Transcatheter closure of patent arterial duct has become a standard of care over last two decades since the advent of the Amplatzer family of devices in the mid-1990s. Although multiple devices are available for patent arterial duct closure,Reference Backes, Rivera and Bridge1,Reference El-Said, Bratincsak and Foerster2 the Amplatzer duct occluder is the most widely used for this purpose. While suited for older children, Amplatzer duct occluder use in infants with large patent arterial ducts often causes coarctation due to protrusion of the retention disc into the aortic lumen.Reference Schwartz, Glatz, Rome and Gillespie3–Reference Pass, Hijazi, Hsu, Lewis and Hellenbrand8 Similarly, in large tubular ducts, the pulmonary end of the Amplatzer duct occluder may not reach and flare out at the pulmonary artery end resulting in aortic embolisation of the device.Reference Sen, Jain and Dalvi9 The Amplatzer duct occluder II was designed to close tubular ducts in small children.Reference Baspinar, Irdem and Sivasli5,Reference Pepeta and Dippenaar10 However, the large disc at the pulmonary end of the Amplatzer duct occluder II is known to cause left pulmonary artery stenosis in small babies.Reference Baspinar, Irdem and Sivasli5,Reference Pepeta and Dippenaar10,Reference Thanopoulos, Eleftherakis, Tzannos and Stefanadis11 Also, in tubular ducts ≥5 mm diameter, the Amplatzer duct occluder II may not adequately occlude the patent arterial duct with significant risk of embolisation.
Due to these inherent limitations of Amplatzer duct occluder and Amplatzer duct occlude II in small children with large tubular ducts, the Amplatzer vascular plug I and Amplatzer vascular plug II have been successfully used as alternatives.Reference Schwartz, Glatz, Rome and Gillespie3,Reference Sant’Anna, da Costa and Ribeiro12–Reference VanLoozen, Sandoval and Delaney15 The purpose of this study is to share our experience with the use of Amplatzer vascular plug II in a selected group of infants with large and long ducts.
Materials and methods
Study design
This is a single centre, prospective, observational study performed between April, 2014 and April, 2018. All infants, who fulfilled the selection criteria and underwent transcatheter patent arterial duct closure using Amplatzer vascular plug II, were enrolled in the study. Pre-procedural clinical, echocardiographic, and angiographic data were evaluated along with the feasibility, safety, and efficacy of Amplatzer vascular plug II in closing these ducts.
Selection criteria
These were age less than 1 year, presence of symptoms and/or continuous murmur, ductal diameter of ≥75% of the body weight in kilogram, and ductal length of ≥6 mm.
The exclusion criteria were the presence of aortic coarctation, presence of left pulmonary artery stenosis, a short duct (<6 mm), and presence of any other cardiac anomaly requiring surgical repair.
The pre-procedural evaluation included detailed clinical assessment, chest X-ray, and thorough systematic echocardiographic evaluation. Apart from routine segmental analysis of the cardiovascular system, special attention was given to the duct measurements using a ductal view (Fig 1a). They comprised its size at the aortic and the pulmonary ends and its length, the direction of the flow across the duct, absence of aortic coarctation and left pulmonary artery stenosis, and the difference between the aortic and pulmonary artery pressure on spectral Doppler (Fig 1a).

Figure 1. Central illustration. (a) Two-dimensional echo with colour flow imaging shows a large and long patent arterial duct with well-defined aortic and pulmonary ends and the length and shape of the patent arterial duct (arrow). (b) Continuous wave Doppler showing near systemic pulmonary artery pressure. (c) Baseline aortogram in lateral projection showing a large and long ductus (arrow). (d) Two-dimensional echo with colour flow imaging prior to release shows the AVP II lying entirely within the ductal ampulla except the proximal disc which is in the MPA (arrow). The LPA flow is unobstructed. (e) Aortogram in lateral projection with device still on cable confirming the distal disc of the AVP II sitting well within the ductal ampulla (arrow). (f) Main pulmonary artery angiogram in PA projection with device still on the cable showing non-obstructive flow to the pulmonary artery branches. (g) Post device closure aortogram in lateral projection shows the entire device (arrow) within the ampulla except the proximal disc of AVP II which is in the MPA. AA = ascending aorta; AO = aorta; AVP = Amplatzer vascular plug; DAO = descending aorta; LPA = left pulmonary artery; MPA = main pulmonary artery; RPA = right pulmonary artery; PA = posteroanterior.
Procedure
An informed consent was taken from the parents/guardians of all the infants prior to the procedure. All the procedures were done under general anaesthesia with fluoroscopic and echocardiographic guidance. Intravenous Cefuroxime 30 mg/kg/dose was given 1 hour before, and two doses were given every 8 hours after the procedure. A femoral artery and vein were accessed using Seldinger technique. After the arterial puncture, heparin was administered in the dose of 100 U/kg. Additional heparin (50 U/kg) was given after 1 hour every 30 minutes if the procedure duration was prolonged beyond an hour. Activated clotting time was not monitored in any of the patients.
After obtaining the systemic and pulmonary artery pressures, descending thoracic aortography was done in lateral (Fig 1c) and/or right anterior oblique projections to define the ductal anatomy which included estimation of ductal size at the pulmonary and aortic end, ductal length, and its shape. The duct was crossed from the pulmonary end using a Judkins right coronary catheter and a straight tipped 0.035″ Teflon coated guide wire. The size of Amplatzer vascular plug II selected was approximately 150–200% of the minimum duct diameter, which was measured at the pulmonary artery end in all our patients. It was delivered through a delivery sheath or a coronary guide catheter under fluoroscopic and echocardiographic guidance. The technique of deployment consisted of releasing the aortic disc and the central lobe into the aorta just opposite the ampulla, then letting the plug fall into the ampulla and finally deploying the pulmonary disc by unsheathing the device without pulling onto the cable. This prevented the elongation of the central lobe which was crucial to keep the entire device within the duct ampulla. With this technique, majority of the devices remained within the ampulla, with the ductal wall offering the retaining force. Prior to release, an echo-Doppler (Fig 1d) and angiographic evaluation was done to confirm the position of the device and to rule out any obstruction to the aortic isthmus (Fig 1e) and the left pulmonary artery (Fig 1f). Thereafter, a gentle tug was given to the loading cable to confirm the stability of the device, and the device was released using the plastic vise. Repeat aortogram was done 10 minutes after the release (Fig 1g) to confirm the final position of the plug and assess the presence of any residual shunt.
The children were observed in the ICU for 24 hours and then discharged after confirming the device position on echo. The follow-up evaluation was done after 6 weeks, 6 months, and annually thereafter.
Statistics
The continuous variables were summarised as mean ± standard deviation or median with range as deemed appropriate, and the discrete variables were presented as percentage.
Results
From April, 2014 to April, 2018, 18 infants underwent patent arterial duct closure using Amplatzer vascular plug II. During this study period, 2 patients with a suspicion of associated coarctation, 1 with mild left pulmonary artery stenosis, and 12 patients with large but short ducts were considered not suitable for the device closure and hence were referred for surgical intervention.
Of these 9 (50%) were boys. The demographic variables and symptoms at the time of presentation are shown in Table 1.
Table 1. Demographic variables and relevant clinical data.

F = female; M = male; SD = standard deviation.
Angiographically, all patients had either Type A or C duct as per Krichenkos classification.Reference Krichenko, Benson, Burrows, Moes, McLaughlin and Freedom16 The patent arterial duct morphological data obtained on echo along with device characteristics and baseline haemodynamic data obtained at the time of cardiac catheterisation are summarised in Table 2 and Table 3, respectively.
Table 2. Patent arterial duct echocardiographic data and outcomes.
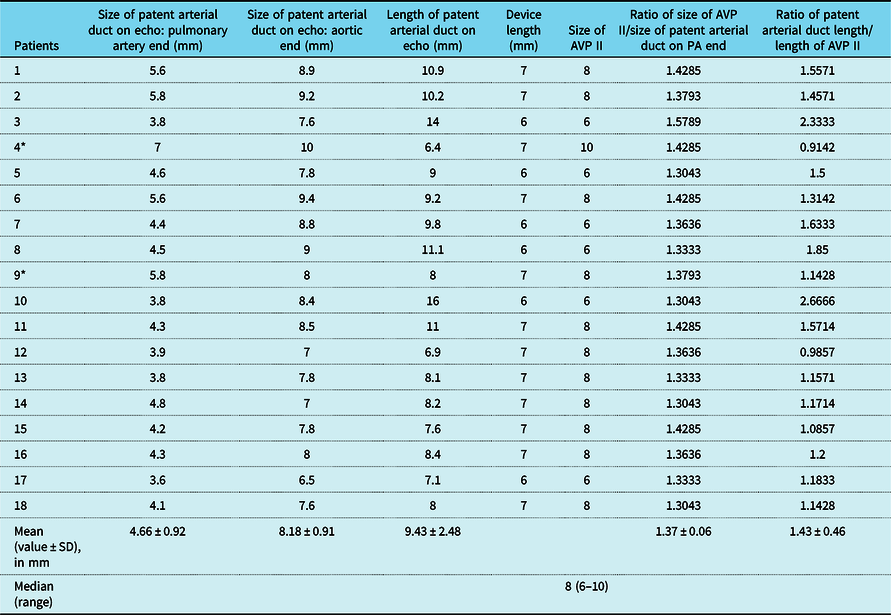
AVP = Amplatzer vascular plug; PA = pulmonary artery; SD = standard deviation.
* Patient (4) had device instability and patient (9) had device embolisation.
Table 3. Baseline haemodynamic data obtained during cardiac catheterisation.
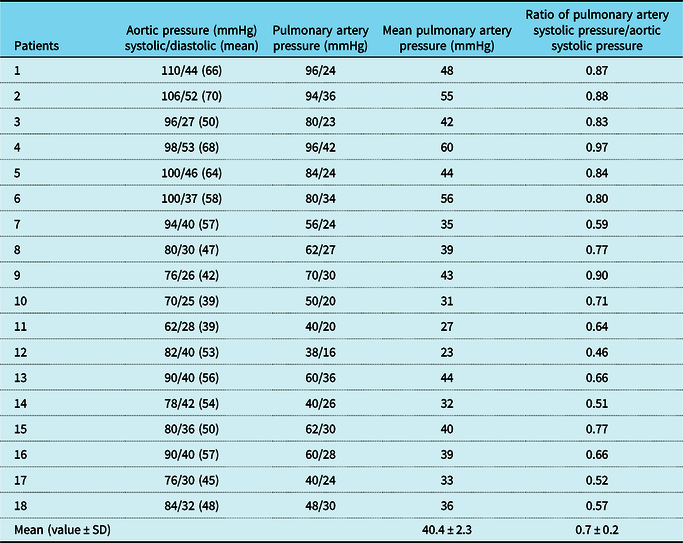
SD = standard deviation.
The sizes of Amplatzer vascular plug II used for attempting to close these ducts (n = 18) ranged from 6 to 10 mm. An 8-mm Amplatzer vascular plug II was used most commonly in 11, 6 mm in 6 and 10 mm in 1 patient, respectively. A 2-month-old patient with a 7-mm-long duct measuring 7 mm at posteroanterior end was closed using 10-mm Amplatzer vascular plug II which had a length of 7 mm. The device embolised immediately after releasing. The patient was operated successfully with removal of the device and ligation of the duct. The second patient, aged 4 months, had a duct measuring 5.8 mm at the pulmonary artery end and a ductal length of 8 mm. An attempt was made to close it with a 8-mm Amplatzer vascular plug II. The device persistently got elongated at the time of deployment with a small part of the central lobe extending beyond the ampulla. The device was found to be unstable at the time of the gentle tug, and hence, it was retrieved before releasing. He also underwent successful surgical closure of the patent arterial duct. The ratio of Amplatzer vascular plug II size to the pulmonary end diameter in cases with successful device closures was 1.65 ± 0.27 and in the failed cases the ratio was 1.40 ± 0.03. Similarly, the mean ratio of patent arterial duct length to Amplatzer vascular plug II length in successful cases was 1.48 ± 0.46, whereas in the two failed cases it was 1.02 ± 0.16.
The pre-discharge echo showed complete closure of the ductus in all 16 patients, no significant flow acceleration in the aortic isthmus, or the left pulmonary artery. There was no clinical evidence of haemolysis.
The follow-up was available in all the 16 babies for a period of 10.3 ± 6.37 months. They remained asymptomatic during the follow-up period with echo showing stable device position, complete closure of the duct in all (100%) without any evidence of obstruction to the aortic isthmus or the left pulmonary artery.
Discussion
The successful use of Amplatzer vascular plug II in small and moderate sized tubular ducts in small children has been described earlier.Reference Schwartz, Glatz, Rome and Gillespie3,Reference Sant’Anna, da Costa and Ribeiro12–Reference VanLoozen, Sandoval and Delaney15,Reference Barwad, Ramakrishnan and Kothari17 Most of the failures in these reports were related to large Type C ductsReference Sant’Anna, da Costa and Ribeiro12,Reference VanLoozen, Sandoval and Delaney15,Reference Barwad, Ramakrishnan and Kothari17 which form the basis of the present study. Our cohort comprises infants with large, long, and pulmonary hypertensive ducts which are technically most challenging to close with transcatheter techniques. With the use of appropriately sized Amplatzer vascular plug II deployed without elongation of the central lobe, the success rate in our study was found to be 88.8% with only one major complication in the form of device embolisation. Although the previous reportsReference Garay, Aguirre, Cardenas, Springmuller and Heusser14,Reference VanLoozen, Sandoval and Delaney15 have much larger cohorts, the patient population is significantly variable in terms of age, weight, duct morphology, and haemodynamics. The children are much older, much bigger with mean/median duct size being much smaller. Also, both the studies are retrospective in nature. Another unique feature of our cohort was the fact that no other device apart from Amplatzer vascular plug II could have been used in majority of the infants looking at the size and morphology of the ducts, haemodynamics, and the age and weight of the patients. Their only other option would have been surgery. This does not, in our opinion, appear to be the case in the other two studies.
Although some of these ducts could have been closed using PFM Nit occlud coils. However, the problem about pfm coils in type C ducts was their stability, given the size and the flow through them and in type A ducts the concern was regarding the residual shunt and resultant haemolysis.
Why Amplatzer vascular plug II in this subset?
Infants with long and large patent arterial ducts pose a technical challenge with routinely used devices viz Amplatzer duct occluder and Amplatzer duct occluder II. The retention disc of Amplatzer duct occluder is stiff and if left protruding into the aorta can cause significant coarctation.Reference Jang, Son, Lee, Lee and Kim4–Reference Pass, Hijazi, Hsu, Lewis and Hellenbrand8 More importantly, the retention disc position may look optimum angiographically when the device is attached to the delivery cable with no gradient recorded during the pull back from the ascending to the descending aorta; but significant device recoil during release can alter the device position resulting in severe aortic coarctation.Reference Jang, Son, Lee, Lee and Kim4–Reference Pass, Hijazi, Hsu, Lewis and Hellenbrand8 Intraductal or intra-ampullary placement of the retention disc is an option but may be technically challenging in these large ducts with device having propensity to herniate across the duct into the pulmonary artery. Even if one is successful in intra-ampullary deployment, this may result in globular deformation of the retention disc which has a higher chance of getting milked out of the ampulla with resultant embolisation to the pulmonary artery.
In comparison to Amplatzer duct occluder, the aortic disc of Amplatzer duct occluder II can be delivered more predictably close to the aortic end of the ampulla and being less stiff is unlikely to cause significant obstruction to the aortic lumen even if there is a mild protrusion. Also, the Amplatzer duct occluder II cable is much thinner and softer; as a result, there is insignificant recoil of the device after release. This usually prevents significant complication at the aortic end. However, the pulmonary disc of the Amplatzer duct occluder II is far too large for this subset of small patients, and it does not take its final position till it is released from the loading cable. So while on cable, the disc may appear non-obstructive on echo or angiogram but after its release, it can get sucked into the left pulmonary artery origin producing significant left pulmonary artery stenosis which may be progressive.Reference Baspinar, Irdem and Sivasli5,Reference Pepeta and Dippenaar10,Reference Thanopoulos, Eleftherakis, Tzannos and Stefanadis11
The Amplatzer vascular plug II can overcome both these problems due to absence of protruding discs on either side as was shown in our study where none of the patients had these complications. Moreover, unlike Amplatzer vascular plug I, the second generation Amplatzer vascular plug II with two peripheral and one central disc of equal diameter with multi-layered mesh lobes creates six occlusive planes for patent arterial duct closure, thereby reducing the incidence of residual shunt. This was exemplified in the present study, wherein all the patients had complete closure of the duct at the time of their last follow-up.
Why the ductal length is crucial for using Amplatzer vascular plug II
In anatomically small patent arterial ducts with a small shunt flow, Amplatzer vascular plug II can remain in position and stability is not a concern. However, in large ducts where the flow is torrential as was the case in this study, Amplatzer vascular plug II embolisation remains the main concern. As shown in the present cohort, in two patients with a relatively shorter ducts, the Amplatzer vascular plug II either embolised (n = 1) or was found to be unstable (n = 1).
With no protruding retention discs, the only retaining force for the Amplatzer vascular plug II is the tight apposition of the central lobe and two peripheral discs to the ductal wall. Hence, there is a need for the ductal length to be adequate to accommodate almost the entire device length (up to 10 mm) allowing snug contact with the ductal wall. That is the reason why Amplatzer vascular plug II should be deployed in a way that does not elongate the central lobe and that the device length remains shorter than the ductal length. This point has been emphasised in the earlier studiesReference Schwartz, Glatz, Rome and Gillespie3,Reference Sant’Anna, da Costa and Ribeiro12–Reference VanLoozen, Sandoval and Delaney15,Reference Barwad, Ramakrishnan and Kothari17 as well.
Sizing the Amplatzer vascular plug II
The Amplatzer vascular plug II should be at least 150–200% of the maximum ductal diameter. This helps in stenting the ductal wall and stabilising the device in the absence of protruding retention discs. However, with oversizing, the device deforms and elongates. Hence, the length of the fully expanded device needs to be taken into consideration. For example, the length of a 10-mm Amplatzer vascular plug II is 7 mm. However, if used to occlude a 5.5-mm patent arterial duct, this device will logically elongate further to around 10-mm length. If this elongation causes protrusion of the peripheral discs outside the length of the duct, there is a higher potential for embolisation and left pulmonary artery stenosis. Instead, an 8-mm Amplatzer vascular plug II, also with a fully expanded length of 7 mm, may be more satisfactory, as it might only elongate to 8 mm and remain intraductal. Some authors have described pushing the elongated device against an expanded balloon in the aorta, thereby preventing undue lengthening of the central lobe (Amin Z, personal communication).
Method of deployment of the Amplatzer vascular plug II
We believe our method of deployment also played an important role in the accurate and predictable intraductal positioning of the Amplatzer vascular plug II. Unlike the Amplatzer duct occluder where the retention disc and body of the device are sequentially deployed, with tension maintained on the cable as the device is unsheathed, we employed an alternative technique for the Amplatzer vascular plug II deployment as described above. This method of device delivery prevented undue elongation of the device resulting in the intraductal position and allowing better and closer apposition of the discs, with adequate support from the ductal wall. Ideally, if all three discs are intraductal, the surface area of contact with the long ductal walls is maximum resulting in improved stability of the device. Since such a position has a theoretically higher chance of aortic embolisation, we preferred that at least some part of the proximal disc remains in the pulmonary artery.
Conclusions
Amplatzer vascular plug II is a safe, reliable, and an effective option for closure of large and tubular ducts in young infants. The absence of retention discs in the Amplatzer vascular plug II gives an added benefit of avoiding device-induced left pulmonary artery stenosis and aortic coarctation. The size of the Amplatzer vascular plug II >150% of the duct diameter and more importantly the length of the duct more than the length of the deployed device are probably necessary to ensure stable device position.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors have no conflict of interest related to this manuscript and have no financial dealings with any device company.
Ethical Standards
This research does not include human experimentation and describes cases from routine clinical practice.






