxbaum, Byerley, Byrne, Caesar, Cahn, Cantor, Casas, Chakravarti, Chambert, Choudhury, Cichon, Cloninger, Collier, Cook, Coon, Cormand, Corvin, Coryell, Craig, Craig, Crosbie, Cuccaro, Curtis, Czamara, Datta, Dawson, Day, De Geus, Degenhardt, Djurovic, Donohoe, Doyle, Duan, Dudbridge, Duketis, Ebstein, Edenberg, Elia, Ennis, Etain, Fanous, Farmer, Ferrier, Flickinger, Fombonne, Foroud, Frank, Franke, Fraser, Freedman, Freimer, Freitag, Friedl, Frisen, Gallagher, Gejman, Georgieva, Gershon, Geschwind, Giegling, Gill, Gordon, Gordon-Smith, Green, Greenwood, Grice, Gross, Grozeva, Guan, Gurling, De Haan, Haines, Hakonarson, Hallmayer, Hamilton, Hamshere, Hansen, Hartmann, Hautzinger, Heath, Henders, Herms, Hickie, Hipolito, Hoefels, Holmans, Holsboer, Hoogendijk, Hottenga, Hultman, Hus, Ingason, Ising, Jamain, Jones, Jones, Jones, Tzeng, Kahler, Kahn, Kandaswamy, Keller, Kennedy, Kenny, Kent, Kim, Kirov, Klauck, Klei, Knowles, Kohli, Koller, Konte, Korszun, Krabbendam, Krasucki, Kuntsi, Kwan, Landen, Langstrom, Lathrop, Lawrence, Lawson, Leboyer, Ledbetter, Lee, Lencz, Lesch, Levinson, Lewis, Li, Lichtenstein, Lieberman, Lin, Linszen, Liu, Lohoff, Loo, Lord, Lowe, Lucae, MacIntyre, Madden, Maestrini, Magnusson, Mahon, Maier, Malhotra, Mane, Martin, Martin, Mattheisen, Matthews, Mattingsdal, McCarroll, McGhee, McGough, McGrath, McGuffin, McInnis, McIntosh, McKinney, McLean, McMahon, McMahon, McQuillin, Medeiros, Medland, Meier, Melle, Meng, Meyer, Middeldorp, Middleton, Milanova, Miranda, Monaco, Montgomery, Moran, Moreno-De-Luca, Morken, Morris, Morrow, Moskvina, Muglia, Muhleisen, Muir, Muller-Myhsok, Murtha, Myers, Myin-Germeys, Neale, Nelson, Nievergelt, Nikolov, Nimgaonkar, Nolen, Nothen, Nurnberger, Nwulia, Nyholt, O'Dushlaine, Oades, Olincy, Oliveira, Olsen, Ophoff, Osby, Owen, Palotie, Parr, Paterson, Pato, Pato, Penninx, Pergadia, Pericak-Vance, Pickard, Pimm, Piven, Posthuma, Potash, Poustka, Propping, Puri, Quested, Quinn, Ramos-Quiroga, Rasmussen, Raychaudhuri, Rehnstrom, Reif, Ribases, Rice, Rietschel, Roeder, Roeyers, Rossin, Rothenberger, Rouleau, Ruderfer, Rujescu, Sanders, Sanders, Santangelo, Sergeant, Schachar, Schalling, Schatzberg, Scheftner, Schellenberg, Scherer, Schork, Schulze, Schumacher, Schwarz, Scolnick, Scott, Shi, Shilling, Shyn, Silverman, Slager, Smalley, Smit, Smith, Sonuga-Barke, St Clair, State, Steffens, Steinhausen, Strauss, Strohmaier, Stroup, Sutcliffe, Szatmari, Szelinger, Thirumalai, Thompson, Todorov, Tozzi, Treutlein, Uhr, van den Oord, Van Grootheest, Van Os, Vicente, Vieland, Vincent, Visscher, Walsh, Wassink, Watson, Weissman, Werge, Wienker, Wijsman, Willemsen, Williams, Willsey, Witt, Xu, Young, Yu, Zammit, Zandi, Zhang, Zitman, Zollner, Devlin, Kelsoe, Sklar, Daly, O'Donovan, Craddock, Sullivan, Smoller, Kendler and Wray2013), which identify specific risk loci associated with case status.
While the exact causes of PPD remain unknown, a genetic contribution is supported by the emerging literature demonstrating that the heritability of PPD is greater than MDD outside of the perinatal period (Treloar et al. Reference Treloar, Martin, Bucholz, Madden and Heath1999; Viktorin et al. Reference Viktorin, Meltzer-Brody, Kuja-Halkola, Sullivan, Landen, Lichtenstein and Magnusson2016). The high prevalence of PPD among Black and Latina women in particular warrants further examination to identify the role of psychosocial contributions in addition to genetic risk. For example, other risk factors, such as daily stressors, adverse life events, and reproductive hormones have been shown to exert their effects via epigenetic changes (Guintivano et al. Reference Guintivano, Arad, Gould, Payne and Kaminsky2014; Kimmel et al. Reference Kimmel, Clive, Gispen, Guintivano, Brown, Cox, Beckmann, Kornhuber, Fasching, Osborne, Binder, Payne and Kaminsky2016). Despite the knowledge that Black and Latina women carry a disproportionately higher burden of exposure to the cumulative stress associated with adversity and trauma, these racial and ethnic groups are consistently underrepresented in studies that examine the predictors and associated contributors to PPD (Stockman et al. Reference Stockman, Hayashi and Campbell2015). Therefore, we sought to address this critical gap in the literature by conducting a rigorous large case–control study of PPD in Black, Latina, and European women to examine the contributions of genetic ancestry, adverse life events, psychological, and biological underpinnings to the development of PPD.
Methods
Participant recruitment and screening
We followed the 2010 US Census terminology for describing the self-reported ‘race’ and ‘ethnicity’ (Hispanic or Non-Hispanic) of subjects. We refer to the participants as Latina (‘of Latino, Hispanic, or Spanish origin’), Black (or African-American), and White (i.e. European ancestry and non-Hispanic). Women who reported being Black and Hispanic (n = 15) were categorized as Black for analysis.
Recruitment of postpartum women aged 17–45 years occurred from 9/2012 to 6/2016 in four outpatient obstetrical clinics (University of North Carolina Women's Hospital, Wake County Health Department, Alamance County Health Department, East Carolina University School of Medicine) during routine 6-week postpartum visits (±1–2 weeks). Detailed recruitment procedures can be found in Supplemental Methods. All women attending these clinics completed the Edinburgh Postnatal Depression Scale (EPDS). The 10-item EPDS is a commonly used PPD screening instrument with EPDS scores consistent with a PPD diagnosis by structured clinical interview (Cox et al. Reference Cox, Holden and Sagovsky1987; Gibson et al. Reference Gibson, Mckenzie-Mcharg, Shakespeare, Price and Gray2009). Women with high EPDS scores (⩾11) or low EPDS scores (⩽7) were invited to participate. While the strict cut-off for PPD in the literature is >12 (Wisner et al. Reference Wisner, Parry and Piontek2002), we included scores of 11 and 12 that may be considered minor depression, which is nonetheless associated with considerable morbidity and hence is clinically relevant. All cases were then compared with MINI diagnosis. For a full list of inclusion/exclusion criteria, see Supplemental Methods. Briefly, all participants had no indication of MDD during the first or second trimesters of pregnancy, singleton pregnancy, and live term birth (⩾34 weeks gestation). This study was approved by the University of North Carolina Institutional Review Board Committee for the Protection of Human Subjects. All subjects provided written informed consent and signed the Health Insurance Portability and Accountability Act release.
Subject assessments
All participants were administered the MINI International Neuropsychiatric Interview (MINI-Plus, version 6.0), a structured clinical interview for the assessment of psychiatric disorders (Sheehan et al. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998; Otsubo et al. Reference Otsubo, Tanaka, Koda, Shinoda, Sano, Tanaka, Aoyama, Mimura and Kamijima2005). Experienced and certified (κ > 0.8 v. criterion ratings) psychiatric research coordinators working in each clinic administered the MINI-Plus. Cases for this study were defined by having current MDD as assessed by the MINI-Plus. Controls did not have current MDD using the MINI-Plus. All study procedures could be performed in Spanish with a native speaker.
Subjects completed a battery of self-report instruments that are widely used and have proven validity (available in English or Spanish). These included the following: Abuse and Trauma Inventory (history of sexual or physical abuse) (Leserman et al. Reference Leserman, Drossman and Li1995; Leserman et al. Reference Leserman, Drossman, Li, Toomey, Nachman and Glogau1996; Leserman, Reference Leserman2005; Meltzer-Brody et al. Reference Meltzer-Brody, Leserman, Zolnoun, Steege, Green and Teich2007), Everyday Stressors Index (ESI) (Hall et al. Reference Hall, Kotch, Browne and Rayens1996), and Postpartum Bonding Questionnaire (PBQ) (Brockington et al. Reference Brockington, Fraser and Wilson2006). See Supplemental Methods for further details.
Biological sampling
The Supplemental Methods section has full protocol details. Briefly, peripheral blood was sampled and immediately processed on-site at the time of subject assessment. All plasma and serum samples were then snap-frozen and kept at −80 °C until analysis. Estradiol and progesterone were measured by radioimmunoassay from serum. Serum BDNF and allopregnanolone, and plasma oxytocin were each assayed using enzyme-linked immunosorbent assays (ELISA). Genomic DNA was extracted from aliquots of whole blood using Qiagen Autopure LS, which utilized Qiagen Puregene chemistry.
SNP genotyping and genetic ancestry determination
Genotypes were assessed using the Illumina Multi-Ethnic Genome Arrays (MEGA; Illumina, San Diego, CA, USA) through the Illumina Fast Track Genotyping service. GenomeStudio software version 2.0 (Illumina, San Diego, CA, USA) was used to call genotypes from raw Illumina data. We have described our quality control procedures for SNPs elsewhere (International Schizophrenia Consortium et al. Reference Purcell, Wray, Stone, Visscher, O'DONOVAN, Sullivan and Sklar2009; Wang et al. Reference Wang, Liu and Aragam2010). Briefly, SNPs are removed for bad genome mapping, missingness (>0.01), and low MAF (<0.01). Any individual with high missingness was excluded. Genetic ancestry was determined using EigenSoft smartpca (Price et al. Reference Price, Patterson, Plenge, Weinblatt, Shadick and Reich2006) and fastStructure (Raj et al. Reference Raj, Stephens and Pritchard2014). Smartpca was run with HapMap (International HapMap Consortium, 2005) phase 3 reference populations. Subsequent principal components were standardized using z-score transformation. FastStructure was run with a range of K = 1 through K = 10. Optimum model complexity was chosen using chooseK within fastStructure. Percent ancestry for each K population subgroups was estimated for each participant.
Statistical analyses
Analyses were conducted using SAS (v9.3, Cary, NC). Using a Shapiro–Wilk test, all distributions of data that rejected the null hypothesis of normality were subsequently evaluated with non-parametric tests. Descriptive statistics are reported using percentages for categorical variables and medians with interquartile ranges (IQR) for continuous variables. Bivariate analyses were conducted using χ2 statistics for categorical variables and analysis of variance for continuous variables. Univariate and multivariate logistic regression was performed to identify association with PPD and predictors of case status. A stringent significance threshold of p < 0.001 was used to account for multiple testing.
Results
Genetic ancestry and self-reported race
A total of 1517 women were included in these analyses (549 PPD cases, 968 controls). Participants self-identified with the following race/ethnicity: 67.4% Black, 14.4% Latina, and 18.2% White. To identify its role as a risk factor for PPD, genetic ancestry was estimated from genotype data in two ways: principal components analysis (PCA) and fastStructure. Principal components (PC) 1 and 2 are a standard metric to estimate genetic ancestry (Patterson et al. Reference Patterson, Price and Reich2006; Price et al. Reference Price, Patterson, Plenge, Weinblatt, Shadick and Reich2006) and showed similar distribution of cases and controls (Fig. 1a), indicating PC1 and PC2 were not segregating our samples by case status. Instead, PC1 and PC2 showed expected associations with Hapmap reference populations, providing validity of our genetic ancestry measures (Fig. 1b). FastStructure determined three genetic groups within our cohort, which appear to represent three ancestry populations: African (AFA), European (EUA), and Mexican (MXA) (International HapMap Consortium, 2005; Joubert et al. Reference Joubert, North, Wang, Mwapasa, Franceschini, Meshnick and Lange2010; Li et al. Reference Li, Glusman, Hu, Shankaracharya, Caballero, Hubley, Witherspoon, Guthery, Mauldin, Jorde, Hood, Roach and Huff2014). These estimated populations can also be observed with Hapmap references in Fig. 1b. Ancestry proportions for each of these subgroups were estimated for each individual (Fig. 1c) and for each self-reported racial group (Fig. 1d). Self-report had varying degrees of association with genetic ancestry: self-reported Black women had mean proportions of 86.2% AFA, 13.1% EUA, and 0.7% MXA ancestry; self-reported Latinas had mean proportions of 61.1% MXA, 32.9% EUA, and 6% AFA ancestry; and self-reported White women had mean proportions of 97.2% EUA, 1.7% AFA, and 1.1% MXA ancestry (Fig. 1d).

Fig. 1. Genetic Ancestry of Study Participants. (a) Principal Component plot of cases (orange), controls (blue), and HapMap references (red). (b) Principal Component plot of self-reported race: Black (green), Latina (purple), White (orange), and HapMap references (red). (c) Ternary plot of fastStructure estimated percent ancestry for each participant. (d) Average genetic ancestry composition for each self-reported racial group. Abbreviations: African ancestry in Southwest USA (ASW); Utah residents with Northern and Western European ancestry (CEU); Han Chinese in Beijing, China (CHB); Chinese in Metropolitan Denver, CO (CHD); Gujarati Indians in Houston, TX (GIH); Japanese in Tokyo, Japan (JPT); Luhya in Webuye, Kenya (LWK); Mexican ancestry in Los Angeles, CA (MEX); Toscani in Italia (TSI); Yoruba in Ibadan, Nigeria (YRI).
There was a significant difference between cases and controls in terms of self-reported race/ethnicity and genetic ancestry. With regards to self-reported race/ethnicity, cases comprised 58.8% Black, 14.7% Latina, and 26.6% White compared with controls with 72.2% Black, 14.3% Latina, and 13.5% White (![]() ${\rm \chi} _2^2 = 21.85$; p = 8.17E-10). There was an association with PPD case status using genetic ancestry: PC1 (
${\rm \chi} _2^2 = 21.85$; p = 8.17E-10). There was an association with PPD case status using genetic ancestry: PC1 (![]() ${\rm \chi} _2^2 = 5.91$; p = 1.51E-02) and PC2 (
${\rm \chi} _2^2 = 5.91$; p = 1.51E-02) and PC2 (![]() ${\rm \chi} _2^2 = 8.85$; p = 2.93E-03), indicating differences in genetic ancestry between cases and controls. To identify the role genetic ancestry plays in association with various risk factors for PPD, we used PC1 and PC2 as covariates in multivariate logistical regression models for all subsequent analyses (denoted as ‘multivariate’) in addition to univariate logistic regression models.
${\rm \chi} _2^2 = 8.85$; p = 2.93E-03), indicating differences in genetic ancestry between cases and controls. To identify the role genetic ancestry plays in association with various risk factors for PPD, we used PC1 and PC2 as covariates in multivariate logistical regression models for all subsequent analyses (denoted as ‘multivariate’) in addition to univariate logistic regression models.
Participant demographics
Participants had a median age of 26.7 years and nearly half (46.5%) of participants reported being married. These women had a high school education (median = 12; IQR: 12–14), were overweight (median BMI = 30.4; IQR: 25.6–36.1), on government sponsored insurance (69.7%), and had given birth multiple times (only 3.6% primiparous). Demographic characteristics for cases and controls are shown in Table 1. Cases and controls did not differ in terms of demography with the exception of education (multivariate OR: 0.91; 95% CI: 0.86–0.96; p = 2.70E-04). The mean difference in education is less than half a year (Δ = −0.41) with cases having less. In addition, the multivariate model showed marital status was also significantly associated with case status (multivariate OR: 0.66; 95% CI: 0.52–0.85; p = 9.55E-04).
Table 1. Demographic and clinical characteristics of study participants

Psychiatric history
There were significantly higher rates of previous psychiatric diagnoses in cases, which include adjustment for genetic ancestry (Fig. 2 and Table 2). Cases had significantly higher EPDS total scores and higher rates of family history of PPD (15.9% v. 4.7%; multivariate OR: 3.74; 95% CI: 2.54–5.59; p = 1.56E-11), which is defined as mother, grandmother, sister, or aunt with lifetime PPD. Cases had significantly higher rates of previous diagnoses of MDD (53.2% v. 14.8%; multivariate OR: 6.28; 95% CI: 4.86–8.13; p = 3.82E-48) and PPD (24.9 v. 5.8%; multivariate OR: 5.39; 95% CI: 3.80–7.73; p = 7.67E-23). Additionally, 46.8% of cases were experiencing their first episode of MDD. Cases had significantly higher rates of suicidality in the month prior to assessment (35.9% v. 1.9%; multivariate OR: 26.15; 95% CI: 16.23–44.72; p = 2.78E-65).
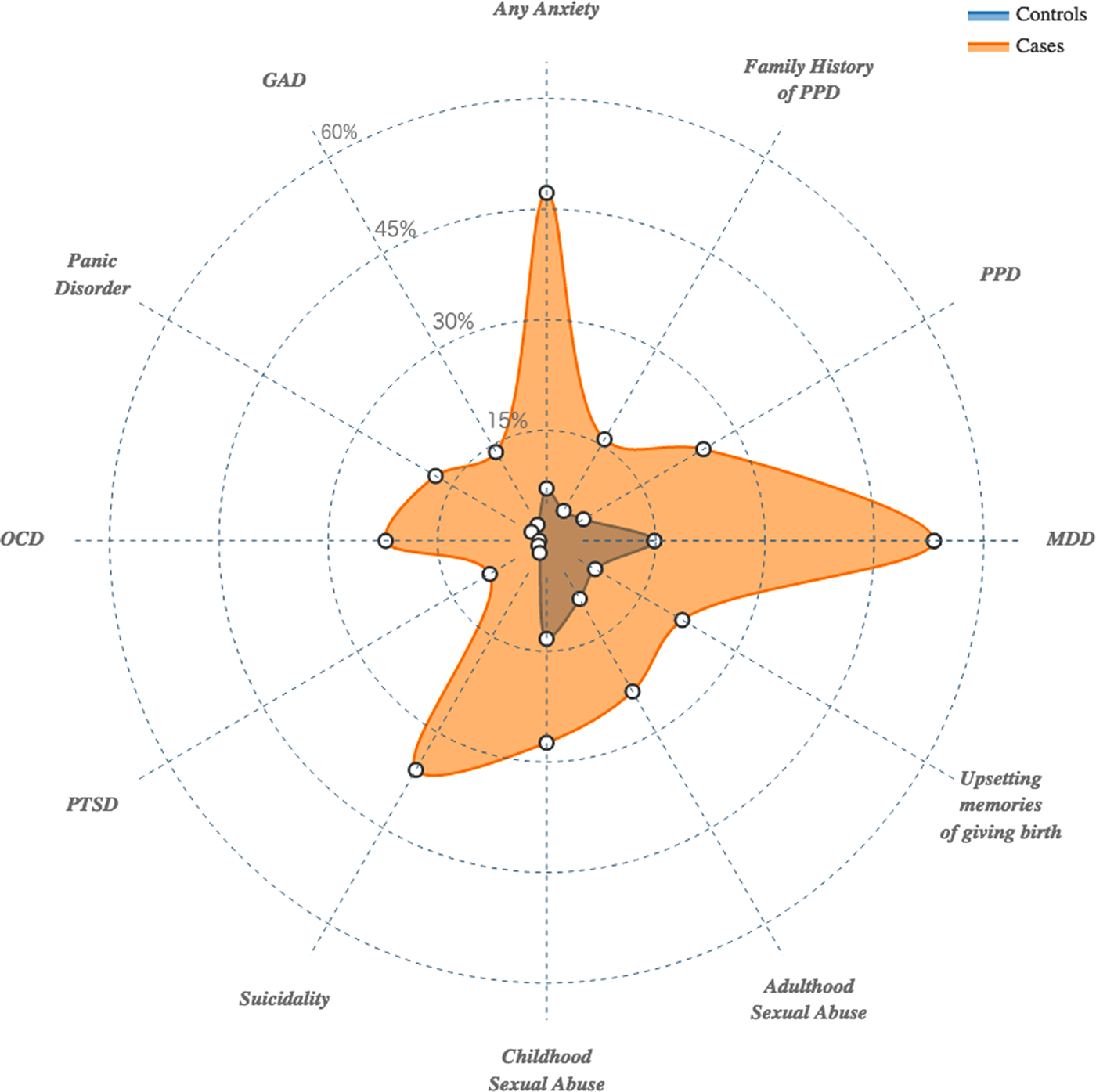
Fig. 2. Radar plot representing the cumulative amount of risk factors (lifetime psychiatric disorders and adverse life events) experienced by cases compared with controls.
Table 2. Psychiatric characteristics and trauma history

History of psychiatric disorders was prominent in PPD cases, and many followed the known comorbidities of MDD (Kessler et al. Reference Kessler, Berglund, Demler, Jin, Koretz, Merikangas, Rush, Walters and Wang2003; Kessler et al. Reference Kessler, Chiu, Demler, Merikangas and Walters2005). Nearly half of all cases (47.2%) had a lifetime anxiety disorder diagnosis compared to 7.1% of controls (multivariate OR: 11.13; 95% CI: 8.11–15.48; p = 2.03E-59). This difference reflects multiple anxiety disorder subtypes: generalized anxiety disorder (13.9% v. 2.5%; multivariate OR: 5.86; 95% CI: 3.59–9.95; p = 9.20E-14), panic disorder (17.6% v.. 2.4%; multivariate OR: 8.13; 95% CI: 5.00–13.82; p = 1.94E-20), obsessive–compulsive disorder (22.1% v. 1.0%; multivariate OR: 22.33; 95% CI: 12.12–46.08; p = 5.32E-37), and post-traumatic stress disorder (9.0 v. 1.3%; multivariate OR: 6.88; 95% CI: 3.62–14.19; p = 2.50E-10).
Abuse and trauma history
Participants reported high rates of abuse and trauma with 66.6% reporting a history of at least one traumatic event. Figure 2 and Table 2 depict the rates of abuse and trauma in cases and controls. These events were prominent in cases. Cases had a greater proportion of multiple events (64.93% v. 38.35%). This is reflected in the summary score of the abuse and trauma inventory, which can be interpreted as the total number of traumatic events experienced. Cases had significantly higher summary scores (median of 2 v. 1; multivariate OR: 1.36; 95% OR: 1.29–1.45; p = 1.39E-27). Those with PPD also had significantly higher scores for most items (nine out of 14) on the abuse and trauma inventory (Supplemental Table 1).
Women who experienced multiple adverse life events were three times more likely to have PPD (multivariate OR = 3.30; 95% CI: 2.49–4.39; p = 9.90E-18) compared with those who did not experience any. Multiple adverse life events also significantly increased the risk for previous MDD (multivariate OR = 4.06; 95% CI: 2.94–5.60; p = 1.40E-17), any lifetime anxiety disorder (multivariate OR = 3.68; 95% CI: 2.57–5.38; p = 8.60E-14), generalized anxiety disorder (multivariate OR = 2.78; 95% CI: 1.56–5.29; p = 3.32E-04), panic disorder (multivariate OR = 2.74; 95% CI: 1.61–4.90; p = 1.27E-04), obsessive–compulsive disorder (multivariate OR = 3.94; 95% CI: 2.30–7.23; p = 1.09E-07), and post-traumatic stress disorder (OR = 3.84; 95% CI: 1.81–9.51; p = 2.40E-04).
Everyday stressors
Women with PPD had a median ESI total score more than three times higher than controls (19 v. 6; multivariate OR: 1.15; 95% CI: 1.13–1.17 p = 6.83E-99). Table 3 depicts the total scores and five items from the ESI that show the largest case–control difference. The most significant stressors experienced by cases were ‘too many responsibilities,’ ‘not enough time,’ and ‘problems with marital status.’ For the full list of items see Supplemental Table 2.
Table 3. Everyday stressors, mother–infant attachment, and levels of steroid hormones and neurosteroids

Mother–infant relationship
The PBQ assesses the degree of disordered mother–infant relationships (Table 3). Previously defined (Brockington et al. Reference Brockington, Fraser and Wilson2006) thresholds were used to determine disordered mother–infant relationships. Although infrequent, there were a significantly higher proportion of dysfunctional mother–infant relationships among cases (7.71% v. 0.35%; multivariate OR: 21.58; 95% CI: 7.70–90.04; p = 3.97E-14). This pattern holds across all four factors with factor 1 having the largest proportion among cases, as well as the largest difference, compared to controls (16.33 v. 4.43; multivariate OR: 4.42; 95% CI: 2.90–6.88; p = 5.11E-14). The complete list of PBQ items and responses can be viewed on Supplemental Table 3.
Reproductive hormones and neurosteroids
Five hormones/neurosteroids (estradiol, progesterone, oxytocin, BDNF, and ALLO) were assayed. As shown in Table 3, there were no significant differences between cases and controls for any of the five hormones. After adjustment for factors that may influence reproductive hormone or neurosteroid levels (maternal age, genetic ancestry, menstrual status, breastfeeding method, days since childbirth), logistic regression showed no association between case status and any of the hormones.
Predictors of PPD onset and severity
Adverse life events, family history of PPD, marital status, and a previous history of an anxiety disorder or MDD were all significantly associated with the case status in our previous logistic regression models (Tables 1 and 2). Multivariable logistic regression was performed using these independent factors along with PC1 and PC2 representing genetic ancestry. After an iterative process, the final model did not include PC1, PC2, family history of PPD, or marital status because they were not associated with case status (p > 0.001). Lifetime anxiety disorder (OR: 7.54; 95% CI: 5.39–10.64; p = 1.25E-34), previous MDD (OR: 3.23; 95% CI: 2.39–4.37; p = 4.01E-14), and adverse life events summary score (OR: 1.17; 95% CI: 1.09–1.25; p = 6.06E-06) were all significantly predictive of PPD status. With this model, we accounted for 23.68% of the variance associated with case status with a misclassification rate of 21.98%.
Using generalized linear modeling with EPDS total score as the outcome variable, lifetime anxiety diagnosis (![]() ${\rm \chi} _1^2 = 222.24$; p = 2.90E-50), previous MDD (
${\rm \chi} _1^2 = 222.24$; p = 2.90E-50), previous MDD (![]() ${\rm \chi} _1^2 = 70.38$; p = 4.90E-17), and adverse life events summary score (
${\rm \chi} _1^2 = 70.38$; p = 4.90E-17), and adverse life events summary score (![]() ${\rm \chi} _1^2 = 31.79$; p = 1.72E-08), were also identified as predictors of EPDS score, a measure of PPD severity. This multivariate model is significant (
${\rm \chi} _1^2 = 31.79$; p = 1.72E-08), were also identified as predictors of EPDS score, a measure of PPD severity. This multivariate model is significant (![]() ${\rm \chi} _3^2 = 552.03$; p = 3.00E-119) and provides the best fit for association with EPDS score (AIC = 8313.38) compared with any predictor alone or in other combination.
${\rm \chi} _3^2 = 552.03$; p = 3.00E-119) and provides the best fit for association with EPDS score (AIC = 8313.38) compared with any predictor alone or in other combination.
Conclusions
The purpose of this study was to examine the contribution of genetic ancestry, adverse life events, psychological, and biological factors to PPD in a case–control sample of minority and low-income women, two groups that have been understudied to date (Liu & Tronick, Reference Liu and Tronick2014). We believe our findings represent the largest and most robustly phenotyped cohort of minority women with PPD. This study was also the first to examine genetic ancestry as it contributes to PPD risk. We found that genetic ancestry was not predictive of case status, despite the previously reported increased prevalence in Black and Latina women. Rather psychiatric history and exposure to adverse life events were predictors of PPD when examined in conjunction with genetic ancestry and other previously reported risk factors for PPD. Additionally, increased levels of perceived stress and altered mother–infant relationships were significantly associated with PPD case status.
We examined the role of genetic ancestry played in association with PPD case status. While self-reported race/ethnicity may have been used, this approach does not account for individual variations in population stratification and may also be a reflection of environmental or cultural associations (Mersha & Abebe, Reference Mersha and Abebe2015). Our study did however have high correlation between self-reported race/ethnicity and genetic ancestry (Fig. 1c and d) for Black and White women, while Latinas were more ancestrally mixed. This discrepancy between self-report and genetic ancestry and the utility of PCA as a more accurate measure for population stratification (Price et al. Reference Price, Patterson, Plenge, Weinblatt, Shadick and Reich2006) was the impetus for using genetic ancestry as the main covariate in association with PPD case status. Correction for population stratification is important in any genetic epidemiological study, but even more important here due to differences in genetic ancestry (PC1 and PC2) between cases and controls. While we show that genetic ancestry does not appear to be predictive of case status, this does not rule out genetic contributors for PPD. Differences in genetic ancestry will affect the frequency of risk alleles present in certain genetic populations. Future studies will work to identify specific genetic variants that underlie PPD, just as they have for other psychiatric disorders (Sullivan, Reference Sullivan2010).
Over half of all cases (53.2%) have a history of past MDD or PPD, which is significantly higher than previous depressive episodes observed in controls (14.8%). This finding is consistent with the current literature demonstrating that a prior history of either PPD or MDD outside of the perinatal period is associated with an increased risk for PPD onset (O'Hara & Swain, Reference O'Hara and Swain1996; Gaynes et al. Reference Gaynes, Gavin, Meltzer-Brody, Lohr, Swinson, Gartlehner, Brody and Miller2005). In addition, histories of any type of anxiety disorders are much more prevalent among cases. Our finding of high rates of lifetime psychiatric disorders is consistent with the literature (Postpartum Depression: Action Towards Causes and Treatment Consortium, 2015) and may be partially explained by high genetic correlations among various psychiatric disorders (Bulik-Sullivan et al. Reference Bulik-Sullivan, Finucane, Anttila, Gusev, Day, Loh, Reprogen, Duncan, Perry, Patterson, Robinson, Daly, Price and Neale2015). Genetic vulnerability is believed to be a major contributor to the pathophysiology of PPD. The high heritability of PPD, along with its shared heritability with non-perinatal MDD, has only recently been examined (Treloar et al. Reference Treloar, Martin, Bucholz, Madden and Heath1999; Viktorin et al. Reference Viktorin, Meltzer-Brody, Kuja-Halkola, Sullivan, Landen, Lichtenstein and Magnusson2016) but supports a genetic basis for PPD. This is further supported in our data by a nearly fourfold increased risk for PPD with a family history of PPD.
In this study, several types of adverse life events were assessed, with childhood (multivariate OR = 2.51) and adult sexual abuse (multivariate OR = 2.87) and life-threatening attack (multivariate OR = 4.27) among the most predictive of case status (Supplemental Table 1). These findings support the existing literature that documents adverse life events increasing risk for PPD (O'Hara & McCabe, Reference O'Hara and Mccabe2013; Gaillard et al. Reference Gaillard, Le Strat, Mandelbrot, Keita and Dubertret2014; Sorbo et al. Reference Sorbo, Grimstad, Bjorngaard, Lukasse and Schei2014; Qobadi et al. Reference Qobadi, Collier and Zhang2016). Adverse life events were prevalent among all study participants, though multiple exposures predicted PPD case status (multivariate OR = 3.30), previous MDD (multivariate OR = 4.06), any lifetime anxiety disorder (multivariate OR = 3.68), generalized anxiety disorder (multivariate OR = 2.78), panic disorder (multivariate OR = 2.74), obsessive–compulsive disorder (multivariate OR = 3.94), and post-traumatic stress disorder (multivariate OR = 3.85). The observed rates of adverse life events may be due in part to the overall lower socioeconomic status of the cohort (Brady & Matthews, Reference Brady and Matthews2002), which may be estimated from government sponsored insurance status and years of education (Shavers, Reference Shavers2007). Lower socioeconomic status may have had an effect on perceived stressors (Supplemental Table 2), which may have contributed to PPD severity and duration, but neither insurance nor education status distinguished cases from controls even when controlling for genetic ancestry. This may be the driver behind the observed increases in prevalence among Black and Latina women in the literature due to the concurrent increased prevalence of adverse life events among these populations (Roberts et al. Reference Roberts, Gilman, Breslau, Breslau and Koenen2011; Laskey et al. Reference Laskey, Stump, Perkins, Zimet, Sherman and Downs2012; Assari & Lankarani, Reference Assari and Lankarani2016), despite genetic ancestry not providing increased for PPD. One plausible mechanism linking adverse life events to PPD is via epigenetic modification. For example, adverse life events have been proposed to alter HPA-axis function via epigenetic changes (Kuhlman et al. Reference Kuhlman, Geiss, Vargas and Lopez-Duran2015; Kimmel et al. Reference Kimmel, Clive, Gispen, Guintivano, Brown, Cox, Beckmann, Kornhuber, Fasching, Osborne, Binder, Payne and Kaminsky2016). Epigenetic changes may compound underlying genetic vulnerability to PPD. Additionally, these molecular marks may persist long after the adverse life events (Kimmel et al. Reference Kimmel, Clive, Gispen, Guintivano, Brown, Cox, Beckmann, Kornhuber, Fasching, Osborne, Binder, Payne and Kaminsky2016) and contribute to what is referred to as allostatic load, or cumulative somatic effects of lifetime stress (Geronimus et al. Reference Geronimus, Hicken, Keene and Bound2006; Myers et al. Reference Myers, Wyatt, Ullman, Loeb, Chin, Prause, Zhang, Williams, Slavich and Liu2015).
Consistent with the current literature (Okun et al. Reference Okun, Luther, Prather, Perel, Wisniewski and Wisner2011; Studd, Reference Studd2011), hormone levels did not distinguish cases from controls. However, we did not assess changes in neurosteroid levels, which have been shown to differ between cases and controls in some previous reports (Romeo et al. Reference Romeo, Strohle, Spalletta, Di Michele, Hermann, Holsboer, Pasini and Rupprecht1998; Strohle et al. Reference Strohle, Romeo, Hermann, Pasini, Spalletta, Di Michele, Holsboer and Rupprecht1999; Stuebe et al. Reference Stuebe, Grewen and Meltzer-Brody2013; Cox et al. Reference Cox, Stuebe, Pearson, Grewen, Rubinow and Meltzer-Brody2015; Christian et al. Reference Christian, Mitchell, Gillespie and Palettas2016; Gao et al. Reference Gao, Wang, Yao, Cai and Cheng2016). Further, women who developed PPD may have an altered response to the normal perinatal fluctuations in reproductive hormones. The transition from pregnancy to the postpartum period is characterized by a particularly large fluctuation in reproductive hormone levels during the reproductive life cycle (Schiller et al. Reference Schiller, Johnson, Abate, Rubinow and Schmidt2016). In the current study, more than half of the cases experienced a recurrence of PPD. This group of women may be more genetically susceptible to the effects of reproductive hormones, resulting in PPD and perhaps other reproductive mood disorders (Guintivano et al. Reference Guintivano, Arad, Gould, Payne and Kaminsky2014).
This study has several strengths on which we capitalize in this analysis. Not only do these include the sample size and diversity of our cohort, but also, we collected data on a large number of both self-report and clinician-administered scales for each study participant, allowing for a comprehensive assessment of PPD in an understudied population. Prior studies that focused primarily on women of European ancestry may not be generalizable by failing to address differences that can arise with ancestry and culture (Di Florio et al. Reference Di Florio, Putnam, Altemus, Apter, Bergink, Bilszta, Brock, Buist, Deligiannidis, Devouche, Epperson, Guille, Kim, Lichtenstein, Magnusson, Martinez, Munk-Olsen, Newport, Payne, Penninx, O'HARA, Robertson-Blackmore, Roza, Sharkey, Stuart, Tiemeier, Viktorin, Schmidt, Sullivan, Stowe, Wisner, Jones, Rubinow and Meltzer-Brody2017). This study complements and extends the existing literature by providing a robust characterization of PPD in a population of women where such data were previously lacking. More importantly, this study shows that genetic ancestry does not appear to play as large a role in predicting PPD compared with exposures such as previous psychiatric episodes or abuse and trauma. Other previously identified risk factors, such as marital status or socioeconomic factors (insurance status, level of education) (O'Hara & McCabe, Reference O'Hara and Mccabe2013), for PPD do not associate with case status when assessed in conjunction with these more significant predictors.
Our findings highlight the diversity of women who experience PPD and lead the way for genetic studies in the field. We have identified phenotypes that future studies should investigate to elucidate the genetic components of this disorder, just as they have for other psychiatric conditions (Cross-Disorder Group of the Psychiatric Genomics Consortium et al. Reference Lee, Ripke, Neale, Faraone, Purcell, Perlis, Mowry, Thapar, Goddard, Witte, Absher, Agartz, Akil, Amin, Andreassen, Anjorin, Anney, Anttila, Arking, Asherson, Azevedo, Backlund, Badner, Bailey, Banaschewski, Barchas, Barnes, Barrett, Bass, Battaglia, Bauer, Bayes, Bellivier, Bergen, Berrettini, Betancur, Bettecken, Biederman, Binder, Black, Blackwood, Bloss, Boehnke, Boomsma, Breen, Breuer, Bruggeman, Cormican, Buccola, Buitelaar, Bunney, Buxbaum, Byerley, Byrne, Caesar, Cahn, Cantor, Casas, Chakravarti, Chambert, Choudhury, Cichon, Cloninger, Collier, Cook, Coon, Cormand, Corvin, Coryell, Craig, Craig, Crosbie, Cuccaro, Curtis, Czamara, Datta, Dawson, Day, De Geus, Degenhardt, Djurovic, Donohoe, Doyle, Duan, Dudbridge, Duketis, Ebstein, Edenberg, Elia, Ennis, Etain, Fanous, Farmer, Ferrier, Flickinger, Fombonne, Foroud, Frank, Franke, Fraser, Freedman, Freimer, Freitag, Friedl, Frisen, Gallagher, Gejman, Georgieva, Gershon, Geschwind, Giegling, Gill, Gordon, Gordon-Smith, Green, Greenwood, Grice, Gross, Grozeva, Guan, Gurling, De Haan, Haines, Hakonarson, Hallmayer, Hamilton, Hamshere, Hansen, Hartmann, Hautzinger, Heath, Henders, Herms, Hickie, Hipolito, Hoefels, Holmans, Holsboer, Hoogendijk, Hottenga, Hultman, Hus, Ingason, Ising, Jamain, Jones, Jones, Jones, Tzeng, Kahler, Kahn, Kandaswamy, Keller, Kennedy, Kenny, Kent, Kim, Kirov, Klauck, Klei, Knowles, Kohli, Koller, Konte, Korszun, Krabbendam, Krasucki, Kuntsi, Kwan, Landen, Langstrom, Lathrop, Lawrence, Lawson, Leboyer, Ledbetter, Lee, Lencz, Lesch, Levinson, Lewis, Li, Lichtenstein, Lieberman, Lin, Linszen, Liu, Lohoff, Loo, Lord, Lowe, Lucae, MacIntyre, Madden, Maestrini, Magnusson, Mahon, Maier, Malhotra, Mane, Martin, Martin, Mattheisen, Matthews, Mattingsdal, McCarroll, McGhee, McGough, McGrath, McGuffin, McInnis, McIntosh, McKinney, McLean, McMahon, McMahon, McQuillin, Medeiros, Medland, Meier, Melle, Meng, Meyer, Middeldorp, Middleton, Milanova, Miranda, Monaco, Montgomery, Moran, Moreno-De-Luca, Morken, Morris, Morrow, Moskvina, Muglia, Muhleisen, Muir, Muller-Myhsok, Murtha, Myers, Myin-Germeys, Neale, Nelson, Nievergelt, Nikolov, Nimgaonkar, Nolen, Nothen, Nurnberger, Nwulia, Nyholt, O'Dushlaine, Oades, Olincy, Oliveira, Olsen, Ophoff, Osby, Owen, Palotie, Parr, Paterson, Pato, Pato, Penninx, Pergadia, Pericak-Vance, Pickard, Pimm, Piven, Posthuma, Potash, Poustka, Propping, Puri, Quested, Quinn, Ramos-Quiroga, Rasmussen, Raychaudhuri, Rehnstrom, Reif, Ribases, Rice, Rietschel, Roeder, Roeyers, Rossin, Rothenberger, Rouleau, Ruderfer, Rujescu, Sanders, Sanders, Santangelo, Sergeant, Schachar, Schalling, Schatzberg, Scheftner, Schellenberg, Scherer, Schork, Schulze, Schumacher, Schwarz, Scolnick, Scott, Shi, Shilling, Shyn, Silverman, Slager, Smalley, Smit, Smith, Sonuga-Barke, St Clair, State, Steffens, Steinhausen, Strauss, Strohmaier, Stroup, Sutcliffe, Szatmari, Szelinger, Thirumalai, Thompson, Todorov, Tozzi, Treutlein, Uhr, van den Oord, Van Grootheest, Van Os, Vicente, Vieland, Vincent, Visscher, Walsh, Wassink, Watson, Weissman, Werge, Wienker, Wijsman, Willemsen, Williams, Willsey, Witt, Xu, Young, Yu, Zammit, Zandi, Zhang, Zitman, Zollner, Devlin, Kelsoe, Sklar, Daly, O'Donovan, Craddock, Sullivan, Smoller, Kendler and Wray2013). Preliminary work regarding the role of epigenetic mechanisms in PPD onset has shown promise, but has been characterized by small sample sizes (Guintivano et al. Reference Guintivano, Arad, Gould, Payne and Kaminsky2014). Our data show that many environmental factors, such as cumulative adverse life events (which may alter epigenetic marks and increase allostatic load), contribute to PPD onset. The interaction of genetic risk with environmental (via epigenetic) changes can help explain the phenotypic heterogeneity of PPD identified in this study.
This study also has limitations that should be kept in mind when interpreting the results. First, our cohort is cross-sectional, examining PPD during the 6-week postpartum visit. We are unable to speak to the duration of illness, as there was no prospective data collected, and we relied on patient report and review of obstetrical records to generate data. This approach also excluded some women during recruitment that may have exhibited PPD symptoms at an earlier time point (earlier than 6 weeks postpartum) and were euthymic at the 6-week visit. Second, there are significantly different proportions of race and ethnicity between cases and controls. This difference was likely due to this cohort being a clinically ascertained sample with no strict matching rather than a strict epidemiological sample. We likely have ascertainment bias due to our inability to recruit matched controls for all cases. However, using logistic regression, we were able to show that this difference in proportions did not account for large variation (1.5%) in determining case status.
Clinical and public health implications
This work contributes important information to our understanding of the complex factors associated with the development of PPD in a racially and ethnically diverse sample of women. Chiefly, we show that genetic ancestry was not predictive of case status despite reported rates of PPD differing among racial groups (Yonkers et al. Reference Yonkers, Ramin, Rush, Navarrete, Carmody, March, Heartwell and Leveno2001; Zayas et al. Reference Zayas, Cunningham, Mckee and Jankowski2002; Zayas et al. Reference Zayas, Jankowski and Mckee2003; Kuo et al. Reference Kuo, Wilson, Holman, Fuentes-Afflick, O'Sullivan and Minkoff2004; Lucero et al. Reference Lucero, Beckstrand, Callister and Sanchez Birkhead2012; Liu & Tronick, Reference Liu and Tronick2014). Rather, among all the phenotyping that was performed in this cohort, the best predictors for PPD are lifetime psychiatric disorders and history of abuse and trauma. These findings have clinical implications. The data provides a set of risk factors that together are significantly associated with case status and should be utilized in screening new mothers. These risk factors could be incorporated into the recent U.S. Preventive Services Task Force recommendations for increased depression screening during the perinatal period (Siu et al. Reference Siu, Force Us, Bibbins-Domingo, Grossman, Baumann, Davidson, Ebell, Garcia, Gillman, Herzstein, Kemper, Krist, Kurth, Owens, Phillips, Phipps and Pignone2016). A brief, single assessment for previous psychiatric and abuse/trauma history during the perinatal period, along with regular mood monitoring, may increase a clinicians’ ability to predict the onset of PPD.
While genetic ancestry does not play a role in our study in determination of case status, there are important ethnic and cultural differences that have been shown to have a meaningful role in the patient's acceptance of different depression treatment options (Cooper et al. Reference Cooper, Gonzales, Gallo, Rost, Meredith, Rubenstein, Wang and Ford2003). For example, Latinas and Black women may be less likely to find antidepressants an acceptable form of therapy (Cooper et al. Reference Cooper, Gonzales, Gallo, Rost, Meredith, Rubenstein, Wang and Ford2003), which would mean also offering an array of psychotherapeutic treatment options. However, before any form of mental health treatment can be initiated, the patient must have appropriate access to care. There are currently significant barriers associated with access to mental health care in the USA that are highly influenced by race and socioeconomic status (Alegria et al. Reference Alegria, Chatterji, Wells, Cao, Chen, Takeuchi, Jackson and Meng2008) and will need to be addressed.
In summary, we believe that improving our understanding of the psychological, social, and biological predictors of PPD is vital to ultimately provide optimal care for all women that suffer with PPD. In particular, we must pay attention to the impact of cumulative adverse life events and past psychiatric history. Our findings demonstrate that the risk factors for PPD only account for 23.68% of the variation we observed in cases. Therefore, future work focused on underlying genetics and epigenetics may allow us to more fully understand the biologic triggers for PPD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717002641.
Acknowledgements
This work was supported by the National Institutes of Mental Health (D.R and S.M. grant 5R01MH 095992-04) (J.G. grant 4T32MH093315-05).
Declaration of Interest
None.







