There is considerable interest in the possibility that exposure to events during intrauterine life can influence subsequent development. If early environmental exposures have causal effects on the likelihood of psychopathology later in life, this has clear implications for early intervention and prevention. The teratogenic effects of thalidomide, rubella, high levels of alcohol, and most recently Zika virus infection on the fetus are well known (Rasmussen, Jamieson, Honein, & Petersen, Reference Rasmussen, Jamieson, Honein and Petersen2016; Thapar & Rutter, Reference Thapar and Rutter2009). In more recent years, the effects of exposures to a broader set of prenatal risks on the development of the offspring have been examined. These risks include exposures such as maternal smoking during pregnancy, maternal depression, anxiety and stress during pregnancy, inadequate maternal nutrition, certain types of medication (e.g., antidepressants), toxins (e.g., lead), and maternal physical illness (e.g., autoimmune diseases; Instanes et al., Reference Instanes, Halmoy, Engeland, Haavik, Furu and Klungsoyr2017). The hypothesized causal mechanisms include direct toxic effects on the fetal brain, hypoxia, disrupted placental function, immune and inflammatory processes, and “developmental programming” that leads to later adult disease. The developmental origins of adult disease (Barker, Reference Barker2007) is a hypothesis that was first considered in relation to ischemic heart disease and type 2 diabetes and subsequently has received considerable attention. It suggests that intrauterine exposure to adversity (e.g., undernutrition) during a sensitive period of development (fetal life) leads to potentially permanent alterations in the structure, physiology, and metabolism of the organism, and this in turn increases susceptibility to later disease (e.g., ischemic heart disease). Nonetheless, as documented in detail elsewhere, there are numerous challenges in establishing whether environmental exposures exert true causal risk effects on developmental outcomes (D'Onofrio, Class, Lahey, & Larsson, Reference D'Onofrio, Class, Lahey and Larsson2014; Gage, Munafo, & Davey Smith, Reference Gage, Munafo and Davey-Smith2016; Rutter, Pickles, Murray, & Eaves, Reference Rutter, Pickles, Murray and Eaves2001; Rutter & Thapar, Reference Rutter, Thapar and Cicchetti2016; Thapar & Rutter, Reference Thapar, Rutter, Thapar, Pine, Leckman, Scott, Snowling and Taylor2015). These include reverse causation, continuing adversity following the initial exposure, and measured and unmeasured confounding. Reverse causation highlights the possibility that the outcome might cause the exposure rather than the other way round. The classic example of this relates to the reinvestigation of socialization effects as child effects on parents (Bell, Reference Bell1968). There are now many examples of instances where children's behavior and psychopathology has effects on parents (Anderson, Hytton, & Romney, Reference Anderson, Hytton and Romney1986; Sellers et al., Reference Sellers, Hammerton, Harold, Mahedy, Potter, Langley and Collishaw2016). Often exposures of interest are associated with continuity over time; for instance, it may be difficult to disentangle the risk effects of exposure to stress in utero from stress exposure later in development (Thapar & Rutter, Reference Thapar and Rutter2009). In the case of confounding, seemingly causal links can be explained by confounding variables that are associated with both exposure and outcome, and it is not necessarily possible to measure or test for all possible confounders, meaning that residual confounding is a serious problem for observational studies. Residual confounding therefore refers to confounding that remains even when the effect of measured confounders is included in statistical analyses and arises because of measurement error in confounders and unmeasured confounding (Fewell, Davey Smith, & Sterne, Reference Fewell, Davey Smith and Sterne2007). This means that erroneous conclusions about causality can be and are drawn from such designs.
One key challenge to rule out is the possibility that an observed association is due to person–environment correlation as this is potentially an important source of confounding in relation to psychopathology, for example, where maternal characteristics influence the exposure (e.g., diet during pregnancy) and outcome variables (e.g., her offspring's behavior). Passive gene–environment correlation (rGE) is a special instance of a person–environment correlation, where the prenatal environment is indexed in part by maternal characteristics including genetic factors that are transmitted to the offspring (mothers and offspring share 50% of their genome; Figure 1).

Figure 1. (Color online) Schematic of passive gene–environment correlation for the prenatal environment. A dashed arrow between prenatal exposure and child outcome and a filled arrow between maternal and child genes illustrates passive gene environment correlation (i.e., that association may arise because of genes shared between mother and child rather than a causal environmental risk effect). Double-headed arrows represent correlations, and directional arrows represent associations.
Thus, observational studies that find association between a prenatal exposure and offspring psychopathology are liable to identifying associations that are not necessarily causal. However, there are designs that enable more robust assessments of causal inference (Davey Smith, Reference Davey Smith2008; Gage et al., Reference Gage, Munafo and Davey-Smith2016; Rutter & Thapar, Reference Rutter, Thapar and Cicchetti2016; Thapar & Rutter, Reference Thapar, Rutter, Thapar, Pine, Leckman, Scott, Snowling and Taylor2015). Genetically informed designs are especially attractive because they separate the genetic and environmental contributions to the association between intrauterine exposure and offspring outcome. The relevance of genetic designs for assessing environmental risk is now widely appreciated in the field of developmental psychopathology. However, it is not always recognized that the designs that distinguish relevant genetic and environmental contributions differ for prenatal and postnatal exposures (see Figure 1 and Table 1); we describe these in detail in this review. The genetic and environmental contributions that need to be separated when investigating prenatal risks are those shared between parents and offspring. For prenatal/intrauterine exposures, the contribution of maternal behaviors and genes is especially important. In this review we focus on the genetically informed family-based comparison designs where either the degree of genetic relatedness differs between types of mother–offspring pair or the genetic relationship is held constant and the intrauterine environment varies (Figure 1; Table 1). These sorts of designs have been used widely to examine questions about the causal relationship between specific prenatal exposures and offspring outcomes, and they allow inferences to be made about separating the contribution of the maternal genome from the intrauterine environment. We note, however, that there are other types of genetically informed designs (e.g., Mendelian randomization and the polygenic transmission disequilibrium test; Davey Smith & Hemani, Reference Davey Smith and Hemani2014; Weiner et al., Reference Weiner, Wigdor, Ripke, Walters, Kosmicki, Grove and Robinson2017) that use information on the specific genetic variants involved in a trait (as opposed to inferring the effects of the entire maternal genome). These sorts of designs have not yet been widely used for prenatal exposures and offspring outcomes and currently capture a small proportion of the genetic variation involved. They are, however, likely to become more important in the future as genome-wide association studies identify increasing numbers of genetic variants that are robustly associated with psychopathology and health-related behaviors. These sorts of designs are also useful for triangulation of evidence.
Table 1. Summary of if and how particular research designs separate prenatal and genetic contributions to offspring outcome

There is good evidence from observational studies, including meta-analyses, that a number of different exposures during prenatal development show association with psychopathology in offspring (Abraham et al., Reference Abraham, Alramadhan, Iniguez, Duijts, Jaddoe, Den Dekker and Turner2017; Rice, Jones, & Thapar, Reference Rice, Jones and Thapar2007; Ruisch, Dietrich, Glennon, Buitelaar, & Hoekstra, Reference Ruisch, Dietrich, Glennon, Buitelaar and Hoekstra2017; Talge, Neal, Glover, & Early Stress Translational Research Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Health, Reference Talge, Neal and Glover2007). One of the most widely examined exposures is maternal smoking during pregnancy, which has been observed to be associated with increased symptoms of attention-deficit/hyperactivity disorder (ADHD) and conduct problems in offspring (Huizink & Mulder, Reference Huizink and Mulder2006; Langley, Rice, van den Bree, & Thapar, Reference Langley, Rice, van den Bree and Thapar2005; Linnet et al., Reference Linnet, Dalsgaard, Obel, Wisborg, Henriksen, Rodriguez and Jarvelin2003). Other studies have focused on severely restricted maternal nutrition, which is associated with an increased risk of psychosis and depression in offspring when they reach adult life (Brown, van Os, Driessens, Hoek, & Susser, Reference Brown, van Os, Driessens, Hoek and Susser2000; St. Clair et al., Reference St. Clair, Xu, Wang, Yu, Fang, Zhang and He2005), and maternal stress, which is associated with a wide range of symptoms of psychopathology in offspring (Rice, Jones, & Thapar, Reference Rice, Jones and Thapar2007; Talge et al., Reference Talge, Neal and Glover2007). Much recent interest has focused on maternal use of medications during pregnancy, including antidepressants and acetaminophen (paracetamol), as well as maternal chronic illnesses (Avella-Garcia et al., Reference Avella-Garcia, Julvez, Fortuny, Rebordosa, Garcia-Esteban, Galan and Sunyer2016; Brown et al., Reference Brown, Gyllenberg, Malm, McKeague, Hinkka-Yli-Salomaki, Artama and Sourander2016, Reference Brown, Ray, Wilton, Lunsky, Gomes and Vigod2017; Grzeskowiak et al., Reference Grzeskowiak, Morrison, Henriksen, Bech, Obel, Olsen and Pedersen2016; Instanes et al., Reference Instanes, Halmoy, Engeland, Haavik, Furu and Klungsoyr2017; Man et al., Reference Man, Chan, Ip, Coghill, Simonoff, Chan and Wong2017; Rai et al., Reference Rai, Lee, Dalman, Newschaffer, Lewis and Magnusson2017; Stergiakouli, Thapar, & Davey Smith, Reference Stergiakouli, Thapar and Davey Smith2016). However, it is unclear to what extent these observed associations are due to prenatal causal risk effects or other factors including familial and genetic confounding. Some investigators explicitly acknowledge this (e.g., Instanes et al., Reference Instanes, Halmoy, Engeland, Haavik, Furu and Klungsoyr2017); others do not. Fortunately, there is growing interest in alternative methods for assessing causality. The importance of considering and testing for the possibility that observed associations between prenatal exposures and offspring outcomes may not be causal has been highlighted for scientific reasons. It is also important for practical and policy reasons, including ensuring that pregnant women receive clear and appropriate as well as accurate advice and guidance, providing antenatal care that is consistent with current scientific evidence, and avoiding the possibility of wasting resources on ineffective intervention (Gage et al., Reference Gage, Munafo and Davey-Smith2016; Rutter, Reference Rutter2007; Thapar & Rutter, Reference Thapar and Rutter2009).
In this review we begin by describing the phenomenon of person–environment correlation and passive rGE in detail and present new data on maternal smoking during pregnancy from the Cardiff in vitro fertilization (IVF) study (Thapar et al., Reference Thapar, Harold, Rice, Ge, Boivin, Hay and Lewis2007) to illustrate key points. Next, we explain the genetically informative research designs that can address familial confounding and passive rGE for prenatal exposures and consider their strengths and limitations. We then systematically assess studies for two prenatal exposures where the plausible hypothesized processes underlying any potential causal association differ. The first is maternal smoking in pregnancy, where any possible causal effect on offspring development and psychopathology seems likely to come about via effects of toxin exposure and/or effects secondary to this such as effects on blood flow or placental functioning that directly affect the developing brain (Ruisch et al., Reference Ruisch, Dietrich, Glennon, Buitelaar and Hoekstra2017; Slotkin, Reference Slotkin2013). The second is maternal stress during pregnancy, where developmental programming of the hypothalamic–pituitary–adrenal axis is hypothesized to underlie any potential causal effect on offspring psychopathology (Talge et al., Reference Talge, Neal and Glover2007). For maternal smoking during pregnancy, a large number of studies have been carried out, and therefore, we selected studies to review that have reported links between maternal smoking in pregnancy and offspring conduct problems and ADHD. The reason for selecting those outcomes is because reported results have been somewhat inconsistent and misinterpreted, meaning a systematic review of the findings from informative study designs would be useful and is important in the context of triangulation of evidence. Triangulation has been described as “the practice of obtaining more reliable answers to research questions through integrating results from several different approaches where each approach has different key sources of potential bias that are unrelated to each other” (Lawlor, Tilling, & Davey Smith, Reference Lawlor, Tilling and Davey Smith2016). Thus, it involves evaluating evidence from different studies that employ different research designs that have differing patterns of strength and weakness: where results converge strengthens the evidence for the reasons for an observed association (causal or not), where they do not requires careful consideration of the evidence, and the likely biases involved and identification of what further research is needed (Lawlor et al., Reference Lawlor, Tilling and Davey Smith2016). This process has some similarities with the concept of “constructive replication,” whereby replication of findings is seen to strengthen evidence only if it removes some weakness in previous studies (Academy of Medical Sciences, 2007). Finally, we highlight areas for future work.
What Is Person–Environment Correlation and Why Is It Important for Prenatal Risk Exposures?
Developmental science shows that people behave in ways that shape their environments, and these environments have important implications for developmental psychopathology. For instance, children with antisocial behavior evoke hostile reactions from others, which serve to further exacerbate that behavior in the child (Anderson et al., Reference Anderson, Hytton and Romney1986; Ge et al., Reference Ge, Conger, Cadoret, Neiderhiser, Yates, Troughton and Stewart1996; Rutter, Moffitt, & Caspi, Reference Rutter, Moffitt and Caspi2006). Individual differences in personality can also affect a persons’ environment; for example, a child concentrating and focusing on an academic task may elicit responses from a teacher that sustains that behavior (Shiner & Caspi, Reference Shiner and Caspi2003). Person–environment correlation also applies to maternal behaviors during pregnancy in that there are measurable differences between mothers that engage in risk behaviors during pregnancy or experience stress and antenatal complications compared to those who do not. For example, mothers who smoke during pregnancy are younger, are more likely to be raising their children in a deprived socioeconomic background, have higher rates of psychopathology (depression and antisocial behavior) and substance use, report greater stress during pregnancy, and are more likely to be nicotine dependent (D'Onofrio, Lahey, Turkheimer, & Lichtenstein, Reference D'Onofrio, Lahey, Turkheimer and Lichtenstein2013; Gilman, Breslau, Subjamian, Ijitsman, & Koenen, Reference Gilman, Gardener and Buka2008; Gustavson et al., Reference Gustavson, Ystrom, Stoltenberg, Susser, Suren, Magnus and Reichborn-Kjennerud2017; Maughan, Taylor, Caspi, & Moffitt, Reference Maughan, Taylor, Caspi and Moffitt2004; Rice et al., Reference Rice, Harold, Boivin, Hay, van den Bree and Thapar2009). Data from the Cardiff IVF sample illustrated in Table 2 also illustrate this point in that mothers who do not smoke, abstain from smoking to prepare for pregnancy, or continue smoking during pregnancy differ on socioeconomic factors, psychopathology, and amount smoked prior to pregnancy. Data on medical complications during pregnancy are also consistent with a different form of person effects on the prenatal environment, in terms of maternal disease liability that could be transmitted to offspring, rather than maternal behavior. For example, women who develop preeclampsia, gestational hypertension, or abruption or infarction of the placenta are at heighted risk for later developing cardiovascular disease and diabetes after pregnancy (Kaaja & Greer, Reference Kaaja and Greer2005; McDonald, Maliniwski, Zhou, Yusuf, & Devereaux, Reference McDonald, Malinowski, Zhou, Yusuf and Devereaux2008; Ray, Vermeulen, Schull, & Redelmeier, Reference Ray, Vermeulen, Schull and Redelmeier2005). This implies therefore that pregnancy may reveal biological vulnerabilities for chronic physical disease that lie dormant before pregnancy. Thus, if women's offspring develop similar illnesses, this could be due to inherited liability not necessarily because of prenatal exposure to the disease. These observations and data then serve to illustrate the point that maternal characteristics influence both the prenatal and the postnatal rearing environment. What implications does this have for research examining the influence of prenatal exposures on offspring development and psychopathology? One major issue is that the factors associated with these differences in the prenatal environment (e.g., for maternal smoking in pregnancy, socioeconomic factors, and psychopathology) are in themselves associated with developmental differences and psychopathology in offspring (D'Onofrio et al., Reference D'Onofrio, Lahey, Turkheimer and Lichtenstein2013; Repetti, Taylor, & Seeman, Reference Repetti, Taylor and Seeman2002). This then raises the issue that confounding may account for associations between prenatal smoking and offspring psychopathology. For instance, it is possible that the association between prenatal smoking and offspring outcome could be due to common confounding causes including genetic ones, as highlighted earlier (see Figure 1). We will illustrate later that including measured confounders (e.g., parent psychopathology) into statistical tests of association does not remove the problem (e.g., D'Onofrio, van Hulle, Goodnight, Rathouz, & Lahey, Reference D'Onofrio, van Hulle, Goodnight, Rathouz and Lahey2012; Gustavson et al., Reference Gustavson, Ystrom, Stoltenberg, Susser, Suren, Magnus and Reichborn-Kjennerud2017; Rice et al., Reference Rice, Harold, Boivin, Hay, van den Bree and Thapar2009; Thapar et al., Reference Thapar, Rice, Hay, Boivin, Langley, van den Bree and Harold2009).
Table 2. Comparison of maternal characteristics in Cardiff IVF sample by smoking before and during pregnancy status

Note: As illustrated above, mothers who smoke during pregnancy differ on socioeconomic factors, psychopathology, and amount smoked prior to pregnancy compared to nonsmoking mothers. It can also be observed that mothers who continue to smoke during pregnancy also differ from those who smoked only in the year before the pregnancy. These data therefore show that maternal characteristics influence both the prenatal and the postnatal rearing environments. Maternal smoking was defined using a combination of antenatal records and maternal retrospective report. For further detail on the sample and the measures included, please see Rice et al., Reference Rice, Harold, Boivin, Hay, van den Bree and Thapar2009, Reference Rice, Harold, Boivin, van den Bree, Hay and Thapar2010; Thapar et al., Reference Thapar, Rice, Hay, Boivin, Langley, van den Bree and Harold2009. *p < .05. **p < .01. ***p < .001.
What Is Passive rGE?
The phenomenon rGE occurs when the genetic and environmental contributors to a trait, behavior, or exposure are correlated. Three key types have been distinguished: passive, evocative, and active (Plomin, DeFries, & Loehlin, Reference Plomin, DeFries and Loehlin1977). Here, we focus on passive rGE, which refers to the special instance in which the child's genotype is correlated with the environment provided by his/her parents. This occurs because parents typically provide both genes and environment to their children. This means that the prenatal and the postnatal rearing environments are correlated with genetic characteristics in the parental generation, and because parents pass genes on to their offspring, also in the child generation (Figure 1). Many postnatal environmental factors that have important risk effects on psychopathology in children such as parenting style and stressful life events are influenced by parent's heritable characteristics (Jaffee & Price, Reference Jaffee and Price2008; Kendler & Baker, Reference Kendler and Baker2007; Reiss, Neiderhiser, Hetherington, & Plomin, Reference Reiss, Neiderhiser, Hetherington and Plomin2000). This is also true for the prenatal environment, and we use the example of maternal smoking during pregnancy to illustrate the point. As described above, there are systematic differences between women who smoke and do not smoke during pregnancy (see also Table 2). Smoking behavior is a heritable trait, with twin studies showing heritability estimates of between 50% and 70% for smoking persistence and nicotine dependence (Kendler et al., Reference Kendler, Neale, Sullivan, Corey, Gardner and Prescott1999; Lessov et al., Reference Lessov, Martin, Statham, Todorov, Slutske, Bucholz and Madden2004; Li, Cheng, et al., Reference Li, Cheng, Ma and Swan2003; Maes, Sullivan, et al., Reference Maes, Sullivan, Bulik, Neale, Prescott, Eaves and Kendler2004), and genome-wide association studies have identified a number of genetic loci that increase susceptibility for smoking-related behaviors (number of cigarettes smoked per day, smoking initiation, and smoking cessation; Tobacco and Genetics Consortium, 2010). The fact that smoking behavior is heritable then raises the possibility that prenatal exposure to smoking, an apparently “environmental” risk factor, is a marker of maternal genetic predisposition, and these same risk genotypes are then transmitted to the next generation and influence risk for psychopathology in the offspring (Figure 1). This supposition is supported by the observation that mothers who smoke and those who do not systematically differ on factors important for children's development (e.g., maternal psychopathology, substance use, and maternal education) and that are heritable. More recent molecular genetic studies also find that genetic risks that contribute to smoking behavior are correlated with those that contribute to psychopathology, including ADHD (Demontis et al., Reference Demontis, Walters, Martin, Mattheisen, Als and Agerbo2017).
Genetically informed designs are valuable for assessing the role of unmeasured or imperfectly measured confounding, including familial and genetic confounding. Confounding, where the exposure and outcome examined have common causes, is a major threat to the validity of observational studies. Where randomization of exposures is not possible or ethical (e.g., randomly exposing offspring to cigarette smoke in utero), then genetically sensitive designs (and other types of natural experiment and quasi-experimental designs) are extremely useful. Next, we provide a description of the sorts of designs that are required to tease apart environmental and genetic factors contributing to the association between prenatal exposures and offspring outcome because they are different from the typical designs used to tease apart genetic and environmental influences relevant to postnatal exposures (Figure 2; Table 1).

Figure 2. (Color online) Schematic of genetically sensitive designs that separate genetic and environmental contributions to prenatal exposure and offspring outcome. In the discordant sibling design, the genetic relationship between biological mother and children is .5. In the children of twins design, identical twin mothers are equally related to their own child (.5) and their sister's child (.5) because identical twin mothers share all their genes in common. In the comparison of maternal and paternal exposures, biological mother and biological father each share .5 of their genes with their child.
Which Genetically Informative Designs Are Helpful for Detecting Familial Confounding and Passive rGE for Prenatal Exposures?
While traditional observational studies cannot distinguish between causal intrauterine effects and rGE, a number of designs are able to separate the prenatal environment from genetic factors shared between parent/mother and offspring (Figure 2; Table 1). First, is the comparison of maternal versus paternal prenatal exposure associations with offspring outcomes. Only in the mother–child association is there a possibility of a direct intrauterine effect, but mothers and fathers both share 50% of their genes with their offspring, meaning that the extent to which association between the prenatal exposure and offspring outcome indexes genetic effects shared between parent and child can be assessed. In effect, the inclusion of data on paternal exposure serves as a negative control (Gage et al., Reference Gage, Munafo and Davey-Smith2016). Taking the example of smoking, in the case of a causal intrauterine effect, no independent association should be observed between paternal smoking and offspring outcome. However, if the association is due to either unmeasured genetic factors or other confounders, the risk to offspring of an adverse outcome should be of similar magnitude regardless of which parent smokes (Langley, Heron, Smith, & Thapar, Reference Langley, Heron, Smith and Thapar2012). Second are sibling comparison designs where differentially exposed sibling pairs are compared, for example, where a mother smoked for one pregnancy and not another. In effect, siblings are matched “by nature” on many confounders, including those that are unmeasured or unknown, making this a convenient method for dealing with confounding (Sjölander & Zetterqvist, Reference Sjölander and Zatterqvist2017). The use of the unexposed sibling group as a control comparison allows the effect of familial confounding for all factors shared within the family to be assessed. A comparison of differentially exposed cousins allows for the control of some shared familial cofounding but less so than for siblings. Third is the children of twins design. For prenatal exposures, the comparison of the offspring of identical mother twins is most informative, where the offspring of identical twin mothers are equally related to their mother (50%) and their aunt (50%) but the cousins experience a different prenatal environment. This design has not yet been widely employed for investigating prenatal exposures on child developmental outcome (see D'Onofrio et al. Reference D'Onofrio, Turkheimer, Eaves, Corey, Berg, Solaas and Emery2003; Knopik et al., Reference Knopik, Marceau, Bidwell, Palmer, Smith, Todorov and Heath2016, as exceptions). Fourth is the IVF design, where related and unrelated mother–offspring pairs are compared; this is a prenatal cross-fostering design, meaning some mothers experience a pregnancy for a child to whom they are not genetically related (by either egg/embryo donation or gestational surrogacy). In unrelated mother–child pairs (where an unrelated mother/surrogate experiences the pregnancy), then association between a prenatal exposure and a child outcome must come about through intrauterine effects because while the mother/surrogate experiences the pregnancy, she shares no genes with the baby, meaning prenatal passive rGE is removed.
What these designs have in common is that they allow the effect of the intrauterine environment to be differentiated from genetic factors that mothers share with their offspring (Table 1; Figure 2). In essence, the designs do this in one of two ways: complete separation of the maternal genome shared with the offspring and the prenatal environment (IVF prenatal cross-fostering design) or by varying the prenatal environment (e.g., across different pregnancies in the same mother or in the separate pregnancies of identical twin mothers) while holding the mother–child genetic relationship constant (Figure 2). These are the crucial aspects of addressing passive rGE for prenatal environmental exposures, and as such the family-based genetically sensitive methods differ for prenatal and postnatal exposures. The comparison of maternal and paternal prenatal exposure associations also provides a useful type of negative control because only in mothers is it plausible that there is an intrauterine effect of the exposure variable (for smoking, in the absence of a substantial passive smoking effect; Gage et al., Reference Gage, Munafo and Davey-Smith2016; Langley et al., Reference Langley, Heron, Smith and Thapar2012). We next explain why the genetically sensitive designs typically used for separating genetic and environmental contributions to postnatal environments (adoption studies after birth and twin studies) are inappropriate for prenatal risks before describing the strengths and limitations of the appropriate prenatal genetically sensitive designs.
Why Adoption After Birth Studies Are Not Informative for Identifying Prenatal Passive rGE
As described above, the key requirement for detecting passive rGE in the case of prenatal exposure variables is that the effect of the intrauterine environment can be isolated from genetic factors that mothers share with their offspring. This requirement means that many of the usual genetically sensitive designs, such as twin studies and adoption studies where children are adopted after birth, are not useful for detecting passive rGE for prenatal environmental exposures. Adoption after birth studies can instead be used to examine whether the postnatal rearing environment has any moderating effect on the relationship between a prenatal exposure and an offspring outcome (Gaysina et al., Reference Gaysina, Fergusson, Leve, Horwood, Reiss, Shaw and Harold2013; Rice, Jones, et al., Reference Rice, Jones and Thapar2007). For standard adoption designs where the genetic mother experiences the pregnancy but the child is adopted after birth, there is no separation of the intrauterine environment from (biological) mother provided genetic effects because biological mother provides genes and the prenatal environment to her offspring, even though she does not provide the postnatal rearing (Table 1). This means that the basic comparison between prenatal exposure and offspring outcome in the genetic mother whose child is then adopted is essentially exactly the same as it would be in a standard observational design. Unfortunately, this failure of adoption studies to address prenatal passive rGE has not always been understood or clearly explicated, meaning that erroneous conclusions may have been made (Dolan et al., Reference Dolan, Geels, Vink, van Beijsterveldt, Neale, Bartels and Boomsma2016; Gage et al., Reference Gage, Munafo and Davey-Smith2016; Gaysina et al., Reference Gaysina, Fergusson, Leve, Horwood, Reiss, Shaw and Harold2013; Slotkin, Reference Slotkin2013). Thus, although adoption studies are thought to lead to the removal of passive rGE, this only refers to passive rGE for the postnatal rearing environment (Rutter et al., Reference Rutter, Pickles, Murray and Eaves2001). It is also known that mothers whose children are adopted are systematically different from mothers whose children are not (Rutter et al., Reference Rutter, Pickles, Murray and Eaves2001). It is likely that this creates differences in the prenatal environment of children who are adopted compared to children who continue to live with their biological parent(s). Thus, mothers whose children are adopted after birth show higher rates of smoking, alcohol use, and illicit substance use during pregnancy and have higher rates of psychopathology, including ADHD and conduct problems, than mothers whose children continue to reside with them after birth (Gaysina et al., Reference Gaysina, Fergusson, Leve, Horwood, Reiss, Shaw and Harold2013). This creates a situation where biological mothers whose offspring are adopted away are a group at high background risk for psychopathology and risky prenatal exposures, and some of this risk will be due to dispositional and genetic factors that biological mothers share with their offspring. This means the degree of familial confounding is potentially higher than is typical. It then follows that if passive rGE for the prenatal environment and child outcome applies, one would expect to see stronger association in an adoption study after birth (when the prenatal exposure is assessed in the biological mother) than in a standard epidemiological design. This is what has been observed for prenatal smoking and offspring conduct problems when those adopted-after-birth, b = 4.27, 95% confidence interval (CI) [–0.90, 9.44]adjusted, are compared to those reared by their biological parents in the same cohort, b = 0.82, 95% CI [0.08, 1.56]adjusted, and from a meta-analysis, b = 2.17, 95% CI [0.72, 3.62]adjusted, reared by adoptive parents; b = 1.13, 95% CI [0.02, 2.24]adjusted, reared by biological parents (Gaysina et al., Reference Gaysina, Fergusson, Leve, Horwood, Reiss, Shaw and Harold2013). This observation therefore provides indirect evidence that there is passive rGE that applies to the link between maternal smoking during pregnancy and offspring conduct problems.
Why Twin Studies Are Not Informative for Identifying Prenatal Passive rGE
The standard twin design involves comparing the phenotypic similarity of identical (monozygotic; MZ) and nonidentical (dizygotic; DZ) twins. MZ twins share all their genes in common, and DZ twins share, on average, half their genes in common. Thus, comparing the similarity of MZ and DZ twins allows the variance of a trait to be decomposed into the proportions due to additive genetic effects, shared environmental effects (environmental influences that make members of a twin pair more similar), and unique or nonshared environmental effects (environmental effects that make members of a twin pair different). In the standard twin design and its extensions such as identical twin differences, it is not possible to identify twin pairs differentially exposed to a prenatal exposure because twins share a prenatal environment (at least as far as is typically measured) and all types of twin share exactly half their genes with their biological mother. This means that each member of a twin pair will be equivalently exposed to a prenatal exposure (e.g., smoking in pregnancy) and that the genetic relationship between mother and twin offspring does not differ across twin pairs. The standard twin design is therefore uninformative for the separation of genetic and environmental contributions to a prenatal exposure and offspring outcome. However, it can be used for assessing the role of perinatal risk factors on which twins may differ, such as birth weight (Tully, Arsenault, Caspi, Moffitt, & Morgan, Reference Tully, Arseneault, Caspi, Moffitt and Morgan2004).
Strengths and Limitations of the Prenatal Genetically Informative Designs
Four genetically sensitive designs for assessing the familial/genetic and environmental contributions to prenatal risk exposures and offspring outcome were described. Each has strengths and limitations, which we review briefly below. The first design, the comparison of prenatal exposures in mothers and fathers, is a convenient approach that controls for the genetic relationship between parent and child as children share exactly half of their genes with each parent. However, it is potentially contaminated by assortative mating, shared couple behaviors, and the shared postnatal family environment, and its assumptions can be violated if the confounding structure of the maternal and paternal exposures differ (Keyes, Davey Smith, & Susser, Reference Keyes, Davey Smith and Susser2014). For some exposures (e.g., cigarette smoking), passive exposure to paternal risks is a potential problem (e.g., father or other household members continue to smoke and mother and baby are exposed). The second design, the sibling comparison study, is a convenient way of controlling for confounding factors shared by family members, and the existence of large population registers in many Scandinavian countries has meant that extremely large sample sizes representative of the general population are available. This is an important strength. However, only siblings who have different prenatal risk exposures contribute to the meaningful comparison in discordant sibling comparisons, and therefore such designs are susceptible to confounding by nonshared factors that might lead to such changes in the mother (Frisell, Öberg, Kula-Halkola, & Sjölander, Reference Frisell, Öberg, Kuja-Halkola and Sjölander2012). In addition, there is the problem of carryover effects where the exposure and outcome of one offspring affects the exposure and outcome of his or her siblings (Sjölander, Frisell, Kuja-Halkola, Öberg, & Zetterqvist, Reference Sjölander, Frisell, Kuja-Halkola, Öberg and Zetterqvist2016). One instance where carryover effects might exist would be if Caesarean section was the exposure variable, where a Caesarean section in one pregnancy might well affect the likelihood of exposure in a subsequent pregnancy. Nonetheless, tests of carryover effects to date have not found this to be an issue for maternal smoking during pregnancy and offspring conduct problems or ADHD (D'Onofrio et al., Reference D'Onofrio, Singh, Iliadou, Lambe, Hultman, Grann and Lichtenstein2010, Reference D'Onofrio, van Hulle, Goodnight, Rathouz and Lahey2012; Skoglund, Chen, D'Onofrio, Lichtenstein, & Larsson, Reference Skoglund, Chen, D'Onofrio, Lichtenstein and Larsson2014). The third design type, the children of twin mothers design, provides an opportunity for investigating the effects of the prenatal environment controlling for shared maternal genes. Strengths include the ability to estimate genetic and environmental influences in the parent and child generation in addition to genetic and environmental transmission paths (using structural equation modeling) without the need for strong assumptions (D'Onofrio et al., Reference D'Onofrio, Turkheimer, Eaves, Corey, Berg, Solaas and Emery2003) and the existence of statistical models to test a variety of extensions to the design (Silberg, Maes, & Eaves, Reference Silberg, Maes and Eaves2010). Limitations include the need to consider paternal effects, assortative mating, and the need for sufficient numbers of similarly aged offspring from identical twin mothers and the need for large sample sizes. The fourth design type, the IVF design, allows unambiguous separation of the prenatal environment from the maternal genome, making it a powerful approach for detecting prenatal passive rGE. One main limitation is generalizability: are those that conceive via IVF similar to the population that conceives naturally? The evidence shows that for parental psychopathology, child psychopathology, and the family environment the answer to this question is yes (Golombok, Reference Golombok2017; Golombok & MacCallum, Reference Golombok and MacCallum2003; Shelton et al., Reference Shelton, Boivin, Hay, van den Bree, Rice, Harold and Thapar2009). However, those conceiving via IVF are at elevated risk of perinatal complications, and the rates of exposure for some prenatal risks (e.g., maternal smoking during pregnancy) are low. Another limitation is that sample sizes of the informative groups (i.e., unrelated mother–child pairs) are also small given the considerable effort involved in identifying these groups. As is the case for all studies, indicators of study quality such as reliability and validity of measurement, adequate sample size, and tests that the assumptions of the design are met also apply to genetically sensitive designs, and consideration of these issues is informative for “triangulation” of findings.
As described elsewhere, each type of quasi-experimental, genetically informed design we have highlighted has a different set of strengths and weaknesses, and none is without limitations (Rutter & Thapar, Reference Rutter, Thapar and Cicchetti2016). Nonetheless, the value of “natural experiments” that tease apart variables that usually go together has been noted as providing important additional leverage in answering questions of environmental causation. A number of other “natural experiment” approaches that do not directly distinguish the intrauterine environment from genetic factors shared between mother and offspring but that can be informative have been discussed in detail elsewhere (Academy of Medical Sciences, 2007; Gage et al., Reference Gage, Munafo and Davey-Smith2016; Rutter, Reference Rutter2007; Rutter & Thapar, Reference Rutter, Thapar and Cicchetti2016; Thapar & Rutter, Reference Thapar, Rutter, Thapar, Pine, Leckman, Scott, Snowling and Taylor2015). These include utilizing naturally occurring situations that have involved universal introduction or removal of prenatal risk. The best example here being the Dutch Hunger Winter and Chinese famine studies, which suggest that extreme prenatal nutritional adversity has likely causal risk effects on later schizophrenia (Lumey, Stein, & Susser, Reference Lumey, Stein and Susser2011; St. Clair et al., Reference St. Clair, Xu, Wang, Yu, Fang, Zhang and He2005; Susser et al., Reference Susser, Neugebauer, Hoek, Brown, Lin, Labovitz and Gorman1996). Other methods not yet mentioned include using changes in policy as natural experiments, instrumental variable approaches other than Mendelian randomization, and cross-cultural comparisons where the confounding structure of exposure variables differs (Davey Smith & Hemani, Reference Davey Smith and Hemani2014; Gage et al., Reference Gage, Munafo and Davey-Smith2016; Thapar & Rutter, Reference Thapar, Rutter, Thapar, Pine, Leckman, Scott, Snowling and Taylor2015). Animal studies that enable experimental design can also be helpful, but here there is the difficulty in assuming that offspring behavior in other species can be equated to child psychopathology (Thapar & Rutter, Reference Thapar, Rutter, Thapar, Pine, Leckman, Scott, Snowling and Taylor2015). Comparing prenatal factors in siblings with and without a psychiatric diagnosis can be informative (Grizenko et al., Reference Grizenko, Fortier, Zadorozny, Thakur, Schmitz, Duval and Joober2012; Oerlemans et al., Reference Oerlemans, Hartman, De Bruijn, van Steijn, Franke, Buitelaar and Rommelse2015), but population-based registers are needed to overcome issues of ascertainment and retrospective recall bias. We do not directly include studies using these methods in our review of prenatal smoking and gestational stress and offspring psychopathology.
Method
The effects of maternal smoking during pregnancy have been examined for a wide range of developmental outcomes, including child psychopathology. For some outcomes, the findings from such studies appear inconsistent. For the outcome of offspring birth weight, findings from a range of genetically informative designs including multiple maternal versus paternal comparisons, discordant sibling studies, a children of twins study, and an IVF study are remarkably consistent and consistent with a causal interpretation in that regardless of familial confounding or genes shared between mother and child, birth weight is reduced in the infants of mothers who smoked during pregnancy (D'Onofrio et al., Reference D'Onofrio, Turkheimer, Eaves, Corey, Berg, Solaas and Emery2003; Ellingson, Goodnight, van Hulle, Waldman, & D'Onofrio, Reference Ellingson, Goodnight, van Hulle, Waldman and D'Onofrio2014; Gilman, Gardener, & Buka, Reference Gilman, Gardener and Buka2008; Kuja-Halkola, D'Onofrio, Larsson, & Lichtenstein, Reference Kuja-Halkola, D'Onofrio, Larsson and Lichtenstein2014; Langley et al., Reference Langley, Heron, Smith and Thapar2012; Obel et al., Reference Obel, Zhu, Olsen, Breining, Li, Gronborg and Rutter2016; Rice et al., Reference Rice, Harold, Boivin, Hay, van den Bree and Thapar2009). We carried out a systematic search for studies of maternal smoking during pregnancy and offspring conduct problems and ADHD where results from genetically informative studies appear to be less consistent. We sought to identify studies using informative research designs (i.e., paternal vs. maternal smoking during pregnancy comparisons; discordant sibling and/or cousin comparisons; IVF design, which includes unrelated “prenatal” mother–child pairs; and children of twin studies). Figure 3 illustrates a flow chart of the search process and full details can be found in Appendix A. The results of the identified genetically informed family-based studies are summarized in Table 3. In our interpretation of results, we consider the following: magnitude of effect sizes, precision of effect sizes (i.e., the width of confidence intervals), the extent to which results are consistent across indicators of the same construct, and analytical options (also referred to as vibration of effects; Button et al., Reference Button, Ioannidis, Mokrysz, Nosek, Flint, Robinson and Munafo2013), and consistency in the pattern of results when negative controls are used.

Figure 3. (a) Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram detailing each stage of the review process for prenatal smoking as an exposure. (b) PRISMA flow diagram detailing each stage of the review process for prenatal stress as an exposure.
Table 3. Summary of papers identified in systematic search of maternal smoking during pregnancy and offspring conduct problems or ADHD
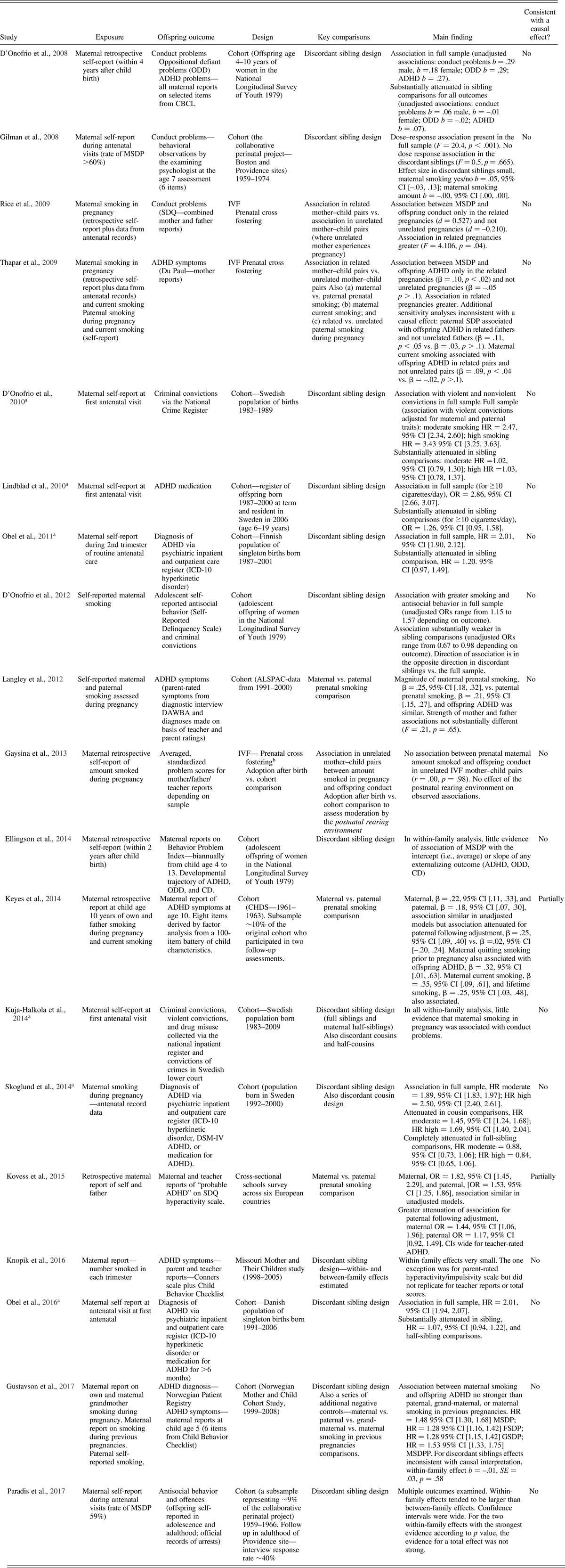
Notes: MSDP, mother smoking during pregnancy. FSDP, father smoking during pregnancy. MSDPP, mother smoking during previous pregnancies. GSDP, grandmother smoking during pregnancy. ODD, oppositional defiant disorder. CD, conduct disorder. ADHD, attention-deficit/hyperactivity disorder. HR, hazard rate. OR, odds ratio. CI, confidence interval. SDQ, Strengths and Difficulties Questionnaire. aA full population cohort. bSame IVF data set as Rice et al., Reference Rice, Harold, Boivin, Hay, van den Bree and Thapar2009 and Thapar et al., Reference Thapar, Rice, Hay, Boivin, Langley, van den Bree and Harold2009.
Results
Maternal smoking during pregnancy and offspring conduct problems: Findings from genetically informed family-based designs
Nine studies utilized an approach that should be robust to genetic and some other sources of confounding and examined offspring conduct problems or antisocial behavior in childhood, adolescence, and adult life. Two publications included the same IVF data set and assessment outcome (Gaysina et al., Reference Gaysina, Fergusson, Leve, Horwood, Reiss, Shaw and Harold2013; Rice et al., Reference Rice, Harold, Boivin, Hay, van den Bree and Thapar2009) meaning that eight independent studies remained, although some studies used the same sample but assessed conduct problems at a later time point (e.g., for childhood, Gilman, Gardener, et al., Reference Gilman, Gardener and Buka2008; for adolescence/adulthood, Paradis, Shenassa, Papandonatos, Rogers, & Buka, Reference Paradis, Shenassa, Papandonatos, Rogers and Buka2017). Differences in how the dependent and independent variables were assessed and in the analytical procedures employed complicate direct comparisons of the effect sizes observed in different studies. In analyses without controls for familial/genetic factors, the studies included in this systematic review report correlation coefficients (or b or β coefficients) between .1 and .3 (D'Onofrio et al., Reference D'Onofrio, van Hulle, Waldman, Rodgers, Harden, Rathouz and Lahey2008; Ellingson et al., Reference Ellingson, Goodnight, van Hulle, Waldman and D'Onofrio2014; Gaysina et al., Reference Gaysina, Fergusson, Leve, Horwood, Reiss, Shaw and Harold2013) and odds ratios (ORs) or hazard ratios (HRs) of between 1.01 and 3.43 depending on the outcome and scaling of the exposure variable (D'Onofrio et al., Reference D'Onofrio, van Hulle, Waldman, Rodgers, Harden, Rathouz and Lahey2008, Reference D'Onofrio, Singh, Iliadou, Lambe, Hultman, Grann and Lichtenstein2010, Reference D'Onofrio, van Hulle, Goodnight, Rathouz and Lahey2012; Kuja-Halkola et al., Reference Kuja-Halkola, D'Onofrio, Larsson and Lichtenstein2014; Paradis et al., Reference Paradis, Shenassa, Papandonatos, Rogers and Buka2017). These effect sizes are similar to that reported in a meta-analysis of observational studies, ODD = 2.06, 95% CI [1.67, 2.54] (Ruisch et al., Reference Ruisch, Dietrich, Glennon, Buitelaar and Hoekstra2017). Seven studies reported that the association between maternal smoking and offspring conduct problems was mainly attributable to familial or genetic confounding. For instance, Rice et al. (Reference Rice, Harold, Boivin, Hay, van den Bree and Thapar2009), using an IVF design (n = 779), observed an association between maternal smoking during pregnancy (defined by an amalgamation of data from self-report and antenatal records) in genetically related mother–child pairs (Cohen's d = 0.527). However, there was no association between maternal smoking during pregnancy and offspring conduct problems in the group of mothers who experienced the pregnancy but were genetically unrelated to their child (Cohen's d = –0.210). The magnitude of association was greater in the related mother–child pairs than in the unrelated mother–child pairs (test for difference in strength of association F = 4.106, p = .04). These results are therefore consistent with the association between maternal smoking during pregnancy and offspring conduct problems being due to passive rGE although the sample size, particularly the unrelated pregnancies exposed to maternal smoking during pregnancy, was unsurprisingly small. In this study as in others (D'Onofrio et al., Reference D'Onofrio, van Hulle, Waldman, Rodgers, Harden, Rathouz and Lahey2008, Reference D'Onofrio, Singh, Iliadou, Lambe, Hultman, Grann and Lichtenstein2010, Reference D'Onofrio, van Hulle, Goodnight, Rathouz and Lahey2012), including measured confounders, such as maternal antisocial behavior, did not alter association findings, highlighting the need for genetically informative designs because including measured confounders in analyses of observational data does not circumvent the problem of passive rGE. In a different analysis of the same IVF data set, Gaysina et al. (Reference Gaysina, Fergusson, Leve, Horwood, Reiss, Shaw and Harold2013) examined the relationship between maternal reported number of cigarettes smoked and offspring conduct problems. These authors also included an adoption-at-birth sample and observational cohort data. Consistent with what had been published previously (Rice et al., Reference Rice, Harold, Boivin, Hay, van den Bree and Thapar2009), in the analysis of the IVF unrelated mother–child pairs, no association between maternal smoking and offspring conduct problems was found and the correlation coefficient was zero for this group (r = .00, p = .98). These observations fail to support the hypothesis that there is a causal effect of maternal smoking during pregnancy on offspring conduct problems and suggest that genes shared between mother and child are important in explaining associations reported in observational studies. However, it is worth noting that the findings reported in one study (Gaysina et al., Reference Gaysina, Fergusson, Leve, Horwood, Reiss, Shaw and Harold2013) have been interpreted by others as being consistent with a causal effect (Dolan et al., Reference Dolan, Geels, Vink, van Beijsterveldt, Neale, Bartels and Boomsma2016; Slotkin, Reference Slotkin2013) despite not reporting data supporting such an interpretation as highlighted by Thapar and Rutter (Reference Thapar, Rutter, Thapar, Pine, Leckman, Scott, Snowling and Taylor2015). This is likely due to confusion in assumptions that data from adoption-after-birth studies enable causal inferences for prenatal exposures; they do not. This misinterpretation highlights the need for systematic review and clear reporting of findings (Academy of Medical Sciences, 2007). As described earlier, the association between prenatal smoking and offspring outcome in an adopted-after-birth study is uninformative regarding differentiating the influences of intrauterine and maternal genetic effects (because the biological mother who shares genes with the adopted away offspring experiences the pregnancy).
Results from six discordant sibling studies (D'Onofrio et al., Reference D'Onofrio, van Hulle, Waldman, Rodgers, Harden, Rathouz and Lahey2008, Reference D'Onofrio, Singh, Iliadou, Lambe, Hultman, Grann and Lichtenstein2010, Reference D'Onofrio, van Hulle, Goodnight, Rathouz and Lahey2012; Ellingson et al., Reference Ellingson, Goodnight, van Hulle, Waldman and D'Onofrio2014; Gilman, Gardener, et al., Reference Gilman, Gardener and Buka2008; Kuja-Halkola et al., Reference Kuja-Halkola, D'Onofrio, Larsson and Lichtenstein2014) report findings that are inconsistent with a causal effect of maternal smoking during pregnancy on offspring conduct problems. Some studies use partly overlapping samples but assess different offspring outcomes. These studies have tended to be based on large samples, including two studies of Swedish population-wide registries (sample sizes of 609,372 and 2,754,626), which are representative of the population as a whole, three of a representative population US sample (sample sizes of 6,066, 10,251, and 11,192), and one of a large US volunteer sample (sample size 52,919). Each of these six studies observes an association between maternal smoking during pregnancy and offspring conduct problems in the full population sample, but for the sibling comparisons that control for shared familial confounding, the association is substantially attenuated. For instance, D'Onofrio et al. (Reference D'Onofrio, Singh, Iliadou, Lambe, Hultman, Grann and Lichtenstein2010, Reference D'Onofrio, van Hulle, Goodnight, Rathouz and Lahey2012) reported results consistent with familial confounding for adult criminal behavior and adolescent antisocial behavior. For adult violent criminal convictions, the HR for association with high levels of maternal smoking during pregnancy was 3.43. In sibling comparison models, the HR was 1.03. For high adolescent antisocial behavior, the HR was 1.34 in the full sample and 0.67 in the sibling comparison. Similarly, Gilman, Gardner, et al. (Reference Gilman, Gardener and Buka2008) reported a dose-response relationship for amount mothers smoked during pregnancy and number of offspring conduct problems in the full sample (F = 20.4, p < .001) but no dose-response relationship in the sibling analysis (F = 0.5, p = .665). Those authors concluded that the results observed suggested that such effects were “not present,” “not readily distinguishable from a broader range of familial factors associated with maternal smoking,” or “were not detectable using the assessment methods available at the time of the study.” The findings from these discordant sibling studies are therefore also inconsistent with inferring a causal effect of maternal smoking during pregnancy on offspring conduct problems. Only one study in Table 3 reported evidence partially consistent with a causal effect of prenatal smoking on offspring antisocial behavior in a genetically informed design (Paradis et al., Reference Paradis, Shenassa, Papandonatos, Rogers and Buka2017), which was a discordant sibling study of a US cohort. That study was a subsample (sample size ranged from 1,883 to 3,447 depending on the outcome) of a much larger study (n = 52,919), which reported results inconsistent with a causal effect of maternal smoking during pregnancy on childhood conduct problems measured at age 7 (Gilman, Gardener, et al., Reference Gilman, Breslau, Subramanian, Hitsman and Koenen2008). In the study by Paradis et al. (Reference Paradis, Shenassa, Papandonatos, Rogers and Buka2017), the within-family effect tended to be larger than the between-family effect for the six offspring antisocial behavior outcomes examined. However, the confidence intervals for the within-family effects were very wide, and results fluctuated depending on the outcome variable and how it was defined. Of note, the total effect tended to be fairly low, and to some extent, this is what would be expected when the exposure variable is common in the general population as was the case in this sample where the prevalence of maternal smoking during pregnancy (in women pregnant between 1959 and 1966) was 59%. Attitudes toward smoking have become less permissive over time, which has had the effect that, in more recent cohorts, smoking behavior has become increasingly associated with psychiatric vulnerability and lower socioeconomic status (Gilman, Breslau, Subramanian, Hitsman, & Koenen, Reference Gilman, Breslau, Subramanian, Hitsman and Koenen2008; Talati, Keyes, & Hasin, Reference Talati, Keyes and Hasin2016; Talati et al., Reference Talati, Wickramaratne, Keyes, Hasin, Levin and Weissman2013). At time periods when maternal smoking during pregnancy was more normative, attenuated associations with offspring antisocial behavior may therefore be expected in the full population. In summary, all but one of the reports based on appropriate genetically informative designs reported no association between maternal smoking during pregnancy and offspring antisocial behavior during childhood, adolescence, and adulthood once familial/genetic confounding had been controlled. These results are therefore inconsistent with a causal effect on prenatal smoking on offspring conduct problems.
Maternal smoking during pregnancy and offspring ADHD problems: Findings from genetically informed family-based research designs
Twelve informative studies examined offspring ADHD as an outcome (Table 3). In analyses without controls for familial/genetic factors, the studies included in this systematic review report correlation coefficients (or β or b coefficients) between .10 and .32 and ORs or HRs of between 1.48 and 2.86 depending on the outcome and scaling of the exposure variable. These effect sizes are similar to that reported in a pooled analysis of observational studies, OR = 2.39, 95% CI [1.61, 3.52] (Langley et al., Reference Langley, Rice, van den Bree and Thapar2005). Eleven studies reported no evidence of a causal association between maternal smoking during pregnancy and offspring ADHD diagnosis or symptoms. These include an IVF study, eight discordant sibling studies, and two maternal versus paternal comparisons. A study using the IVF design reported results inconsistent with a causal effect of maternal smoking during pregnancy on offspring ADHD (Thapar et al., Reference Thapar, Rice, Hay, Boivin, Langley, van den Bree and Harold2009). These authors observed association between maternal smoking during pregnancy and offspring ADHD in the genetically related mother–child pairs only. The magnitude of association was greater in the related compared to unrelated mother–child pairs (test for difference in strength of association β = –.10, p < .05). In addition, the study by Thapar et al. (Reference Thapar, Rice, Hay, Boivin, Langley, van den Bree and Harold2009) also carried out sensitivity analyses of paternal smoking during pregnancy for related and unrelated fathers (a different set of parent couples to the previous analysis) and reported findings consistent with a shared genetic influence on paternal smoking and offspring ADHD (similar to that for maternal smoking during pregnancy) such that there was only an association between paternal smoking during pregnancy and offspring ADHD when the father was genetically related to the child. These results are therefore consistent with passive rGE.
Eight discordant sibling studies report results showing that the association between maternal smoking during pregnancy and offspring ADHD was largely due to familial or genetic confounding (D'Onofrio et al., Reference D'Onofrio, van Hulle, Waldman, Rodgers, Harden, Rathouz and Lahey2008; Ellingson et al., Reference Ellingson, Goodnight, van Hulle, Waldman and D'Onofrio2014; Gustavson et al., Reference Gustavson, Ystrom, Stoltenberg, Susser, Suren, Magnus and Reichborn-Kjennerud2017; Knopik et al., Reference Knopik, Marceau, Bidwell, Palmer, Smith, Todorov and Heath2016; Lindblad & Hjern, Reference Lindblad and Hjern2010; Obel et al., Reference Obel, Olsen, Henriksen, Rodriguez, Jarvelin, Moilanen and Gissler2011, Reference Obel, Zhu, Olsen, Breining, Li, Gronborg and Rutter2016; Skoglund et al., Reference Skoglund, Chen, D'Onofrio, Lichtenstein and Larsson2014). These include discordant sibling studies of whole population registries and extremely large samples that are representative of the local population as a whole (sample sizes between 100,000 and 1,000,000; Gustavson et al., Reference Gustavson, Ystrom, Stoltenberg, Susser, Suren, Magnus and Reichborn-Kjennerud2017; Obel et al., Reference Obel, Olsen, Henriksen, Rodriguez, Jarvelin, Moilanen and Gissler2011, Reference Obel, Zhu, Olsen, Breining, Li, Gronborg and Rutter2016; Skoglund et al., Reference Skoglund, Chen, D'Onofrio, Lichtenstein and Larsson2014). For instance, in the study by Obel et al. (Reference Obel, Zhu, Olsen, Breining, Li, Gronborg and Rutter2016) of a Danish national register-based cohort, in the full sample, the adjusted HR of ADHD contingent on exposure to maternal smoking during pregnancy was 2.01, 95% CI [1.94, 2.07]. In contrast, in the discordant sibling comparison (where the rate of ADHD in the exposed and unexposed siblings are compared), the HR was substantially attenuated 1.07, 95% CI [0.94, 1.22]. This suggests that most of the observed association between maternal smoking during pregnancy and offspring ADHD is due to familial confounding. Similar results were reported in a Swedish national register-based cohort (Skoglund et al., Reference Skoglund, Chen, D'Onofrio, Lichtenstein and Larsson2014) such that the level of maternal smoking during pregnancy substantially increased risk of offspring ADHD in conventional observational tests (HRs 1.89moderate smoking; 2.50high smoking). This association was reduced somewhat when controlling statistically for measured confounds, but was substantially attenuated for cousin (HRs 1.45moderate smoking; 1.69high smoking) and sibling comparisons (HRs 0.88moderate smoking; 0.84high smoking). Gustavson et al. (Reference Gustavson, Ystrom, Stoltenberg, Susser, Suren, Magnus and Reichborn-Kjennerud2017) found similar results in using a discordant sibling design. There are four published studies that have used the comparison of maternal and paternal smoking during pregnancy (Gustavson et al., Reference Gustavson, Ystrom, Stoltenberg, Susser, Suren, Magnus and Reichborn-Kjennerud2017; Keyes et al., Reference Keyes, Davey Smith and Susser2014; Kovess et al., Reference Kovess, Keyes, Hamilton, Pez, Bitfoi, Koc and Susser2015; Langley et al., Reference Langley, Heron, Smith and Thapar2012). Two studies report findings inconsistent with a causal effect of maternal smoking during pregnancy on offspring ADHD (Gustavson et al., Reference Gustavson, Ystrom, Stoltenberg, Susser, Suren, Magnus and Reichborn-Kjennerud2017; Langley et al., Reference Langley, Heron, Smith and Thapar2012), and two studies report findings that are at least partially consistent with a causal effect (Keyes et al., Reference Keyes, Davey Smith and Susser2014; Kovess et al., Reference Kovess, Keyes, Hamilton, Pez, Bitfoi, Koc and Susser2015). In the large study by Gustavson et al., three negative control variables were included (paternal smoking during pregnancy, maternal grandmother smoking during pregnancy, and maternal smoking during previous pregnancies). Results showed that associations between maternal smoking during pregnancy (where a intrauterine effect is plausible) on offspring ADHD diagnosis were of a similar magnitude when compared to each of the three negative control variables, HRmaternal smoking = 1.48, 95% CI [1.30, 1.68]; HRpaternal smoking 1.28, 95% CI [1.16, 1.42]; HRmaternal grandmother smoking = 1.28, 95% CI [1.15, 1.42]; HRmaternal previous smoking = 1.53, 95% CI [1.33, 1.75]. These results are therefore inconsistent with a causal intrauterine effect of maternal smoking during pregnancy on offspring ADHD because a similar effect size is seen regardless of which parent smoked and the timing of maternal smoking (during the index pregnancy or a different pregnancy). Similarly, the UK study by Langley et al. (Reference Langley, Heron, Smith and Thapar2012) showed no difference in the magnitude of association of maternal, β = .25, 95% CI [.18, .32], and paternal smoking, β = .21, 95% CI [.15, .27]; test for difference in strength of association F = 0.21, p = .65, during pregnancy and offspring ADHD, and results were therefore inconsistent with a true intrauterine effect. These two studies included data on mothers’ and fathers’ own reports (where available) of their smoking behavior assessed contemporaneously during pregnancy (Gustavson et al., Reference Gustavson, Ystrom, Stoltenberg, Susser, Suren, Magnus and Reichborn-Kjennerud2017; Langley et al., Reference Langley, Heron, Smith and Thapar2012). While the results of Keyes et al. (Reference Keyes, Davey Smith and Susser2014) were inconsistent with a potentially causal effect when no statistical adjustments for measured confounders. The magnitude of association for maternal, β = .22, 95% CI [.11, .33], and paternal smoking, β = .18, 95% CI [.07, .30], was very similar. When statistical adjustments for measured confounders were made, there was an attenuation of the association between paternal smoking during pregnancy, β = .25, 95% CI [.09, .40] for maternal; β = .02, 95% CI [–.20, .24] for paternal. That finding therefore suggest that maternal smoking during pregnancy may be more important than paternal smoking during pregnancy, which is consistent with a causal hypothesis. Nevertheless, in the same study, maternal quitting smoking prior to pregnancy was associated with offspring ADHD to the same extent as maternal smoking during pregnancy. That finding is therefore consistent with dispositional factors that affect the likelihood of women smoking being important in the association with offspring ADHD as opposed to a true intrauterine risk effect. In conclusion, the findings from that study are ambiguous. Results from the study by Kovess et al. (Reference Kovess, Keyes, Hamilton, Pez, Bitfoi, Koc and Susser2015) are similarly difficult to interpret in that the authors observed an association for both maternal and paternal smoking during pregnancy and offspring ADHD in unadjusted associations, OR = 1.82, 95% CI [1.45, 2.29] for maternal; OR = 1.53, 95% CI [1.25, 1.86] for paternal, which were attenuated in both groups, OR = 1.44, 95% CI [1.06, 1.96] for maternal; OR = 1.17, 95% CI [0.92, 1.49] for paternal (slightly more so in the fathers) when statistical adjustment for potential confounders was made. The adjusted association between maternal (and paternal) smoking during pregnancy were attenuated further when teacher reports of ADHD problems were used: OR = 1.33, 95% CI [0.96, 1.84] for maternal; OR = 1.10, 95% CI [0.86, 1.40] for paternal. One methodological issue to note is that these two studies relied on maternal retrospective reports of paternal smoking during pregnancy at child age 10 (Keyes et al., Reference Keyes, Davey Smith and Susser2014) and in a sample of children aged 6–11 years (Kovess et al., Reference Kovess, Keyes, Hamilton, Pez, Bitfoi, Koc and Susser2015). The reliability of a mother retrospectively reporting on her partner's smoking behavior during pregnancy once a relatively long period has elapsed is not known.
In summary, a body of evidence from a series of studies using innovative research designs suggests that it is unlikely that there is a substantial environmental causal effect of maternal smoking during pregnancy on offspring ADHD or conduct problems. Nevertheless, it is important to note that the results of maternal and paternal comparison studies are inconsistent, and methodological tests of the reliability and validity of maternal retrospective reports of paternal smoking during pregnancy are required. There is a need for further studies that include reports on smoking behavior from mothers and fathers assessed during pregnancy rather than after the child is born. The vast majority of genetically informative studies use maternal reports of smoking behavior. There is good evidence that these are reliable and valid: maternal retrospective reports of smoking status correlate highly with contemporaneous reports during pregnancy (Rice, Lewis, et al., Reference Rice, Lewis, Harold, van den Bree, Boivin, Hay and Thapar2007) and with plasma cotinine levels which index recent exposure to nicotine in tobacco smoke (George, Granath, Johansson, & Cnattingius, Reference George, Granath, Johansson and Cnattingius2006). The best evidence for the validity of maternal reported smoking is the consistent evidence for correlations with objective measures of infant birth weight. There is strikingly consistent evidence from genetically sensitive study designs that maternal smoking during pregnancy reduces offspring birth weight in a way that is consistent with a causal effect (Gustavson et al., Reference Gustavson, Ystrom, Stoltenberg, Susser, Suren, Magnus and Reichborn-Kjennerud2017; Langley et al., Reference Langley, Heron, Smith and Thapar2012; Obel et al., Reference Obel, Zhu, Olsen, Breining, Li, Gronborg and Rutter2016; Rice et al., Reference Rice, Harold, Boivin, Hay, van den Bree and Thapar2009; Thapar et al., Reference Thapar, Rice, Hay, Boivin, Langley, van den Bree and Harold2009), illustrating that studies using these methods are able to detect potentially causal intrauterine effects when they are present. This same pattern of findings for maternal smoking during pregnancy and infant birth weight emerges from studies using alternative methods (with differing patterns of bias) including randomized controlled trials of smoking cessation and Mendelian randomization (Tyrrell et al., Reference Tyrrell, Huikari, Christie, Cavadino, Bakker and Brion2012; Veisani, Jenabi, Delpisheh, & Khazaei, Reference Veisani, Jenabi, Delpisheh and Khazaei2017).
Maternal prenatal stress
Our search identified only one genetically informative study that examined maternal prenatal stress and offspring psychopathology in humans (Rice et al., Reference Rice, Harold, Boivin, van den Bree, Hay and Thapar2010). That study used an IVF design, a retrospective measure of perceived maternal stress during pregnancy, which showed reliability (using test–retest methods) and examined childhood anxiety, ADHD, and conduct problems as continuous outcomes (rated by mothers). Results differed for each childhood outcome examined. For ADHD, results were consistent with shared genetic effects as associations were observed in related prenatal mother–child pairs only. For offspring conduct, there was some evidence consistent with a causal intrauterine effect because similarly sized effects were observed in related and unrelated prenatal mother–child pairs. For offspring anxiety, while associations with maternal prenatal stress were observed in both groups, results appeared to be primarily attributable to postnatal anxiety, and postnatal anxiety mediated observed associations in both the related and unrelated groups. This study showed that results differed depending on the child outcome examined. Limitations include the retrospective assessment of perceived stress, which requires validation and the possibility of shared method variance because mothers rated both the exposure and the outcome variable. There is clearly a need for further genetically informative studies focusing on maternal stress in pregnancy given the dearth of such studies. These will need to consider continuity of maternal stress to the postnatal rearing environment (O'Donnell & Meaney, Reference O'Donnell and Meaney2017; Rice et al., Reference Rice, Harold, Boivin, van den Bree, Hay and Thapar2010)
Discussion
After undertaking a systematic review of genetically informative studies, the findings suggest that the prenatal risk factor of maternal smoking during pregnancy has likely causal effects on infant birth weight and prematurity but that there is minimal evidence to support a causal effect on offspring ADHD or conduct problems and much evidence to suggest associations reflect familial confounding and passive rGE. There are too few genetically informative studies of maternal stress in pregnancy to draw firm conclusions in spite of a substantial observational literature on the topic. It seems reasonable to conclude that relatively less attention has been paid to genetically informed studies that include either extremely large samples or extremely informative comparisons but report negative results compared to studies reporting apparently positive results (e.g., Obel et al., Reference Obel, Zhu, Olsen, Breining, Li, Gronborg and Rutter2016; Slotkin, Reference Slotkin2013; Thapar et al., Reference Thapar, Rice, Hay, Boivin, Langley, van den Bree and Harold2009), a problem that applies to the whole of science (Ahmed, Sutton, & Riley, Reference Ahmed, Sutton and Riley2012; Easterbrook, Berlin, Gopalan, & Matthews, Reference Easterbrook, Berlin, Gopalan and Matthews1991). Clear reporting is needed to address this (Academy of Medical Sciences, 2007). The uptake of common analytical strategies is also likely to be helpful. For prenatal risks, there are examples of designs where current evidence using appropriate designs supports causal effects, such as exposure to extreme maternal undernutrition during pregnancy and offspring psychosis risk (Mackay, Dalman, Karlsson, & Gardner, Reference Mackay, Dalman, Karlsson and Gardner2017). In these cases, it will be important to understand the mechanisms through which such exposures influence offspring risk for maladaptive outcomes. In our view, for certain prenatal exposures and outcomes, especially maternal smoking in pregnancy and ADHD or conduct problems, further reports of association from observational designs will be unhelpful because of contributions of person–environment correlation, passive rGE, and the problem of residual confounding. A key reason for identifying if early environmental exposures have causal effects on the likelihood of psychopathology later in life is to guide prevention and early intervention. Effective strategies for reducing maternal smoking remain an appropriate public health target because of the deleterious effects of smoking on fetal growth and with obstetric and perinatal complications including prematurity and miscarriage. Given that maternal smoking in pregnancy is already recognized as a health hazard, we therefore urge researchers in the field of developmental psychopathology to investigate other environmental risk factors amenable to change. Some may be especially or exclusively relevant for mothers in low- and middle-income settings, and studies in these contexts is a priority. Findings from genetically informative and quasi-experimental designs that are well designed will however continue to be important. Avoiding the expenditure of resources on preventive interventions that do not work becomes even more important in low-resource settings. Where studies with different sets of strengths and limitations find converging evidence, this adds to confidence about inferring causal effects. However, what happens when findings from such studies do not “triangulate,” as illustrated by the data presented on maternal smoking in pregnancy? Here it remains crucial that, in addition to considering the key sources of bias, the usual criteria regarding careful consideration of the scientific quality of each of the published quasi-experimental studies prevail, including appropriate design, adequate measurement of exposure and outcome, sample size, and evidence that the assumptions of the method are met. Future directions in this area include a greater use of genetically informed approaches that utilize information on genetic variants associated with psychopathology to test causal inferences. As understanding of the genetic variants contributing to psychiatric disorders and health-related traits increases, such approaches can potentially be applied to a large number of exposures. Genome-wide association studies for psychiatric disorders have now identified many variants robustly associated with disorder. This then provides the opportunity for this information to be used to test hypotheses relevant to the causal contribution of prenatal risk factors to offspring development and psychopathology. One important such approach is Mendelian randomization, which uses genetic variants as instrumental variables to facilitate causal inference within observational data, avoiding bias due to confounding and reverse causality (Davey Smith & Ebrahim, Reference Davey Smith and Ebrahim2003; Davey Smith & Hemani, Reference Davey Smith and Hemani2014; Gage et al., Reference Gage, Munafo and Davey-Smith2016). A number of recommendations and extensions of Mendelian randomization for testing the specific situation of prenatal exposures on offspring outcomes have been developed, including examining data from fathers and offspring as well as the use of maternal genetic instrumental variables where the mother's allele is not transmitted to the offspring (Lawlor et al., Reference Lawlor, Richmond, Warrington, McMahon, Davey Smith, Bowden and Evans2017). However, Mendelian randomization relies on a number of assumptions (Davey Smith & Ebrahim, 2003, Reference Davey Smith and Ebrahim2004; Davey Smith & Hemani, Reference Davey Smith and Hemani2014), and these are not always met for psychopathology. In particular pleiotropy, where the same genetic variant has independent effects on different outcomes, may well exist with respect to psychopathology and complicates interpretation. Care is therefore needed in conducting and interpreting Mendelian randomization findings, and triangulation of evidence is again important. Other potential future directions include application of techniques such as the polygenic transmission disequilibrium test (Weiner et al., Reference Weiner, Wigdor, Ripke, Walters, Kosmicki, Grove and Robinson2017) and examination of placental functioning in the context of genetically informed designs.
Appendix A
Systematic Review Methods
A systematic electronic search of the NCMI PubMed database was conducted on July 10, 2017, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Liberati et al., Reference Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche, Ioannidis and Moher2009). This search (see Figure 3 and Table 2), identified journal articles that investigated the relationship between maternal smoking during pregnancy and offspring attention-deficit/hyperactivity disorder (ADHD) or conduct problems in humans using the following genetically informative designs: in vitro fertilization (IVF) studies, children of twin studies, sibling comparison studies, and maternal and paternal comparison studies. The following search terms were used: (a) (((ADHD OR conduct) AND smoking AND (prenatal OR pregnan* OR maternal) AND (discordant sibling OR sibling))); (b) ((((ADHD OR conduct) AND smoking AND (prenatal OR pregnan* OR maternal) AND (IVF OR assisted reproduct*)); (c) ((ADHD OR conduct) AND smoking AND (prenatal OR pregnan* OR maternal) AND children of twin); and (d) (ADHD OR conduct) AND smoking AND (prenatal OR pregnan* OR maternal) AND (maternal AND paternal AND (comparison OR compared)). In addition to the electronic search, a manual citation search of the studies that passed full-text screening and studies known to the authors were also included. Having removed non-English language articles and duplicates, two authors (K.L. and C.W.) excluded irrelevant articles by title and abstract, blind to the others’ classification with 95% agreement. All differences were resolved on discussion. The full text of 37 articles was screened for final inclusion by two authors (F.R. and K.L.) with 100% agreement. In total, 19 studies were included in the final review. Details of this process are illustrated in Figure 3a. A separate search of studies in humans using the same genetically informative research designs to examine the relationship between maternal stress during pregnancy and offspring psychopathology was carried out on September 5, 2017. This expanded set of offspring outcomes was chosen to expand the studies identified. The following search terms were used: (a) (((ADHD OR conduct OR anx* OR depress* OR emotion*) AND stress AND (prenatal OR pregnan* OR maternal) AND (discordant sibling OR sibling))); (b) (((ADHD OR conduct OR anx* OR depress* OR emotion*) AND stress AND (prenatal OR pregnan* OR maternal) AND (children of twin))); (c) (((ADHD OR conduct OR anx* OR depress* OR emotion*) AND stress AND (prenatal OR pregnan* OR maternal) AND (IVF OR assisted reproduct*))); and (d) (((ADHD OR conduct OR anx* OR depress* OR emotion*) AND stress AND (prenatal OR pregnan* OR maternal) AND (maternal AND paternal AND (comparison OR compared)). Two authors (K.L. and F.R.) excluded irrelevant articles by title and abstract, blind to the others’ classification with 100% agreement. The full text of 5 articles was screened for final inclusion by two of the authors (F.R. and K.L.) also with 100% agreement. One study was included in the final review. In addition to the electronic search, a manual citation search of the studies that passed full-text screening and studies known to the authors were also included. However, no additional studies were identified using this method. The full details of this search can be seen in Figure 3b.








